![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
184 Cards in this Set
- Front
- Back
|
Acute Kidney Injury- Definition |
1. A rapid decline in renal function, with an increase in serum creatinine level (a relative increase of 50% or an absolute increase of 0.5 to 1.0 mg/dL). The Cr my be normal despite a markedly reduced GFR in the early stages of AKI due to the time it takes for Cr to accumulate in the body.
-- this condition is also called Acute Renal Failure (AFR) |
|
|
RIFLE Criteria for defining Acute Kidney Injury stages
|
1. Risk - 1.5 fold increase in the serum Cr or GFR decrease by 25% or the urine output <0.5 mL/kg/hour for 6 hours
2. Injury- Twofold increase in the serum Cr or GFR decrease by 50% or UOP <0.5 mL/kg/hour for 12 hours 3. Failure- threefold increase in the serum Cr or GFR dec by 75% or UOP of <0.5 mL/kg/hour for 24 hours, or anuria for 12 hours 4. Loss- complete loss of kidney function (i.e. requiring dialysis) for more than 4 weeks 5. ESRD- complete loss of kidney function (i.e. requiring dialysis) for more than 3 months |
|
|
What are the most common clinical findings associated with Acute Kidney Injury?
|
Weight gain and edema due to a positive water and sodium balance
|
|
|
Labs in Acute Kidney Injury
|
Azotemia- elevated BUN and creatinine
- Elevated BUN is also seen with catabolic drugs (e.g. steroids), GI/soft tissue bleeding and dietary protein intake - elevated Cr is also seen with increased muscle breakdown and various drugs. **The baseline Cr varies proportionately with muscle mass. ** |
|
|
Prognosis of Acute Kidney Injury
|
- More than 80% of patients with AKI will recover completely, however the prognosis varies widely depending on the severity of renal failure and the presence of comorbid conditions
- The older the patient and the more severe the insult, the lower the likelihood of complete recovery - The most common cause of death is infection (75% of all deaths), followed by cardiorespiratory complications |
|
|
Categories of Acute Kidney Injury
|
1. Prerenal - most common cause of AKI
2. Renal 3. Postrenal |
|
|
Prerenal acute kidney injury
1. how common 2. reversible? 3. etiology |
- most common cause of AKI
- potentially reversible - caused by a decrease in systemic arterial blood volume or renal perfusion- can complicate any disease that causes hypovolemia, low cardiac output and systemic vasodilation - hypovolemia- dehydration, excessive diuretic use, poor fluid intake, vomiting, diarrhea, burns, hemorrhage - CHF - Hypotension- systolic BP below 90 mmHg from sepsis, excessive antihypertensive meds, bleeding and dehydration - renal artery obstruction - kidney is hypoperfused despite elevated BP - cirrhosis, hepatorenal syndrome - In patients with decreased renal perfusion, NSAIDs (constrict afferent arteriole), ACE inhibitors (cause efferent arteriole vasoconstriction) and cyclosporin can precipitate renal failure |
|
|
Pathophysiology of acute kidney injury
|
1. Renal blood flow decreases enough to lower GFR, which leads to decreased clearance of metabolites (BUN, Cr, uremic toxins)
2. because the renal parenchyma is undamaged, tubular function ( and therefore concentrating ability) is preserved. Therefore the kidney responds appropriately, conserving as much sodium and water as possible. - this form of AKI is reversible on restoration of blood flow, but if hypoperfusion persists, ischemia results and can lead to Acute Tubular Necrosis (ATN) |
|
|
Clinical features of prerenal acute kidney injury
|
Signs of volume depletion (dry mucous membranes, hypotension, tachycardia, decreased tissue turgor, oliguria/anuria)
|
|
|
Lab findings in prerenal acute kidney injury
|
- Oliguria is always found in prerenal failure (this is to preserve volume)
- Increased BUN: Cr ratio (>20:1 is the classic one) because the kidney can absorb urea - Increased urine osmolality (>500mOsm/kg H2O) - because the kidney is able to reabsorb water - decreased urine Na+ (<20mEq/L with fractional excretion of sodium (FENa) < 1% because Na+ is actively reabsorbed - Increased urine-plasma Cr ratio (>40:1) because much of the filtrate is reabsorbed- but not the creatinine - Bland urine sediment |
|
|
Intrinsic renal failure
1. definition 2. causes 3. clinical features |
1. kidney damage is such that GFR and tubular function are significantly impaired. The kidneys are unable to concentrate urine effectively
2. Causes - a. tubular disease (ATN)- can be caused by ischemia (most common cause), nephrotoxins b. glomerular disease - acute glomerulonephritis, goodpasture's syndrome, wegener's granulomatosis, PSGN, lupus c. vascular disease- renal artery occlusion, TTP, HUS d. Interstitial disease - allergic interstitial nephritis, often due to hypersensitivity reaction to medication 3. clinical features depend on the cause. Edema is usually present. Recovery may be possible but takes longer than in prerenal failure |
|
|
Laboratory findings in renal causes of acute kidney injury
|
- Decreased BUN:Cr ratio (<20:1, typically closer to 10:1) in comparison with prerenal failure. Both BUN and Cr levels are still elevated, but less urea is reabsorbed than in prerenal failure
- Increased urine Na+ (>40mEq/L with FENa > 2-3%) because Na+ is poorly reabsorbed - decreased urine osmolality (<350 mOsm/kg H2O) - because renal water reabsorption in impaired - decreased urine-plasma Cr ratio (<20:1) because filtrate cannot be reabsorbed |
|
|
Postrenal failure
1. how common 2. causes 3. reversibility? 4. complications |
1. Least common cause of AKI
2. obstruction of any segment of the urinary tract (with intact kidney) leads to increased tubular pressure (urine produced cannot be excreted), which leads to decreased GFR. Blood supply and renal parenchyma are intact. note that BOTH kidney must be obstructed for creatinine to rise 3. renal function is restored if obstruction is relieved before the kidneys are damaged 4. if untreated, can lead to ATN |
|
|
Causes of postrenal failure
|
1. Urethral obstruction secondary to enlarged prostate (BPH) is the most common cause
2. obstruction of a solitary kidney 3. nephrolithiasis 4. obstructing neoplasm (bladder, cervix, prostate and so on) 5. retroperitoneal fibrosis 6. ureteral obstruction is an uncommon causes because obstruction would have to be BILATERAL to cause renal failure |
|
|
Three basic tests for postrenal failure
|
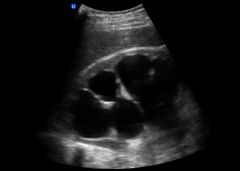
1. physical exam- palpate the bladder
2. US - look for obstruction, hydronephrosis 3. catheter - look for large volume of urine |
|
|
Diagnosis of AKI
|
- diagnosis is usually based on lab findings- elevated BUN and Cr because the patient is typically asymptomatic
- check electrolytes (Na+, K+, Ca2+ and PO43-, albumin levels, CBC with diff - UA - a dipstick positive for protein (3+,4+) suggests intrinsic renal failure due to glomerular insult - microscopic exam of the urine sediment is helpful (hyaline casts are devoid of contents and seen in prerenal failure), RBC casts indicate glomerular disease, WBC casts indicate renal parenchymal inflammation, fatty casts indicate nephrotic syndrome) - urine chemistry- to distinguish between forms of AKI (Urine Na+, Cr and osmolality, urine Na+ depends on dietary intake), FENa : collect urine and plasma electrolytes at the same time - FENa is most useful if oliguria present - urine cultures and sensitivities - renal bx if suspect acute GN or acute allergic insterstitial nephritis - renal arteriography- for Renal art occlusion |
|
|
Role of imaging in diagnosis of AKI
|
- renal US - useful for evaluating kidney size and for excluding urinary tract obstruction (i.e. postrenal failure) - presence of bilateral hydronephrosis or hydroureter. Order for most patients with AKI- unless the cause is obvious and is NOT postrenal
- CT Scan abd/pelvis- may be helpful in some cases, usually done if the renal US shows an abnormality such as hydronephrosis |
|
|
Complications of Acute Kidney Injury
|
1. ECF volume expansion and resulting pulmonary edema- treat with diuretic (furosemide)
2. Metabolic- hyperkalemia- due to decreased excretion of K+ and movement of K+ from the ICF into the ECF due to tissue destruction and acidosis - metabolic acidosis- with increased anion gap- due to decreased excretion of hydrogen ions, if severe (below 16 mEq/L), then correct with sodium bicarb - hypocalcemia - loss of ability to form active vitamin D and rapid development of PTH resistance - hyponatremia may occur if water intake is greater than body losses or if a volume depleted person consumes excessive hypotonic solutions - hyperphosphatemia - hyperuricemia 3. Uremia- toxic end products of metabolism accumulate (esp from protein metabolism) 4. Infection - a common and serious complication of AKI (occurs in 50-60% of cases). The causes is probably multifactorial, but uremia itself is thought to impair immune functions- e.g. pneumonia, UTI, wound infection, and sepsis |
|
|
Treatment of AKI
1. general measures |
1. avoid meds that decrease renal blood flow (NSAIDs) or are nephrotoxic (aminoglycosides, and radiocontrast agents)
2. Adjust med dosages for level of renal function 3. Correct fluid imbalance - If the patient is volume depleted, give IV Fluids. However many patients with AKI are volume overloaded and require diuresis - the goal is to strick a balance between correcting the volume deficits and avoiding volume overload (while maintaining adequate UOP) - monitor fluid balance by daily weight measurements (most accurate estimates) and I&Os - be sure to take into account a patient's cardiac history when considering tx options for fluid imbalance (do not give excessive fluids in a patient with CHF) - correct electrolyte disturbances - optimize CO. BP should be 120-140/80-90. - order dialysis if symptomatic uremia, intractable acidemia, hyperkalemia, or volume overload develop |
|
|
Treatment of prerenal failure
|
1. treat the underlying disorder
2. Give normal saline to maintain euvolemia and restore BP- do not give to patients with edema or ascites. Stop anti-hypertensive meds 3. Eliminate and offending agents (NSAIDs, ACE-Is etc) 4. If the patient is unstable, Swan-Ganz monitoring is indicated for accurate assessment of intravascular volume |
|
|
Treatment of Instrinsic renal failure
|
1. Once ATN develops, therapy is supportive. Eliminate the cause/offending agent.
2. If the patient is oliguric, a trial of furosemide may help increase urine flow. This improves fluid balance |
|
|
Treatment of postrenal failure
|
1. a bladder catheter may be inserted to decompress the urinary tract. Consider urology consult
|
|
|
diagnostic approach to AKI
|
1. history and physical
2. the first thing to do is determine the duration of the renal failure. A baseline Cr level provides this info 3. The second task is to determine whether AKI is prerenal, intrarenal or postrenal. This is done via a combination of history, physical, and labs- signs of volume depletion and CHF suggest prerenal etiology. Signs of allergic reaction (rash) suggest acute interstitial nephritis (intrarenal etiology). A suprapubic mass, BPH or bladder dysfunction suggests a postrenal etiology. 4. Medication review 5. UA 6. Urine chem - FENa, osmolality, urine Cr 7. Renal US (to r/o obstruction) |
|
|
Prerenal failure vs ATN
1. Urine osmolality 2. Urine Na+ 3. FENa 4. urine sediment |
Prerenal failure
1. >500 2. <20 3. < 1% 4. Scant ATN 1. > 350 2. >40 3. > 1% 4. full brownish pigment, granular casts with eipthelial casts |
|
|
Monitoring a patient with AKI
|
1. daily weights, intake and output
2. BP 3. serum electrolytes 4. Watch Hb and Hct for anemia (kidney secretes erythropoeitin-Epo) 5. Watch for infection |
|
|
Rhabdomyolysis
1. definition and cause 2. pathophysiology 3. Lab findings 4. treatment |
1. Skeletal muscle breakdown caused by trauma, crush injuries, prolonged immobility, seizures, snake bites etc
2. release of muscle fiber contents (myoglobin) into bloodstream. Myoglobin is toxic, which can lead to AKI 3. Lab findings include markedly elevated creatine phosphokinase (CPK), hyperkalemia, hypocalcemia, and hyperuricemia 4. Treatment with IV Fluids, mannitol (osmotic diurectic) and bicarbonate (drives K+ back into the cells) |
|
|
Causes of Acute Tubular Necrosis (ATN)
1. Ischemic AKI 2. Nephrotoxic AKI |
1. Ischemic AKI- secondary to severe decline in renal blood flow, as in shock, hemorrhage, sepsis, DIC and HF. Ischemia results in the death of tubular cells
2. Nephrotoxic- Injury secondary to substances that directly injure renal parenchyma and result in cell death. Causes include antibiotics (aminoglycosides, vancomycin), radiocontrast agents, NSAIDs - constrict afferent arteriole (especially in the setting of CHF), poisons, myglobulinuria (from muscle damage, rhabdomyolysis, strenuous exercise), hemoglobinuria (from hemolysis), chemo drugs (cisplatin), and kappa and gamma light chains produced in multiple myeloma |
|
|
Course of Acute Tubular Necrosis (ATN)
|
- onset (insult)
- oliguric phase (azotemia and uremia) - average length 10-14 days. Urine output <400-500 mL/day - Diuretic phase- begins when UOP > 500 mL/day. High UOP due to the following: fluid overload (excretion of retained salt, water and other solutes, that were retained during the oliguric phase), osmotic diuresis due to retained solutes during the oliguric phase; tubular cell damage (delayed recovery of epithelial cell function relative to GFR) - recovery phase - recovery of tubular function |
|
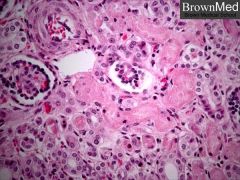
Histology of Acute Tubular Necrosis (ATN)
|
Note the necrosis and sloughing of the epithelial cells of the proximal convoluted tubules, while the glomeruli and distal convoluted tubules are preserved
|
|
|
Urine osmolality
|
1. This is a measure of urine concentration. The higher the osmolality, the more concentrated the urine
2. Dehydration in a healthy person leases to an increase in urine concentration (osmolalitY) as follows: Dehydration causes low intravascular volume, which triggers ADH release, which stimulates the reabsorption of water from the kidney to fill the vasculature. Increased water reabsorption leads to more concentrated urine 3. In ATN, the tubule cells are damaged and cannot absorb water or sodium, so the urine cannot be concentrated, which leads to low urine osmolality |
|
|
What should you always obtain in any patient with AKI?
|
1. UA
2. Urine chem 3. Serum electrolytes (Na+, K+, BUN, Cr), CBC 4. bladder catheterization to r/o obstruction (diagnostic and therapeutic) 5. Renal US to look for obstruction |
|
|
UA Findings in Renal Failure
1. Prerenal 2. Intrarenal a. ATN, b. Acute GN, c. Acute Interstitial Nephritis 3. Postrenal |
1. Prerenal - benign sediment (few hyaline casts), negative for blood and protein
2. Intrarenal a. ATN - "muddy brown" casts, renal tubular cells/casts, granular casts with trace protein and negative blood b. Acute glomerulonephritis- dysmorphic RBCS, RBCs with casts, WBCs with casts, fatty casts, 4+ protein, and 3+ blood c. Acute interstitial nephritis (AIN)- RBCs, WBCs with casts, eosinophils, 1+ protein and 2+ blood 3. Postrenal- benign, may or may not see RBCs and WBCs, negative for blood and protein |
|
|
In the early phases of AKI, what are the most common causes of mortality?
|
1. hyperkalemic cardiac arrest and pulmonary edema
|
|
|
Prognostic factors in AKI
1. severity of renal failure 2. Underlying health of the patient 3. Clinical circumstances |
1. Magnitude of increase in Cr, presence of oliguria, FENa, requirement of dialysis, duration of severe renal failure, marked abnormalities on UA
2. Age and presence, severity and reversibility of the underlying disease 3. cause of renal failure, severity and reversibility of acute process(es), number and type of other failed organ systems, development of sepsis and other complications |
|
|
Differentiation of AKI vs CKD
1. factors that favor chronic kidney disease 2. factors that favor acute kidney injury |
1. history of kidney disease, HTN, abnormal UA and edema, small kidney size on renal US, hyperkalemia, acidemia, hyperphosphatemia, anemia, UA with broad casts (ie more than two or three WBCs in diameter)
2. Acute- return of renal function to normal with time, hyperkalemiam acidemia, hyperphosphatemia and anemia, Urine output <500 mg/day without uremia symptoms |
|
|
Chronic kidney disease - general characteristics
1. definition 2. causes |
1. CKD - defined as either decreased kidney function (GFR <60mL/min) or kidney damage (structural or functional abnormalities)
2. Causes- diabetes is the most common cause (30% of cases), HTN is responsible for 25% of cases, and chronic glomerulonephritis causes 15% of cases. Interstitial nephritis, PCKG, obstructive uropathy. Any of the causes of AKI may lead to CKD if prolonged and/or treatment is delayed |
|
|
Pathophysiology and Epidemiology of CKD
|
1. plasma Cr varies inversely with GFR
2. Cr clearance is the most common clinical measure of GFR 3. any increase in plasma Cr indicates progression, whereas a decrease suggests recovery of renal function (assuming muscle mass has not changed). Most labs not show an estimated GFR each time a Cr is ordered - More common in african american patients than whites |
|
|
Clinical features of CKD
1. Cardio 2. GI 3. Neuro 4. skin |
1. Cardiac- HTN secondary to salt and water retention, decreased GFR stimulates RAAS, which leads to inc BP. Renal failure is the most common cause of secondary HTN.
- CHF - due to volume overload, HTN and anemia - pericarditis (uremia) 2. GI - usually due to uremia --> n/v, decreased appetite 3. Neuro- lethargy, somnolence, confusion, peripheral neuropathy, and uremic seizures. Physical findings include weakness, asterixis, and hyperreflexia. Patients may show "restless legs: - neuropathic pain in the legs that is only relieved by movement 4. pruritis and calciphylaxis (due to calcium and phosphate binding together and depositing in the skin) |
|
|
Hematologic Clinical features of CKD
|
1. Normocytic normochromic anemia (secondary to deficiency of erythropoeitin) - may be severe. Bleeding secondary to platelet dysfunction (due to uremia). Platelets do not granulate in uremic environment
|
|
|
Endocrine/metabolic consequences of CKD - calcium/phosphate disturbances and consequences of this
|
1. Calcium-phosphorus disturbances- decreased renal clearance of phosphate leads to hyperphosphatemia, which results in decreased renal production of active vitamin D (1,25 dihydroxy vitamin D). This leads to hypocalcemia, which causes secondary hyperparathyroidism
- hypocalcemia and hyperphosphatemia are usually seen, but long-standing secondary hyperparathryoidism and calcium-based phosphate binders may sometimes cause hypercalcemia - secondary hyperparathyroidism causes renal osteodystrophy, which causes weakening of the bones and possibly fractures - hyperphosphatemia may cause calciym and phosphate to precipitate, which causes vascular calcifications that may result in necrotic skin lesions. This is call calciphylaxis |
|
|
Sexual/reproductive symptoms seen in CKD
|
Decreased testosterone in men and amenorrhea, infertility, and hyperprolactinemia in women.
- These sx are due to HPA axis disturbances and gonadal response to sex hormones |
|
|
Fluid and electrolyte problems observed in CKD
|
1. Volume overload - watch for pulmonary edema
2. hyperkalemia - due to decreased urinary secretion 3. hypermagnesemia- secondary to decreased urinary loss 4. hyperphosphatemia 5. metabolic acidosis- due to loss of renal mass (and thus decreased ammonia production) and the kidney's inability to excrete H+ |
|
|
Immunologic complications of CKD
|
Uremia inhibits cellular and humoral immunity
|
|
|
Diagnosis of CKD
|
1. UA - examine sediment
2. Measure Cr clearance to estimate GFR 3. CBC (anemia and thrombocytopenia) 4. Serum electrolytes- (e.g. K+, Ca2+, PO43-, serum protein) 5. Renal US - evaluate size of kidneys and r/o obstruction - small kidneys are suggestive of chronic renal insufficiency with little chance of recovery - presence of normal-sized or large kidneys does not exclude CKD - renal biopsy- in select cases to elucidate the cause of CKD |
|
|
Treatment of CKD
1. diet 2. BP control |
1. Low protein diet - 0.7-0.8 g/kg body weight/day. Low salt diet if HTN, CHF or oliguria are present. restrict potassium, phosphate, and magnesium intake
2. strict control decreases the risk of disease progression. ACE-inhibitors are the preferred agents, although sometimes multiple drugs including diuretics are needed. |
|
|
Treatment of CKD
1. ACE-inhibitors 2. glycemic control |
1. ACE-Is dilate the efferent arteriole of the glomerulus. If used early on, they reduce the risk of progression to ESRD because they slow the progression of proteinuria. Use these with great caution though because it can cause hyperkalemia (dec aldo --> not kicking out K+)
|
|
|
What are the most common complications of CKD that require urgent intervention?
|
1. symptomatic volume overload
2. severe hyperkalemia |
|
|
Life-threatening complications of CKD
|
1. hyperkalemia- obtain ECG (be aware that potassium levels can be high even with a normal ECG)- may see peaked T waves
2. pulmonary edema secondary to volume overload- look for recent weight gain 3. Infection (e.g. pneumonia, UTI, sepsis)- most common cause of death |
|
|
Treatment of CKD
1. lifestyle changes 2. correction of electrolyte abnormalities 3. anemia 4. pulmonary edema 5. pruritis 6. what is the only cure? |
1. smoking cessation
2. correct hyperphosphatemia with calcium citrate (a phosphate binder). Most patients will need long term treatment with oral vit D and calcium to prevent secondary hyperparathyroidism and uremic osteodystrophy. Acidosis-- treat the underlying cause (renal failure). Patients may require oral bicarbonate replacement 3. anemia- give Epo 4. pulmonary edema- attempt diuresis, dialysis if this fails 5. try capsaicin cream or cholestyramine and UV light 6. kidney transplant |
|
|
Dialysis- general characteristics
|
- artificial mechanism by which fluid and toxic solutes are removed from the circulation when the kidneys cannot do so sufficiently
- In all forms of dialysis, the blood interfaces with an artificial solution resembling plasma (called dialysate) and diffusion of fluid and solutes occurs across a semi-permeable membrane - two major methods of dialysis are hemodialysis and peritoneal dialysis - the majority of dialysis patients in the Us receive hemodialysis at hospitals and dialysis centers, but more and more are opting for chronic ambulatory peritoneal dialysis - for patients with life threatening complications of AKI, continuous renal replacement therapy can be used for constant renal support |
|
|
Absolute indications for dialysis
|
AEIOU
A- acidosis- significant, intractable metabolic acidosis E- electrolytes- severe persistent hyperkalemia I- intoxications- methanol, ethylene glycol, lithium, aspirin O- overload- hypervolemia not managed by other means U- uremia (severe) - based on clinical presentations, not lab values (uremic pericarditis is an absolute indication for dialysis) - NOTE - not all drugs can by dialyzed in the setting of overdose - Cr levels are NOT an absolute indication for dialysis |
|
|
Settings in which dialysis is considered
1. CKD 2. AKI 3. overdose of medications or substances cleared by the kidneys |
1. CKD- dialysis serves as a bridge for renal transplantation or a permanent treatment when the patient is not a candidate for transplant
2. AKI- dialysis is often required as a temporary measure until the patient's renal function improves 3. Overdose of medications and ingestions- not all meds and toxins can be dialyzed-- some common ones that can be include methanol, ethylene glycol, lithium, aspirin and magnesium containing laxatives |
|
|
Non-emergent indications for dialysis
|
1. Cr and BUN levels are NOT an absolute indication for dialysis.
2. Symptoms of uremia include n/v, lethargy/deterioration in mental status, encephalopathy, seizures and pericarditis |
|
|
Emergent indications for dialysis
|
1. life-threatening manifestations of volume overload- pulmonary edema, and hypertensive emergency refractory to antihypertensive agents
2. severe, refractory electrolyte disturbances - e.g. hyperkalemia and hypermagnesemia 3. severe metabolic acidosis 4. drug toxicity/ingestions (particularly in patients with renal failure)- methanol, ethylene glycol, lithium, acetylsalicylic acid (aspirin) |
|
|
Hemodialysis
1. process 2. frequency |
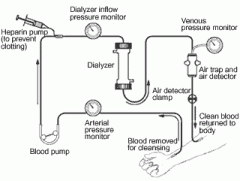
1. the patient's blood is pumped by an artificial pump outside of the body through the dialyzer, which typically consists of fine capillary networks of semipermeable membranes. The dialysate flows outside of these netowrks and fluid and soluted diffuse across the membrane
2. most hemodialysis patients require 3-5 hours of dialysis 3 days/week |
|
|
Hemodialysis access
|
1. a central catheter placed using the seldinger technique is most often used in the subclavian or jugular vein for temporary access
2. tunneled catheters are placed under the skin which leads to a lower rate of infection. These catheters are often suitable for up to 6 months. 3. Arteriovenous fistula- this is the best form of permanent dialysis access. It requires vascular surgery to connect the radial or brachial artery to veins in the forearm. These needs time to mature. An audible bruit over the fistula indicates that it is patent. |
|
|
Alternatives to traditional hemodialysis
|
1. continuous arteriovenous hemodialysis (CAVHD) and continuous venovenous hemodialysis (CVVHD) are often used in hemodynamically unstable patients such as those in the ICU with AKI- they cannot handle large fluid shifts associated with traditional dialysis
|
|
|
Advantages of hemodialysis
|
1. it is considered more efficient than peritoneal dialysis. high flow rates and efficient dialyzers shorten the period of tiem needed for dialysis
2. it can be initiated more quickly than peritoneal dialysis, using temporary vascular access in the emergent setting |
|
|
Disadvantages of hemodialysis
|
1. It is less similar to the physiology of natural kidney function than is peritoneal dialysis, predisposing the patient to: hypotension due to rapid removal of intravascular volume leading to rapid shifts from the extravascular space into cells and hypo-osmolality due to solute removal
- requires vascular access |
|
|
Peritoneal dialysis
1. process 2. frequency |
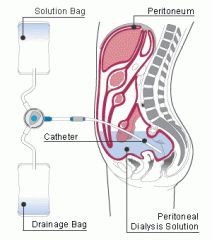
1. The peritoneum serves as a dialysis membrane. Dialysate fluid is infused into the peritoneal cavity, then fluids and solutes from the peritoneal capillaries diffuse into the dialysate fluid, which is then drained from the abdomen.
- a hyperosmolar (high-glucose) solution is used and water is removed from the blood via osmosis 2. frequency- dialysate fluid is drained and replaced every hour in acute peritoneal dialysis but only once every 4-8 houts in chronic per. dialysis |
|
|
Peritoneal dialysis access
|
With CAPD, dialysate is infused into the peritoneal fluid via an implanted catheter. A temporary catheter is used for acute peritoneal dialysis
|
|
|
Advantages of peritoneal dialysis
|
1. The patient can learn how to perform dialysis on his or her own
2. it mimics the physiology of the normal kidney function more closely than hemodialysis in that it is more continuous |
|
|
Disadvantages if peritoneal dialysis
|
1. high gluose load may lead to hyperglycemia and hypertriglyceridemia
2. peritonitis is a significant potential complication' 3. The patients must be highly motivated to self-administer 4. Cosmetic- there is increased abdominal girth due to the dialysate fluid |
|
|
Limitations and complications of hemodialysis
|
1. Limitations- dialysis does not replicate the kidney's synthetic functions. Therefore dialysis patients are still prone to erythropoietin and vit D deficiency
2. Complications of hemodialysis- hypotension- may result in MI, fatigue and so on. The relative hypo-osmolality of the ECF compared with the brain may result in n/v, HA and rarely seizures or coma. The first-use syndrome - chest pain, back pain, and rarely anaphylaxis can occur after a patient uses a new dialysis machine. Complications associated with anticoagulation- hemorrhage, hematoma, etc. Infection of the vascular access site-- may lead to sepsis. Hemodialysis associated amyloidosis of b2 microglobulin in bones and joints |
|
|
Limitations and complications of peritoneal dialysis
|
1. Limitations- dialysis does not replicate the kidney's synthetic functions. Therefore dialysis patients are still prone to erythropoietin and vit D deficiency
2. Complications- peritonitis, often associated with fever and abdominal pain--usually can be treated with intraperitoneal antibiotics. (cloudy peritoneal fluid is the key sign). - abdominal/inguinal hernias- increased risk due to elevated intra-abdominal pressures - hyperglycemia- esp in diabetics - protein malnutrition |
|
|
Proteinuria
1. definition 2. Classes/causes |
1. urinary excretion of >150 mg protein/24 hours
2. a. Glomerular- due to increase glomerular permeability to proteins, can lead to nephrotic syndrome, may be seen in all types of GN. Protein loss tends to be more severe than in nonglomerular causes b. Tubular- small proteins normally filtered at the glomerulus then reabsorbed by the tubules appear in the urine because of abnormal tubules (i.e. due to decreased tubular reabsorption). Proteinuria tends to be less severe. Causes include sickle cells disease, urinary tract obstruction, and interstitial nephritis. c. overflow proteinuria- increased production of small proteins overwhlems the tubules; ability to reabsorb them (e.g. Bence Jones proteins in multiple myeloma) d. other causes- UTI, fever, heavy exertion, stress, CHF, pregnancy, orthostatic proteinuria- occurs when the patient is standing but not when recumbent |
|
|
Nephrotic syndrome
|
1. Urine protein excretion rate > 3.5 g/24 hours
2. hypoalbuminemia- hepatic albumin synthesis cannot keep up with these urinary protein losses. The result is decreased plasma oncotic pressure, which leads to edema 3. Edema- this is often the initial complaint (from pedal edema to periorbital edema to anasarca, ascites, pleural effusion). It results from hypoalbuminemia. Increased aldosterone secretion leads an exacerbation of the problem (increased sodium reabsorption) 4. Hyperlipidemia and lipiduria- increased hepatic synthesis of LDL and VLDL because the liver is revving up albumin synthesis 5. hypercoaguable state (due to loss of certain anticoagulants in the urine)-- increased risk of thromboembolic events - DVT PE, renal vein thrombosis 6. Increased incidence of infection- results from immunoglobulins in the urine - nephrotic syndrome usually indicates significant glomerular disease (either primary or secondary to systemic illness)- the underlying cause is abn glomerular permeability |
|
|
Causes of nephrotic syndrome
|
1. primary glomerular disease (50-75% of cases of nephrotic syndrome) - membranous nephropathy is the most common in adults (40% of cases), followed by focal segmental glomerulosclerosis (FSGS- 35%), and membranoproliferative GN (15%). Minimal change disease is the most common cause in children (75% of cases)
2. Systemic disease0 diabetes, collagen vascular disease, SLE, RA, HSP, PAN, Wegener's granulomatosis 3. amyloidosis, cryoglobulinemia 4. drugs/toxins- captopril, heroin, heavy metals, NSAIDs, penicillamine (used to treat Wilson's disease) 5. Infection- bacterial, viral, protozoal 6. Multiple myeloma, malignant HTN, transplant rejection |
|
|
Diagnosis of nephrotic syndrome
|
1. Urine dipstick (read color changes) - specific for albumin- detects concentrations of 30 mg/dL or higher. Graded ), trace, 1+ (15-30 mg/dL), through 4+ (>500 mg/dL). More sensitive to albumin than immunoglobulins, thus can lead to false negative results when the prominent urinary protein loss is globulin (e.g. multiple myeloma)
2. UA- initial test once proteinuria is detected by dipstick. Examine urine sediment. RBC casts suggests GN, WBC casts suggest pyelonephritis and interstitial nephritis. Fatty casts suggests nephrotic syndrome (lipiduria). If UA confirms the presence of protein, then a 24 hour urine collection for albumin and Cr should be done 3. test for microalbuminuria- corresponds to albumin excretion of 30-300 mg/day. This is below the range of sensitivity of standard dipsticks. If test is positive then perform a radioimmunoassay. This may be an early sign of diabetic nephropathy 4. Other tests - Cr clearance, BUN and Cr, CBC for anemia due to renal failure, serum albumin, renal US- to detect obstruction, masses and cystic disease, Intravenous Pyelogram (IVP)- to detect chronic pyelonephritis, ANA (lupus) anti-GBM, hepatitis serology, antistreptococcal antibodies, complement levels, cryoglobulin studies, serum and urine electrophoresis (myeloma), renal bx- if no cause is identified by less invasive means |
|
|
Treatment of nephrotic syndrome
|
1. Asymptomatic proteinuria- if it is transient, no further workup or treatment is necessary. If it is persistent, then start by checking BP and examining urine sediment. Treat the underlying condition and associated problems (e.g. hyperlipidemia)
2. symptomatic proteinuria- further testing is always required. Treat the underlying disease (DM, multiple myeloma, SLE etc). ACE Inhibitors or ARB - these decrease the urinary albumin loss. Tehy are an essential part of the treatment for diabetics with HTN and should be started before fixed albuminuria is present. Diuretics if edema isp reseny. Limit dietary protein and sodium. Treat hypercholesterolemia (using diet or lipid-lowering agent). Vaccinate against influenza and pneumococcus- there is an increased risk of infection in these patients |
|
|
Urinalysis (UA)
|
pH- normal is 6
specific gravity- directly proportional to urine osmolality - protein - proteinuria is defined as > 150mg/day, nephrotic syndrome >300 mg/day - glucose- absence of glucosuria does not r/o DM - blood - ketones- DKA or starvation - Nitrite- suggests presence of bacteria in urine - leukocyte esterase- suggests presence of WBC in urine- if neg then infection is unlikely - microscopic examination of urine sediment to look for casts, cells, bacteria, WBCs, RBCs, crystals - pic : WBC Cast |
|
|
Hematuria
1. definition 2. microscopic hematuria vs gross hematuria 3. What should be considered the diagnosis of all painless hematuria until proven otherwise? 4. complications |
1. > 3 erythroctyes/HPF on UA
2. more commonly glomerular in origin, whereas gross hematuria is more commonly nonglomerular or urologic in origin 3. bladder or kidney cancer until proven otherwise. Gross hematuria is a common presenting sign in patients with bladder cancer (85%) and patients with RCC up to 40%) 4. obstruction if large clots form in the lower GU tract. Excessive blood loss can lead to IDA |
|
|
Causes of hematuria
|

1. kidney stones
2. infection (UTI, urethritis, pyelonephritis) 3. Bladder or kidney cancer 4. glomerular disease, IgA nephropathy 5. Trauma (foley, trauma, invasive procedures) 6. strenuous exercise- marathon running, fever, - hematuria is generally harmless 7. systemic diseases (SLE, rheumatic fever, HSP, Wegener's granulomatosis, HUS, Goodpastures, PAN) 8. Bleeding disorders (hemophilia, thrombocytopenia) 9. sickle cell disease 10. medications (cyclophosphamide, anticoagulants, salicylates, sulfonamides) 11. analgesic nephropathy 12. polycystic kidney disease, simple cysts 13. BPH- rarely causes isolated hematuria |
|
|
Diagnosis and treatment of hematuria
|
1. urine dipstick- sensitivity in identifying hematuria is > 90%
2. UA- crucial. Examine urine sediment. If RBC cases and proteinuria are also present, a glomerular causes is almost always present (usually GN), If pyuria is present, send for urine culture. If dipstick is positive for blood but UA does not reveal microscopic hematuria (no RBCs), hemoglobinuria or myoglobulinuria is likely present). 3. Urine specimen for cytology- to detect cancers like bladder ca. If suspicion for malignancy is high, perform a cystoscopy to evaluate regardless of cytology results 4. 24 hr urine - test for Cr and protein to assess renal function. Collect if proteinuria is present (if it is heavy- likely glomerular disease pathology) 5. Blood tests- coag studies, CBC, BUN/Cr 6/ IVP, CT scan, US- if no cause is identified by the above tests. Look for stones, tumors, cysts, urethral strictures or vascular malformations 7. renal biopsy- if there is suspicion of glomerular disease 8. tx- treat the underlying cause and maintain urine volume |
|
|
Glomerulonephropathies - general characteristics
1. 2 classes of disease 2. disease progression |
1. glomerular disease can be primary (intrinsic renal pathology) or secondary (to a systemic disease). Two important categories are diseases that present with nephrotic vs nephritic syndrome
2. There is a wide range of disease progression, varying from days to weeks of acute glomerular diseases, to years in the chronic disorders. |
|
|
Causes of glomerular disease
|
1. glomerulonephritis is caused by immune-mediated mechanisms
2. other mechanisms include metabolic and hemodynamic disturbances |
|
|
Clinical features of glomerular disease
|
1. impairment in selective filtration of blood, resulting in excretion of larger substances such as plasma proteins and blood cells. As disease advances, GFR decreases proportionately, leading to renal failure and the possible need for dialysis and/or transplantation.
2. proteinuria, hematuria or both. Nephrotic range proteinuria (>300mg/day) is pathognomonic for glomerular disease |
|
|
Diagnosis of glomerular disease
|
1. UA (hematuria, proteinuria, RBC casts)
2. Blood tests (renal function tests) 3. needle biopsy of the kidney |
|
|
Rapidly progressive glomerulonephritis (RPGN)
|
clinical syndrome that includes any type of GN in which there is a rapid deterioration of renal function over weeks to months, leading to renal failure and ESRD
|
|
|
Possible presentations of glomerular disease
|
1. isolated proteinuria
2. isolated hematuria 3. nephritic syndrome- hematuria, HTN, azotemia 4. nephrotic syndrome- proteinuria, edemia, hypoalbuminemia, hyperlipidemia |
|
|
Pathogenesis of nephritic syndrome vs nephrotic syndrome
|
1. nephritic- inflammation of the glomeruli due to any of the causes of glomerulonephritis
2. nephrotic- abnormal glomerular permeability due to a number of conditions |
|
|
Causes of nephritic syndrome
|
- post-streptococcal GN (PSGN)- most common cause, but may be dye to any of the causes of GN
|
|
|
Causes of nephrotic syndrome
|
- many conditions. Membranous GN is the most common in adults, minimal change disease in kids.
- other causes include diabetes, SLE, drugs, infection, FSGS |
|
|
Lab findings in nephritic vs nephrotic syndromes
|
1. nephritic- hematuria, AKI- azotemia and oliguria, proteinuria if present is mild and not in the nephrotic range
2. nephrotic- urine protein rate > 3.5 g/24 hr, hypoalbuminemia, HLP, fatty casts in the urine pic- fatty cast |
|
|
Clinical findings in nephritic vs nephrotic syndrome
|
1. nephritic- HTN and edema
2. Edema, hypercoaguable states (due to loss of clotting proteins), and increased risk of infection (due to loss of immunoglobulins) |
|
|
Glomerular disease vs Tubular disease
|
- tubular disease is usually acute, where as glomerular disease is more chronic
- tubular disease is often caused by toxins (NSAIDs, contrast, myoglobin, drugs), glomerular disease is typically NOT - tubular disease does NOT cause nephrotic syndrome whereas glomerular disease does - tubular disease does NOT need biopsy, where as glomerular disease does - steroids used for glomerular disease, whereas tubular disease not - immunosuppressive medications used for glomerular disease and not tubular disease |
|
|
Primary Glomerular Disorders
|
1. Minimal change disease
2. Focal Segmental Glomerulosclerosis 3. membranous glomerulonephritis 4. IgA nephropathy (Berger's disease) 5. Hereditary nephritis (Alport's syndrome) |
|
|
Minimal Change Disease
1. nephrotic or nephritic? 2. epidemiology and associations 3. histologic appearance 4. prognosis and treatment 5. most likely cause |
1. nephrotic syndrome- most common presentation
2. most common in children- hodgkin's disease and non-hodgkin's lymphoma have been associated with it 3. No histologic abnormalities on light microscopy, fusion of foot processes on electron microscopy 4. excellent prognosis, responsive to steroid therapy (4-8 weeks), although relapses may occur 5. current evidence points to systemic T cell dysfunction as the most likely root cause |
|
|
Focal Segmental Glomerulosclerosis (FSGS)
1. how common and nephritic or nephrotic 2. prognosis 3. treatment |
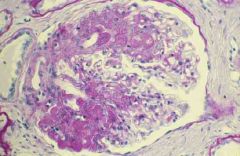
1. This accounts for 25% of the cases of nephrotic syndrome in adults and is more common in blacks. Hematuria and HTN are often present
2. It has a fair to poor prognosis. It is generally resistant to steroid therapy- patients develop renal insufficiency within 5-10 years of diagnosis. The course is progressive 3. The treatment remains controversial, but remission has been achieved in 50% of patietns with the use of cytotoxic agents, steroids, immunosuppressives and ACE-Is/ARBs |
|
|
Membranous Glomerulonephritis
1. presentation 2. cause(s) 3. prognosis and steroid responsiveness |
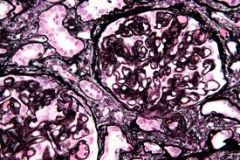
1. usually presents with nephrotic syndrome, glomerular capillary walls are thickened
2. Primary disease is idiopathic. The secondary form is due to infection (hepatitis C, hepatitis B, syphilis, malaria), drugs (gold, captopril, penicillamine), neoplasm or lupus 3. The prognosis is fair to good. The course is variable. Remission is common (in 40% of cases), but renal failure develops in 33%. Steroid therapy does not change the survival rate |
|
|
IgA nephropathy (Berger's disease)
|
1. Asymptomatic recurrent hematuria/mild proteinuria is common. This is the most common cause of glomerular hematuria. Gross hematuria after an upper respiratory infection or exercise is common.
2. renal function is usually normal 3. Mesangial deposition of IgA and C3 on electron microscopy 4. The prognosis in most patients is good with preservation of renal function (renal insufficiency may develop in 25%) 5. Some advocate steroids for unstable disease, but no therapy has been proven effective |
|
|
Hereditary Nephritis (Alport's syndrome)
1. inheritance pattern 2. clinical features 3. treatment |
1. X-linked or autosomal dominant inheritance with variable penetrance
2. features include hematuria, pyuria, proteinuria, high-frequency hearing loss without deafness, progressive renal failure 3. no effective treatment |
|
|
What is the most common cause of ESRD?
|
Diabetic nephropathy
|
|
|
Membranoproliferative Glomerulonephritis
1. cause(s) 2. associations 3. prognosis |
1. Usually due to hepatitis C infection, other causes include hepatitis B, syphilis, and lupus
2. commonly associated with cryoglobulinemia 3. The prognosis is poor. Renal failure develops in 50%. Treatment is rarely effective. |
|
|
Post-streptococcal glomerulonephritis (PSGN)
|
1. This occurs after infection with group A beta-hemolytic streptococcal infection of the upper respiratory tract (or skin- impetigo). The GN develops about 10-14 days after the infection.
2. It primarily affects children (age 2-6) 3. Features include hematuria, edema, HTN, low complement levels and proteinuria 4. Antistreptolysin O (ASO) titer may be elevated indicating a recent strep infection 5. It is self-limited (usually resolves in weeks to months) with an excellent prognosis. Some cases develop in RPGN (more commonly in adults) 6. Therapy is primarily supportive. Antihypertensives, loops diuretics for edema, the use of antibiotics is controversial. Steroids may be helpful in severe cases |
|
|
Goodpasture's syndrome
1-2. clinical features- triad of sx 3. course 4. immunofluorescence pattern 5. treatment |
1. Classic triad of proliferative GN (usually cresenteric), pulmonary hemorrhage, and IgG anti-glomerular basement membrane antibody
2. Clinical features include rapidly progressive renal failure, hemoptysis, cough and dyspnea 3. lung disease precedes kidney disease by days to weeks. It is associated with a variable course 4. renal biopsy shows linear immunofluoresence pattern 5. treat with plasmapheresis to remove circulating anti-IgG antibodies. Cyclophosphamide and steroids can decrease the formation of new antibodies |
|
|
HIV nephropathy
1. characteristics 2. histopathology resembles what other glomerular disease? 3. treatment |
1. proteinuria, edema, and hematuria
2. histopathology most often resembles a collapsing form of FSGS 3. treat with prednisone, ACE-inhibitors, and antiretroviral therapy |
|
|
Acute Interstitial Nephritis (AIN)
1. definition 2. how common 3. causes |
1. inflammation involving the interstitium (tissue that surrounds the glomeruli and tubules).
2. accoutns for 10-15% of cases of AKI 3. Causes - acute allergic reaction to medication is the most common cause- for ex: penicillins, cephalosporins, phenytoin, sulfa drugs, diuretics, anticoags, rifampin, allopurinol, PPIs. Infections (esp in children)- due to a variety of agents, including streptococcus spp and legionella. Collagen vascular disease- e.g. sarcoidosis. Autoimmune disease - for ex: SLE and Sjogrens |
|
|
Clinical features of acute interstitial nephritis (AIN)
|
1. AIN causes AKI and its associated symptoms
2. rash, fever and eosinophilia are the classic findings 3. pyuria and hematuria may be present |
|
|
Diagnosis of acute interstitial nephritis (AIN)
|
1. renal function tests (increased BUN and Cr levels)
2. UA- eosinophils in the urine suggests the diagnosis, given the proper history and findings. Mild proteinuria or microscopic proteinuria may be present. 3. Note that is often impossible to distinguish AIN from ATN on the based on clinical findings alone. Renal biopsy is the only way to distinguish between the two, but it is usually not performed given its invasiveness |
|
|
Treatment of acute interstitial nephritis
|
1. Removing the offending agent is usually enough to reverse the clinical findings.
2. If creatinine continues to increase after stopping the offending agent, steroids may help 3. Treat infection if present |
|
|
Renal Papillary Necrosis
- most common causes - diagnosis - course - appearance - treatment |
- most commonly associated with analgesic nephropathy, diabetic nephropathy, sickle cell disease, urinary tract obstruction, UTI, chronic alcoholism, and renal transplant rejection
- diagnosis is typically made by excretory urogram- note change in papilla or medulla - the course is variable. Some patients have rapid progression, whereas others have a more indolent, chronic course - sloughed, necrotic papillae can cause ureteral obstruction - treat the underlying cause, and stop the offending agents (e.g. NSAIDs) |
|
|
Renal Tubular Acidosis
|
1. RTA is a disorder of the renal tubules that leads to a non-anion gap hyperchloremic metabolic acidosis. Glomerular function is normal
2. it is characterized by a decrease in the H+ excreted in the urine leading to acidemia and urine alkalosis 3. There are three types - 1,2 and 4 - type 3 is a term that is no longer used |
|
|
Type 1 (Distal) Renal Tubular Acidosis (RTA)
1. pathogenesis 2. effects/clinical features 3. type of acid-base disorder 4. symptoms 5. causes 6. treatment |
1. The defect is an inability to secrete H+ at the distal tubule (therefore new bicarb cannot be generated). This inability to acidify the urine results in metabolic acidosis. Although normally the urine pH can be as low as 4.7, in distal RTA the urine pH is never less than 6, regardless of the severity of the metabolic acidosis.
2. It leads to increased excretion of ions (sodium, calcium, potassium, sulfate, phosphate), with the following effects: a. decrease in ECF volume, b. hypokalemia, c. renal stones/nephrocalcinosis (due to increased calcium and phosphate excretion into alkaline urine), d. rickets/osteomalacia in children 3. leads to hypokalemia, hyperchloremic, non-anion gap metabolic acidosis 4. Symptoms are secondary to nephrolithiasis and nephrocalcinosis (up to 70% of patients have kidney stones) 5. causes: congenital, multiple myeloma, nephrocalcinosis, nephrotoxicity (e.g. amphoteracin B), autoimmune diseases (lupus and Sjogrens), medullary sponge kidney and analgesic nephropathy 6. Treatment - correct acidosis with sodium bicarbonate. This can also help prevent kidney stones, which is the major goal of therapy. Administer phosphate salts (promotes excretion of titratable acid) |
|
|
Type 2 - proximal renal tubular acidosis (RTA)
|
1. The defect is in an inability to reabsorb HCO3- at the proximal tubule, resulting in increased excretion of bicarbonate in the urine and metabolic acidosis. The patient loses K+ and Na+ in the urine
2. Characterized by hypokalemic, hyperchloremic non-anion gap metabolic acidosis. (same as in type 1 RTA) 3. Causes- fanconi's syndrome (in children), cystinosis, wilson's disease, lead toxicity, multiple myeloma, nephrotic syndrome, amyloidosis. The excretion of monoclonal light chains is a common feature, so multiple myeloma should always be ruled out in a patient with proximal RTA. 4. Nephrolithiasis and nephrocalcinosis do NOT occur as they do in RTA type 1 5. Treatment: treat the underlying cause. Do NOT give bicarbonate to correct the acidosis because it will excreted in the urine. Sodium restriction increases sodium reabsorption (and thus bicarbonate reabsorption) in the proximal tubule |
|
|
Type 4 renal tubular acidosis
|
1. This can result from any condition that is associated with hyperaldosteronism, or increased renal resistance to aldosterone.
2. It is common in patients with interstitial renal disease and diabetic nephropathy. 3. It is characterized by decreased Na+ absorption and decreased H+ and K+ secretion in the distal tubule 4. Unlike other types of RTA, type 4 results in hyperkalemia and acidic urine-- although a non-anion gap acidosis still occurs. 5. Nephrolithiasis and nephrocalcinosis are rare |
|
|
Fanconi's syndrome
|
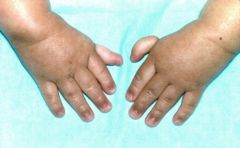
- hereditary or acquired proximal tubule dysfunction that leads to defective transport of some of the following: glucose, amino acids, sodium, potassium, phosphate, uric acid, and bicarbonate.
- It is associated with glucosuria, phosphaturia (leads to skeletal problems: rickets/impaired growth in children; osteomalacia, osteoporosis, and pathologic fractures (in adults), proteinuria, dehydration, type 2 RTA, hypercalciuria, and hypokalemia - treat with phosphate, potassium, alkali and salt supplementation, as well as adequate hydration |
|
|
Hartnup syndrome
|
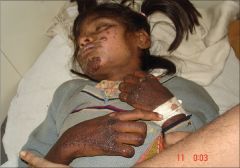
- autosomal recessive inheritance of defective amino acid transporter
- results in decreased intestinal and renal reabsorption of neutral amino acids, such as tryptophan, causing NAD deficiency - clinical features are similar to those of pellagra: dermatitis, diarrhea, ataxia, and psychiatric disturbances - Give supplemental nicotinamide if the patient is symptomatic |
|
|
Autosomal dominant polycystic kidney disease
(ADPKD) -- general characteristics 1. inheritance 2. course |
1. inherited in an autosomal dominant or recessive pattern. ADPKD is the most common genetic cause of chronic kidney disease.
2. The course is variable, but ESRD commonly develops in 50% of the patients (by late 50s to 60s); remainder have a normal lifespan. Renal failure occurs from recurrent episodes of pyelonephritis and nephrolithiasis |
|
|
Clinical features of ADPKD
|
1. Hematuria
2. abdominal pain 3. HTN (in 50% of cases) 4. Palpable kidneys on abdominal exam 5. complications/associated findings a. INTRACEREBRAL BERRY ANEURYSM (in 5-20%) - most do NOT rupture. b. Infection of renal cysts and bleeding into cysts, c. renal failure (late disease), d. kidney stones, e. heart valve abnormalities (especially MVP), f.cysts in other organs (liver, spleen, pancreas, brain), g. diverticula (colon), hernias (abdominal/inguinal) |
|
|
Diagnosis of ADPKD
|
1. ultrasound is confirmatory -- multiple cysts appear on the kidney
2. CT and MRI are alternatives |
|
|
Treatment of ADPKD
|
1. No curative therapy is available
2. Drain cysts if symptomatic 3. Treat infection with antibiotics 4. Control HTN |
|
|
Autosomal Recessive Polycystic Kidney Disease (ARPKD) - general characteristics
1. definition- primary features 2. how common 3. course |
1. previously called infantile polycystic kidney disease. It is characterized by cysts predominantly in the renal collecting ducts as well as hepatic fibrosis
2. It is less common than ADPKD, though the true incidence is unknown since many affected newborns die without proper diagnosis 3. As with ADPKD, there is a wider variability in the level of renal impairment. However, most patients will ultimately experience progressive renal failure |
|
|
ARPKD clinical features
|
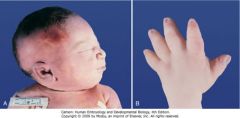
1. LIVER INVOLVEMENT IS ALWAYS PRESENT and may be the dominant clinical feature especially in older individuals
2. Kidneys are increased in size which may cause severe abdominal distention 3. HTN 4. Pulmonary insufficiency secondary to pulmonary hypoplasia and enlarged kidneys limiting diaphragmatic movement may be severe. Pulmonary complications are the leading cause of morbidity and mortality in the neonatal period 5. Newborns with severe ARPKD may present with Potter syndrome, which is a constellation of clinical features associated with decreased amniotic fluid (oligohydramnios). Potter syndrome is characterized by hypoplasia of the lungs, limb abnormalities (e.g. club feet) and characteristic abnormal facies |
|
|
Diagnosis of ARPKD
|
1. some cases are detected prenatally due to widespread use of ultrasound-- oligohydramnios. Less severe cases may not be detected until much later.
2. oligohydramnios during pregnancy usually indicates severe disease 3. US will show characteristics renal cysts in the absence of renal cysts in either parent. US will show hepatomegaly and dilated bile ducts. 4. molecular genetic testing may confirm the disease in cases where diagnosis is unclear |
|
|
Treatment of ARPKD
|
1. No curative therapy is available
2. Manage respiratory issues in newborns, and treat ESRD with renal replacement therapy |
|
|
Medullary Sponge Kidney
|
- characterized by cystic dilation of the collecting ducts
- may present with hematuria, UTIs, or nephrolithiasis, or may be asx - thought to be associated with hyperparathyroidism and parathyroid adenoma - Diagnosed by IVP - No treatment is necessary other than the prevention of stone formation and the treatment of recurrent UTIs |
|
|
Simple Renal Cysts
1. how common, epidemiology 2. course, sx 3. treatment |
1. Very common (50% of people over age 50), incidence increases with age
2. may be single or multiple. Usually asx and discovered incidentally on abdominal ultrasound or other imaging study 3. no treatment is necessary in most cases |
|
|
Renal artery stenosis (renovascular HTN)
|
1. Renal artery stenosis causes a decrease in blood flow to the juxtoglomerular apparatus. As a result the renin-angiotensin-aldosterone system becomes activated, leading to HTN
2. This is the most common cause of secondary HTN 3. Atherosclerosis- accounts for 2/3 of the cases (most often in elderly men), bilateral in up to 1/3 of the cases, smoking and high cholesterol levels are predisposing factors. - fibromuscular dysplasia-- usually seen in young females. Bilateral in 50% of patients |
|
|
Clinical features of renal artery stenosis
|
1. HTN- look for sudden onset of HTN in a patient without a family history. HTN is often severe (may cause malignant HTN) and/or be refractory to medical therapy
2. decreased renal function 3. abdominal bruit (RUQ, LUQ, or epigastrium) is present in 50-80% of patients, it is especially common in patients with fibromuscular dysplasia |
|
|
Diagnosis of Renal Artery Stenosis
|
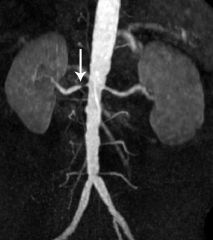
1. Renal arteriogram is the gold standard, but contrast dye can be nephrotoxic-- do NOT use in patients with renal failure
2. MRA has high sensitivity and specificity. The magnetic due is not nephrotoxic so it can be used in patient with renal failure 3. Duplex Doppler US of the renal arteries and contrast enhanced CT may also be helpful in some cases |
|
|
Treatment of Renal Artery Stenosis
|
1. Revascularization with percutaneous transluminal renal angioplasty (PTRA) is the initial treatment in most patients. It has a higher success rate and lower re-stenosis rate with fibromuscular dysplasia than with atherosclerotic type
2. surfery if PRTA is not successful (bypass) 3. conservative medical therapy (ACE-inhibitor, calcium channel blockers) may be tried alone or in combination with revascularization procedures |
|
|
Renal Vein Thrombosis
1. what clinical settings may it be seen in? 2. clinical features 3. diagnosis 4. treatment |
1. May be seen in the following clinical settings: nephrotic syndrome, invasion of the renal vein by RCC, trauma, pregnancy/OCPs, extrinsic compression (retroperitoneal fibrosis, aortic aneurysm, lymphadenopathy) or severe dehydration (in infants)
2. clinical features depend on the acuity and severity of the process and include decreased renal perfusion (can lead to renal failure), flank pain, HTN, hematuria, and proteinuria 3. Diagnostic tests include selective renal venography visualizing the occluding thrombus (definitive study) or IVP 4. Anticoagulate to prevent PE |
|
|
Atheroembolic Disease of the renal arteries
|
- refers to showers of cholesterol crystals that dislodge from plaque in larger arteries and embolize to the renal vasculature
- can occur in other organs as well, such as the retina, brain or skin |
|
|
Hypertensive Nephrosclerosis
1. definition |
1. systemic HTN increases capillary hydrostatic pressure in the glomeruli, leading to benign or malignant sclerosis
|
|
|
Benign nephrosclerosis
|
- thickening of the glomerular afferent arterioles develops in patients with long-standing HTN
- results in mild to moderate increase in Cr levels, microscopic hematuria, and mild proteinuria - advanced disease can lead to ESRD ** nephrosclerosis due to HTN is the second most common cause of ESRD (diabetes is the most common cause)** |
|
|
Malignant Nephrosclerosis
1. when does it occur 2. key features differentiating it from benign nephrosclerosis 3. what patient population is most susceptible 4. clinical manifestations |
- this can develop in a patient with long-standing or benign HTN or in a previously undiagnosed patient
- characterized by a rapid decrease in renal function and accelerated HTN due to diffuse intrarenal vascular injury - African American men are the most susceptible - Clinical manifestations: a rapid increase in Cr, proteinuria, hematuria, RBC and WBC casts in the urine sediment, and sometimes nephrotic syndrome. - microangiopathic hemolytic anemia may also be present |
|
|
Treatment of nephrosclerosis
|
- the most important treatment for both benign and malignant forms is controlling the BP. It is not clear which blood pressure agents should be used in the chronic setting or how effective they are once frank albuminuria is present
- In advance disease, treat as for CKD |
|
|
Sickle Cell Nephropathy
|
- this refers to a sickling of RBCs in the microvasculature, which leads to infarction. In the kidney this occurs mostly in the renal papillae. Recurrent papillary infarction can lead to papillary necrosis, renal failure, and high frequency of UTIs
- nephrotic syndrome can develop (which can lead to renal failure) - it progresses to ESRD in approximately 5% of the patients - ischemic injury to the renal tubules can occur, which increases the risk of dehydration (impaired urine concentration), precipitation sickling crises - ACE inhibitors may be helpful |
|
|
Nephrolithiasis- general characteristics
1. definition 2. sites of obstruction (which is most common) |
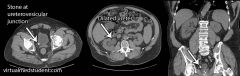
1. the development of stones within the urinary tract
2. a. ureterovesicular junction - most common site of impaction, b. calyx of the kidney, c. ureteropelvic junction, d. intersection of the ureter and the iliac vessels (near the pelvic brim) |
|
|
Risk factors for nephrolithiasis
|
1. Low fluid intake- most common and preventable risk factor
2. family history 3. conditions known to precipitate stone formation (e.g. gout, crohn's disease, hyperparathyroidism, type 1 RTA) 4. medications (e.g. loop diuretics, acetazolamide, antacids, chemotherapy drugs (inc cell lysis-- uric acid stones) 5. male gender (three times more likely to have urolithiasis) 6. UTIs especially with urease-producing bacteria 7. dietary factors- low calcium and high oxalate intake |
|
|
Types of kidney stones
|
1. calcium stones (most common)- 80-85%
2. uric acid stones (second most common) - 10% 3. struvite stones (staghorn stones)- 5-10% 4. cysteine stones - 1% |
|
|
calcium kidney stones
|
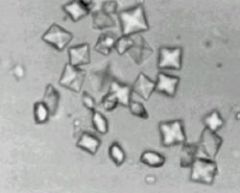
- most common form, 80-85% of urinary stones, composed of calcium oxalate or calcium phosphate (less often) or both
- bypyramidal or biconcave ovals - RADIODENSE - can be seen on plain abd radiograph - secondary to hypercalciuria and hyperoxaluria, which can be due to a variety of causes |
|
|
uric acid urinary stones
|
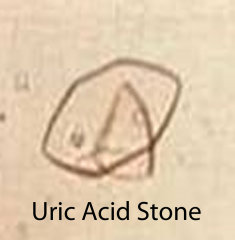
- second most common form - 10% of stones
- a persistently acidic urine pH (<5.5) promotes uric acid stone formation - these are associated with hyperuricemia, secondary to gout or to chemotherapy treatment of leukemias and lymphomas with high cell destruction. The release of purines from dying cells leads to hyperuricemia - flat square plates - stones are radioLUCENT- canNOT be seen on abd radiograph, require CT, US or IVP for detection |
|
|
struvite stones
|
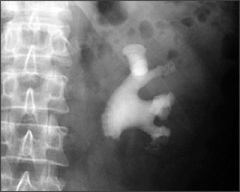
- staghorn stones
- account for 5-10% of stones - radioDENSE (visible on abd xray), rectangular prisms - occur in patients with recurrent UTIs with urease-producing organisms (Proteus, klebsiella, serratia, enterobacter spp) - They are facilitated by alkaline urine: urea-splitting bacteria convert urea to ammonia, this producing alkaline urine - the resultant ammonia combines with magnesium and phosphate to form struvite calculi, which may involve the entire renal collecting system (staghorn stone) |
|
|
Cysteine stones
|
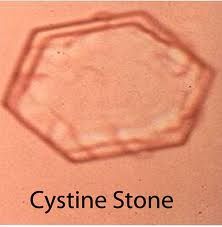
- account for 1% of urinary stones
- genetic predisposition is important (cystinuria- AR) - hexagon-shaped crystals are poorly visualized |
|
|
Clinical course of urinary stones
|
1. if a stone is > 1 cm it is UNLIKELY to pass spontaneously. Stones <0.5 cm usually do pass spontaneously
2. recurrence is common. Up to 50% of the patients have recurrences within in 10 years of having the first stone |
|
|
Clinical features of nephrolithiasis
|
1. Renal colic- pain associated with passing a kidney stone into the ureter, with ureteral obstruction and spasm
- pain begins suddenly and soon may become severe (patient cannot sit still--usually writhes in excruciating pain. Pain may come in waves/paroxysms - location of the pain- begins in the flank and radiates anteriorly toward the groin (i.e. follows the path of the stone) 2. n/v are common 3. hematuria (in over 90% of cases) 4. UTI |
|
|
Diagnosis of nephrolithiasis
1. labs 2. imaging |
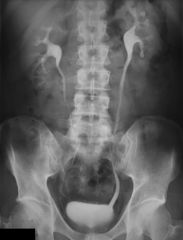
1. UA - reveals either microscopic or gross hematuria. Reveals an associated UTI if pyuria or bacteriuria are present. Examine the urine sediment for crystals (calcium, cysteine, uric acid or struvite crystals). Determine the urinary pH- alkaline urine might indicate the presence of a urease-producing bacteria that can an infection stone. Acidic urine is suggestive of uric Acid stones
b. urine culture c. 24-hour urine- obtain if infection is suspected d. serum chemistry- obtain BUN/Cr levels (for evaluation of renal function) and also calcium, uric acid, and phosphate levels 2. Imaging- Plain radiograph of the kidneys, ureter and bladder (KUB)- initial imaging test for detecting stones (cysteine and uric acid stones are not usually visible on plain films). spiral CT scan without contrast is the gold standard for diagnosis. Most sensitive test for detecting stones. All stones, even radiolucent ones are visible on CT. IVP- most useful test for defining the degree and extent of urinary tract obstruction. This is usually not necessary for the diagnosis of renal calculi. IVP may be appropriate for deciding whether a patient needs procedural therapy. - Renal Us- helps in detecting hydronephrosis or hydroureter. False-negative results are common in early obstruction. Also, there is a low yield in visualizing the stone. Procedure of choice in patient who cannot receive radiation (i.e. pregnant patients) |
|
|
Treatment of nephrolithiasis
1. General measures for all stone types |
1. IV morphine, parenteral NSAIDs (ketorlac), vigorous fluid hydration is always beneficial, abx if infection present, outpatient management for most patients. (see other card for when to hospitalize)
|
|
|
Nephrolithiaisis Treatment
Specific measures depend on the severity of the pain a. mild to moderate pain b. moderate to severe pain |
a. high fluid intake, oral analgesia while waiting for stone to pass spontaneously (give the patient a urine strainer-- want to keep stone for analysis as knowing what it is composed of is helpful for both treatment and prophylaxis)
b. severe pain especially with vomiting-- IV fluids and pain control. Obtain a KUB and IVP to find the site of obstruction. If a stone does NOT pass spontaneously after 3 days, consider a urology consult c. ongoing obstruction and persistent pain not controlled by narcotics- surgery is necessary. Extracorporeal shock wave lithotripsy is the most common method. It breaks the stone apart so that the individual pieces can pass spontaneously. Best for stones > 5mm but <2cm in diameter. If this fails, then percutaneous nephrolithotomy is needed. This is best for all stones > 2cm |
|
|
When should a patient be hospitalized with a diagnosis of nephrolithiasis?
|
1. Pain not controlled with oral medications
2. anuria (usually seen in patients with one kidney) 3. renal colic plus UTI and/or fever 4. large stone (>1 cm) that is unlikely to pass spontaneously |
|
|
Prevention of recurrences in nephrolithiasis
1. Dietary measures 2. pharmacologic measures1 |
1. Dietary measures- high fluid intake is essential (keep urine volume > 2L/day). Limit animal protein intake in patients with hyperuricosuria (uric acid stones). Limit calcium intake only if the patient has calcium stones
2. Pharmacologic measures- thiazide diuretics reduce urinary calcium and have been found to lower recurrence rates, especially in patients with hypercalciuria. Allopurinol is effective in preventing recurrence in patients with high uric acid levels in the urine |
|
|
Urinary Tract Obstruction- general characteristics
1. possible complications 2. what patient population is it most common in? 3. when is AKI most likely to occur? |
1. Can lead to renal insufficiency and hydronephrosis (dilation of urinary tract, specifically the pelvis and calyces)
2. more common in men (due to BPH and prostate cancer) 3. Urinary tract obstruction does not usually cause AKI unless the obstruction is bilateral or there is pre-existing renal damage |
|
|
Classification of urinary tract obstruction
|
1. Acute vs chronic. Acute- clinical features are sudden in onset. Chronic- this causes progressive renal failure/uremia, recurrent infections, and bladder calculi.
2. Lower vs upper tract infection- - lower tract obstruction- below ureterovesical junction- affects urination - upper tract obstruction - above ureterovesical junction- typically causes renal colic 3. complete vs partial obstruction 4. unilateral vs bilateral obstruction (if upper tract) |
|
|
What factors determine the degree of damage to the kidneys and likelihood of return to normal kidney function in urinary tract obstruction?
|
- severity and duration of the obstruction
|
|
|
Causes of lower urinary tract obstruction
|
1. BPH, prostate cancer
2. Urethral stricture, stone 3. neurogenic bladder (MS, diabetes) 4. trauma (pelvic fracture or straddle injury) 5. bladder cancer |
|
|
Causes of upper urinary tract obstruction
1. intrinsic causes 2. extrinsic causes |
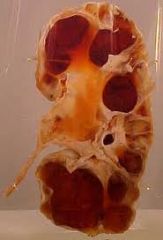
1. intrinsic causes- kidney stones, blood clots, sloughed renal papillae, crystal deposition (uric acid), tumors, strictures, ureteropelvic or ureterovesical junction dysfunction
2. Extrinsic causes- pregnancy, tumors (gynecologic, mets), abdominal aortic aneurysm, retroperitoneal fibrosis, endometriosis, prolapse, hematomas, crohn's disease, diverticulitis ** picture- hydronephrosis pathology specimen |
|
|
Clinical features of urinary tract obstruction
|
** depends on the duration, location, cause and duration of obstruction
1. renal colic and pain- this is more common with acute obstruction (kidney stones, sloughed papilla, blood clot), pain may manifest only during urination. Chronic obstruction may be asx 2. oliguria 3. recurrent UTIs 4. hematuria and proteinuria 5. renal failure |
|
|
Diagnosis of urinary tract obstruction
|
1. Renal US is the initial test- it shows urinary tract dilation. It is very sensitive and specific for identifying hydronephrosis.
2. UA, standard lab tests (CBC, electrolytes, BUN, Cr) 3. KUB- can reveal stones 4. Intravenous urogram - also called IVP- gold standard for diagnosis of ureteral obstruction. contraindicated if the patient is pregnant, allergic to contrast material, or has renal failure 5. voiding cystourethrography - for lower tract obstruction 6. cystoscopy- to evaluate urethra and bladder 7. CT scan to help identify the cause of the obstruction |
|
|
Treatment of urinary tract obstruction
1. what does it depend on 2. location of obstruction a. lower tract b. upper tract 3. Duration and severity of obstruction a. acute complete obstruction b. acute partial obstruction c. chronic partial obstruction |
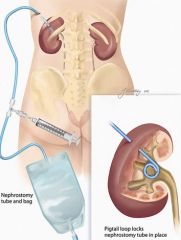
1. Treatment depends on duration, severity, location and cause of the obstruction
2. location of the obstruction a. lower urinary tract obstruction- urethral catheter for acute obstruction. Dilatation or internal urethrotomy - if cause is urethral strictures. Prostatectomy if BPH is the cause b. upper urinary tract obstruction- nephrostomy tube drainage - for acute obstruction. Ureteral stent (through cystoscope) - if ureteral obstruction 3. duration and severity of obstruction a. acute complete obstruction - pain or renal failure may be present. This requires immediate therapy. b. acute partial obstruction- usually due to stones c. chronic partial obstruction - this requires immediate therapy only when infection, severe symptoms, renal failure or urinary retention is present |
|
|
Prostate cancer- general characteristics
1. how common 2. risk factors (what is the most important of these?) |
1. second most common form of cancer in men worldwide. 95% of these are adenocarcinomas
2. risk factors include: age (most important), african-american race, high fat diet, positive family history, exposure to herbicides and pesticides- certain occupations, such as farming and work in industrial chemical industry, present a higher risk |
|
|
Clinical features of prostate cancer
1. early 2. later 3. late |
1. early- it is mostly asx. Cancer begins in the periphery of the gland and moves centrally. Thus obstructive symptoms occur late. In fact, by the time prostate cancer causes urinary tract obstruction, it has often metastasized to bone or lymph nodes
2. later- symptoms are due to obstruction of the urethra. There is difficulty in voiding, dysuria, and increased urinary frequency 3. late- bone pain from mets - most commonly in the vertebral bodies, pelvis and long bones of the legs, weight loss |
|
|
Diagnosis of Prostate Cancer
|
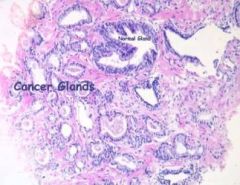
1. Digital Rectal Exam (DRE)
2. PSA (Prostate specific antigen) 3. Transrectal Ultrasound (TRUS) 4. Other tests- bone scan, plain radiographs of the pelvis and spine and a CT of the pelvis and spine to evaluate for mets |
|
|
Digital Rectal Examination (DRE)
|
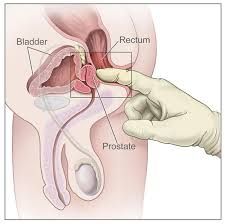
1. carcinoma is characteristically hard, nodular and irregular
2. normal prostate feels like a thenar eminence (group of muscles in the palm of the hand at the base of the thumb), while prostate cancer feels like knuckle. Men with induration, asymmetry or palpable nodularity of prostate need a biopsy, especially if over age 45 c. when palpable, 60-70% have spread beyond the prostate d. if DRE is abnormal, transrectal ultrasound (TRUS) with biopsy is indicated, regardless of the PSA level |
|
|
Prostate Specific Antigen (PSA)
|
** Not routinely used as a screening test, but if the patient requests it then order it. A normal test does NOT r/o prostate cancer
a. PSA is NOT cancer specific. PSA levels also increase as result of: prostatic massage - DRE should not change these, needle biopsy, cystoscopy, BPH, proctatitis, advanced age b. refinements of the PSA assay- some strategies for improving the PSA test include - age-adjusted PSA - because PSA normally increases with age - PSA velocity- analysis of the rate of increase in the level with time. - quantifying free and protein-bound forms of serum PSA- PSA produced by prostate cancer tends to be bound by plasma proteins, whereas PSA produced by normal cells is more likely to free in plasma - PSA density- correlation of PSA levels with prostate volume |
|
|
Transrectal Ultrasound of the prostate with biopsy
|
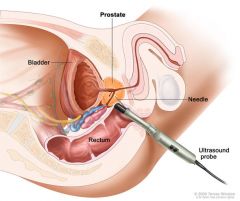
1. may need to repeat biopsies for definitive diagnosis
2. indications - PSA > 10ng/dL (or possibly lower). If PSA > 10, chance of finding cancer is over 50% - PSA velocity > 0.75 per year - abnormal DRE |
|
|
Stages of prostate cancer (A-D)
|
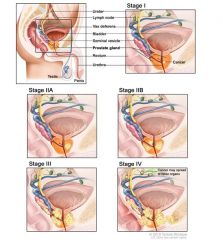
- Stage A- non-palpable and confined to the prostate
- Stage B - palpable nodule, but confined to the prostate - Stage C- extends beyond the capsule without mets - Stage D- metastatic disease |
|
|
Treatment of prostate cancer
1. localized to the prostate 2. locally invasive disease 3. metastatic disease |
1. localized disease (to prostate) - this is usually curable disease. The definitive therapy is a radical prostatectomy. However, watchful waiting is warranted in older men (i.e. those whose remaining life expectancy is < 10 years). The most common complications of prostatectomy are ED and urinary incontinence
2. Locally invasive disease- give radiation therapy plus androgen deprivation (not curative, but decreases the local spread) 3. Metastatic disease- reduces the amount of testosterone with any of the following. - orchiectomy (remove testes)- more common in patients who are noncompliant with medical therapy - antiandrogens - luteinizing hormone-releasing hormone agonists- leuprolide - GnRH antagonists- suppress testosterone by binding receptors in the pituitary without causing a transient surge of LH and FSH (Degarelix) |
|
|
Renal Cell Carcinoma
1. epidemiology- who is more common in? 2. how common as a type of renal cancer 3. cause 4. where does it usually metastasize to? |
1. Twice as common in men as in women
2. RCC comprises about 85% of primary renal cancers (transitional cell is the second most common) 3. Most cases are sporadic, less than 2% are a part of AD Von Hippel-Linday syndrome. The cause is unknown 4. lung, liver, brain and bone. Tumor thrombus can invade the renal vein or IVC, resulting in hematogenous dissemination |
|
|
Risk factors for Renal Cell Carcinoma (RCC)
|
1. Cigarette smoking
2. phenacetin analgesics (high use) 3. ADPKD 4. chronic dialysis (multicystic disease develops) 5. exposure to heavy metals (mercury and cadmium) 6. Hypertension |
|
|
Clinical features of RCC - what is the most common symptom and what are other potential symptoms?
- paraneoplastic syndromes? |
1. hematuria is the most common symptom (gross or microscopic)-- occurs in 70% of the patients
2. abdominal or flank pain - occurs in 50% of patients 3. abdominal (flank) mass- occurs in 40% of patients 4. weight loss, fever 5. paraneoplastic syndromes (uncommon)-- these tumors can ectopically secrete erythropoietin (causing polycythemia), PTH-like hormone (causing hypercalcemia), renin (causing HTN), cortisol (causing Cushing's syndrome), or gonadotropins (causing feminization or masculinization) -- the classic triad of hematuria, flank pain and abdominal mass occurs in less than 10% of patients with RCC |
|
|
Diagnosis of Renal Cell Carcinoma
|
1. Renal US- for detection of renal mass
2. Abdominal CT- with and without contrast. This is the optimal test for diagnosis and staging; perform if ultrasound shows a mass or cysts -- up to 20-30% of RCCs are discovered incidentally on CT scan or US performed for other reasons |
|
|
Treatment of RCC
|
Radical nephrectomy (excision of kidney and adrenal gland, including the Gerota's fascia with excision of nodal tissue along the renal hilum) for stages I through IV
|
|
|
With a combination of DRE and PSA, what percent of prostate cancers can be detected while they are still localized?
|
60%
|
|
|
Bladder cancer- general characteristics
1. how common, what type is most common and where can it occur 2. most common route of spread 3. prognosis s/p bladder excision |
1. bladder carcinoma is the most common type of tumor of the GU tract: 90% of bladder cancers are transitional cell carcinomas. Transitional cell carcinomas can occur anywhere from the kidney to the bladder (e.g. renal pelvis, ureter etc), but 90% of these occur in the bladder. Squamous cell carcinoma (5%) and adenocarcinoma (2%) are also possible
2. the most common route of spread is local extension to surrounding tissues 3. It is likely to recur after removal |
|
|
Risk factors for bladder cancer
|
1. cigarette smoking (major risk factor)
2. industrial carcinogens (aniline dyes, and azo dyes) 3. long-term treatment with cyclophosphamide (may cause hemorrhagic cystitis and increase the risk of transitional cell carcinoma-- so always give it with mesna) |
|
|
Clinical features of bladder cancer
|
1. initial presenting sign is hematuria in most cases (painless hematuria is the classic presentation)
2. Irritable bladder symptoms, such as dysuria and frequency |
|
|
Diagnosis of bladder cancer
|
1. UA and urine culture to r/o infection
2. urine cytology- to detect malignant cells 3. IVP 4. cystoscopy and biopsy (definitive test) 5. chest xray and CT scan- for staging |
|
|
Treatment of bladder cancer by stage
|
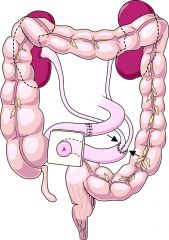
1. stage 0- superficial, limited to the mucosa- carcinoma in situ) - intravesical chemotherapy
2. stage A (involves lamina propria) a. transurethral resection of the bladder tumor b. tends to recur, so frequent cystoscopy and removal of recurrent tumors indicated 3. Stage B (muscle invasion)- radical cystectomy, lymph node dissection, removal of prostate/uterus/ovaries/anterior vaginal wall, and urinary diversion (ileal conduit) 4. stage C- extends to perivesicular fat - treatment is the same as for stage B 5. stage D- mets to LNs, abd organs, or distant sites- consider cystectomy and systemic chemotherapy ** pic- ileal conduit (urinary diversion) |
|
|
Testicular Cancer- general characteristics
1. age most commonly affected 2. prognosis |
1. most common in men aged 20-35 years, but can occur in men of any age
2. has a relatively high cure rate compared with other cancers |
|
|
Types of testicular tumors
|
1. Germ cell tumors (95%)
a. seminomas (35%) b. nonseminomatous (65%) 2. Non-germ cell tumors (5% of all testicular cancers)--usually benign |
|
|
Germ cell tumors of the testicles
|
- These account for 95% of all testicular cancers- most common in men 20-40, curable in > 95% of cases
- seminomas 35%- most common- slow growth and late invasion, most radiosensitive - non-seminomatous- 65%- usually contain cells from at least two of the following four types (mixed cell type): a. embyronal carcinoma (high malignant potentioal, hemorrhage and necrosis are common, mets to the abd LNs and lungs may occur as an early event) b. choriocarcinoma (most aggressive type, rare, mets usually occur by the time of diagnosis) c. teratoma (rarely metastasize) d. yolk sac tumor (rare in men, usually occurs in young boys) |
|
|
Non-germ cell tumors (NGCTs) of the testicles
|
- account for 5% of all testicular cancers
- Leydig cell tumors are hormonally active- most are benign and are treated with surgery. Prognosis is poor if mets occur. They may secrete a variety of steroid hormones, including estrogen and androgens, and associated with precocious puberty in children and gynecomastia in adults - Sertoli cell tumors are usually benign |
|
|
Risk factors for testicular cancer
|
1. cryptochidism- undecended testicle. Surgical correction does NOT eliminate this risk
2. Klinefelter's syndrome (47 X,X,Y) - hypogonadism and sterility, taller, less facial and body hair, larger breasts, weaker bones, broader hips, may have MCI- caused by non-disjunction during meiosis |
|
|
Clinical features of testicular cancer
|
1. Painless mass/lump/firmness of the testicle- because of the lack of pain, it may go unnoticed by the patient until it is advanced
2. gynecomastia may be present because some of the non-seminomatous germ cell tumors produce gonadotropins |
|
|
Diagnosis of testicular cancer
|
1. physical exam (testicular mass)
2. testicular ultrasound - initial test for localizing the tumor 3. Tumor markers- helpful in the diagnosis, staging and monitoring of response to treatment a. b-hcg- always elevated in choriocarcinoma, may be elevated in other types of nonseminomatous germ cell tumors b. AFP- increased in embyronal tumors (in 80% of cases). choriocarcinoma and seminoma never have an elevated AFP 4. CT scan and CXR for staging |
|
|
Differential diagnosis for testicular cancer
|
1. varicocele- dilated, tortuous veins in testicle- bag of worms
2. testicular torsion 3. spermatocele- testicular cyst 4. hydrocele (fluid in the testicle) 5. epididymitis 6. lymphoma |
|
|
Treatment of testicular cancer
|
1. if testicular cancer is suspected based on physical exam or US, the testicle should be removed surgically to confirm the diagnosis. An inguinal approach is used because a scrotal incision may lead to tumor seeding of the scrotum
2. After orchiectomy, perform a CT scan of the chest, abd, pelvis for staging 3. perform b-hcg and AFP measurements after orchiectomy for comparison with the pre-operative values 4. Further treatment depends on the histology of the tumor. Seminoma- inguinal orchiectomy and radiation (very radiosensitive). Nonseminomatous disease- orchiectomy and retroperitoneal lymph node dissection with or without chemo |
|
|
Staging (Boden-Gibb) of testicular cancer
|
1. Stage A- confined to testis/cord
2. Stage B- retroperitoneal lymph node spread (below diaphragm) 3. stage C- distant mets |
|
|
Penile cancer
- when does it most commonly occur? - protective factors? - associations? - presentation? - treatment? |
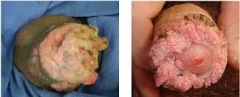
- the peak incidence of this tumor is in men in their 7th decade
- circumcision may have protective effect because penile cancer is very rare in those who have been circumcised - It is associated with HSV and HPV 18 infections - It presents as an exophytic mass on the penis - treatment of the primary disease is local exicision |
|
|
Testicular torsion
1. what is it? 2. when it is usually seen? 3. clinical features? |
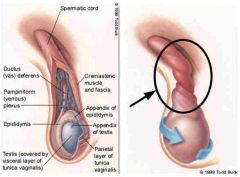
1. twisting of the spermatic cord leading to arterial occlusion and venous outflow obstruction. Ischemia can lead to testicular infarction
2. usually seen in adolescent male patients 3. clinical features include acute severe testicular pain, swollen and tender scrotum and an elevated testicle (as twisting occurs, the testicle moves to a higher position in the scrotum) |
|
|
Treatment of testicular torsion
|
THIS IS A SURGICAL EMERGENCY- the treatment is immediate surgical detorsion and orchiopexy to the scrotum (perform bilaterally to prevent torsion in the contralateral testicle). If surgery is delayed beyond 6 hours, infarction may occur and the testicle may no longer be salvagable
- orchiectomy is appropriate if the testicle is not viable |
|
|
Epididymitis
1. causes by age group 2. clinical features 3. treatment |
- infection of the epididymis. The common offending organism in children and the elderly is E. coli. In young men, STDs are more common (Chlamydia and gonorrhea
- clinical features include a swollen, tender testicle, dysuria, fever/chills, scrotal pain and a scrotal mass - r/o testicular torsion and administer abx - testicular torsion usually has a more abrupt onset and NO fever |

