![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
60 Cards in this Set
- Front
- Back
|
Where does iron absorption occur? |
duodenum |
|
|
Which form of iron is more readily absorbed |
heme (meat) |
|
|
Describe transport and storage of iron |
|
|
|
Causes of Microcytic Anemia |
Problem w/ Normal Hb production 1) Iron deficiency Anemia - Fe deficiency
2) Anemia of Chronic Disease - Fe locked up in Macrophages
3) Sideroblastic Anemia - protoporphyrin def.
4) Thalassemia - Globin defect |
|
|
Most important regulatory step in iron uptake? |
Ferroportin - transport of iron into blood (located on basolateral surface of enterocyte) |
|
|
TIBC |
measures amount of transferrin in blood |
|
|
Serum Iron |
Measure of iron in blood (this iron WILL be bound to transferrin) |
|
|
% Saturation |
percentage of transferrin bound to Fe |
|
|
Serum Ferritin |
amount of iron bound in liver and BM macrophages |
|
|
Lab findings in 1st stage of iron deficiency |
Storage iron is depleted 1st
↑ TIBC b/c liver makes more transferrin to try and pick up more Fe |
|
|
Lab findings in 2nd stage of iron deficiency |
Serum iron is depleted next (2nd)
% Sat of transferrin ↓ |
|
|
Lab findings in 3rd stage of Fe deficiency |
Normocytic anemia |
|
|
Lab findings in 4th stage of iron deficiency |
Microcytic, hypochromic anemia
Erythroblasts divide extra time (Microcytic) in hopes of maintaining close to Normal [Hb] |
|
|
clinical features of iron deficiency |
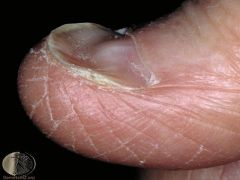
1) Anemia 2) Koilonychia (spoon shaped nails) 3) Pica (chew on abnormal items->eat dirt or other things in search of Fe) |
|
|
Describe RDW & FEP in Iron deficiency anemia |
↑ RDW (RBC Distribution Width) ->spectrum of size of RBC's) due to initial normocytic RBC's mixing with microcytic, so the distribution of width is larger than Normal |
|
|
Plummer-Vinson syndrome |
1) Fe deficiency Anemia presents w/
2) Esophageal webs
3) Atrophic glossitis (smooth tongue) |
|
|
Anemia of Chronic Disease pathophysiology |
chronic inflammation leads to ↑ acute phase reactant: Hepcidin |
|
|
Most common type of anemia in hospitalized patients |
Anemia of chronic disease (associated with chronic inflammation e.g. endocarditis or autoimmune conditions or cancer) |
|
|
Causes of Nutritional Deficiency of Fe |
1. Dietary insufficiency
2. Malabsorption
3. Gastrectomy - ↓Acid b/c part of stomach removed -> less Fe2+ (bioavailable b/c absorbable) since Acid necesary for reducing Fe3+ to Fe2+ |
|
|
Populations suceptible to Fe Deficiency |
1) Infants - Breast feeding (milk is low in Fe)
2) Children - poor diet
3) Adults - ♀- menorrhagia (blood loss) & pregnancy (deficiency b/c fetus uses a lot of Fe) ♂- peptic ulcer dz. (blood loss)
4) Elderly - Rich countries: colon polyps/carcinoma Developing countries: hookworm (Necatur/Ancylostoma) due to blood loss |
|
|
Lab findings in anemia of chronic disease |
↑ ferritin (b/c all Fe is bound up to ferritin)
↓ TIBC - b/c Liver ↓Transferrin levels when Ferritin levels are HIGH ↓ serum Fe - b/c Erythroblasts use up a lot of Fe from blood
↓ %sat - b/c a lot of Fe removed from Transferrin
↑ FEP (Free Erythrocyte Protoporphyrin b/c ↓ heme due to low available Fe) |
|
|
Sideroblastic Anemia pathophysiology |
defective protoporphyrin synthesis
Fe will be transferred from BM Macrophages to Erythroblast Mitochondria and get stuck there b/c Protoporphyrin Deficiency interferes w/ Heme production |
|
|
Rate limiting step in protoporphyrin synthesis? |
In Erythroblast Cytosol
Conversion of: Succinyl-CoA -> Aminolevulinic Acid (ALA)
by the enzyme: Aminolevulinic Acid Synthetase (ALAS)
|
|
|
Classic histological finding in sideroblastic anemia? What causes them? |
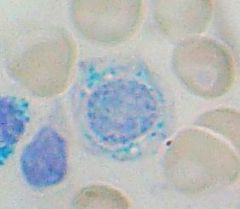
Ringed Sideroblasts
-Fe trapped b/c deficiency of functional Protoporphyrin |
|
|
What stain marks iron? |
Prussian Blue |
|
|
Congenital form of sideroblastic anemia caused by? |
Enzyme defect in ALAS (Aminolevulinic Acid Synthetase): the rate limiting step in Protoporphyrin/Heme Synthesis |
|
|
Acquired form of sideroblastic anemia caused by? |
1) Alcohol - Mitochondrial poison (dissolves mitochon. membranes) that damages production of Protoporphyrin
2) Lead poisoning - Denatures ALAD & Ferrochelatase
3) Vitamin B6 deficiency - ALAS cofactor |
|
|
What drug commonly causes vitamin B6 deficiency? |
Isoniazid treatment (used for TB) |
|
|
Lab findings in sideroblastic anemia |
Fe Overloaded state in Erythroblast Mitoch. Massive Fe stores generate ROS ROS damages erythroblast->cell lysis Fe leaks out Fe picked up by BM MΦ's-> Results in ↑ Ferritin-> ↓ TIBC B/c some Fe leaks out into blood stream-> ↑ serum Fe and ↑% sat
|
|
|
Another Fe Overloaded State w/ very similar lab findings to Sideroblastic Anemia |
Hemochromatosis (so much Fe that it is out of proportion to Normal levels of Protoporphyrin) |
|
|
What disease are people who are carriers for thalassemia protected against? |
Plasmodium falciparum malaria |
|
|
3 types of hemoglobin
|
α2β2 - adult (HbA) |
|
|
genetics of alpha thalassemia... |
4 total alpha globin alleles - 2 on each copy of chromosome 16 |
|
|
cis vs. trans deletion alpha thalassemia |
cis-both deletions occur on same chromosome; seen in Asians (higher risk for severe thalassemia in offspring)
trans-deletion seen in Africans (one deletion occurs on each chromosome) |
|
|
HbH |
Occurs when 3 genes are deleted in alpha thalassemia; B chains form tetramers (HbH) that damage RBCs; HbH is seen on electrophoresis |
|
|
HbBarts
|
Gamma subunit tetramer (B/c insufficient copies of Alpha globin) |
|
|
genetics of beta thalassemia |
|
|
|
Beta thalassemia minor |
|
|
|
What is seen on blood smear in beta thalassemia minor? Electrophoresis? |
Smear: Microcytic, hypochromic RBCs and target cells
Electrophoresis: slightly decreased HbA with increased HbA2 (5%, normal 2.5%) and HbF (2%, normal 1%) |
|
|
Pathophysiology beta thalassemia major |
|
|
|
Consequences of massive erythroid hyperplasia |
|
|
|
What does blood smear show in beta thalassemia major |
microcytic, hypochromic RBCs with target cells and nucleated red blood cells |
|
|
What does electrophoresis show in beta thalassemia major? |
HbA2 and HbF with little or no HbA |
|
|
When does beta thalassemia major present? |
presents with severe anemia a few months after birth (high HbF is temporarily protective) |
|
|
Most common cause of macrocytic anemia |
|
|
|
Where is folate absorbed? |
Jejunum |
|
|
Causes of folate deficiency |
|
|
|
Clinical/lab findings in folate deficiency |
|
|
|
Where is vitamin B12 absorbed? |
Ileum |
|
|
Most common cause of B12 deficiency? |
|
|
|
Clinical/lab findings in B12 deficiency |
|
|
|
Causes of normocytic anemia |
Increased peripheral destruction or underproduction (reticulocyte count helps to distinguish between these) |
|
|
How are young RBCs identified on blood smear? |
Larger cells with bluish cytoplasm (due to residual RNA) |
|
|
Normal reticulocyte count (RC) |
|
|
|
Extravascular hemolysis |
destruction of RBCs by RES (macrophages of spleen, liver, and lymph nodes) |
|
|
Clinical/lab findings in extravascular hemolysis |
|
|
|
Clinical/lab findings in intravascular hemolysis |
|
|
|
Hereditary spherocytosis |
|
|
|
Clinical/lab findings in hereditary spherocytosis |
|
|
|
How is hereditary spherocytosis diagnosed? |
Osmotic fragility test, which reveals increased spherocyte fragility in hypotonic solution |

