![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
|
Erythropoiesis is the production of RBCs in the bone marrow and is dependent on the release of erythropoietin from the kidneys. Name the stimuli for EPO release.
|
Hypoxemia, severe anemia, left-shifted O2 binding curve, high altitude. (Remember: hypoxemia is a decrease in PaO2; hypoxia is inadequate oxygenation of tissue).
|
|
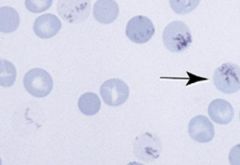
Supravital stain (new methylene blue) of RBCs. What does the arrow point to?
|
Reticulocyte newly released from the bone marrow with thread-like RNA filaments in the cytoplasm. They will mature to RBC in 24 hours.
|
|
|
Define these terms:
1) Hematocrit 2) Microcytic anemia 3) Poikilocytosis 4) Anisocytosis |
1) ~ PCV (packed cell volume): proportion of blood volume that is occupied by RBCs .
2) Decreased size of RBCs. 3) Variation in shape of RBCs. 4) Variation in size of RBCs. |
|
|
What is mean corpuscular volume and what is the equation used to determine it?
What is mean corpuscular hemoglobin concentration and what is the equation used to determine it? |
MCV = Hct / RBCs
MCV is the average volume of RBC. It is used to classify anemia as microcytic, normocytic, or macrocytic. MCHC = Hb / Hct MCHC is the average Hb concentration in RBCs. |
|
|
What would the MCHC, MCV, and MCH of a macrocytic RBC be (elevated, normal, or decreased)?
|
MCHC would be normal.
MCV would be elevated. MCH (weight of hemoglobin in RBC) would be increased. |
|
|
What information does the reticulocyte count give you and how would you correct the calculated reticulocyte count in an anemic patient?
|
Reticulocyte count is a marker of effective erythropoiesis. Normal response is above 3%.
Equation: corrected reticulocyte count = (actual Hct/45)*reticulocyte count |
|
|
What percentage of RBCs in the adult human are HbA, HbA2, and HbF? When does HbA take over for HbF?
|
HbA (2 alpha, 2 beta): 97%
HbA2 (2 alpha, 2 delta): 2.5% HbF (2 alpha, 2 gamma): 0.5% HbA replaces HbF between the first 3-9 months of life. |
|
|
A decrease in MCHC (mean corpuscular hemoglobin concentration) indicates what? How will the RBCs appear?
|
A decrease in MCHC correlates with decreased synthesis of Hb (microcytic anemias). The RBCs will have a greater central area of pallor (hypochromasia). An increase in MCHC indicates spherical RBCs and no central area of pallor.
|
|
|
What microcytic anemia will have an increase RDW?
|
RDW reflects variation in size of RBCs in the peripheral blood (anisocytosis). Iron deficiency is the only microcytic anemia with an increased RDW due to a mixture of normocytic and microcytic RBCs.
|
|
|
Patient has chocolate-colored blood and cyanosis. Administration of O2 doesn't help. Why would methylene blue help this patient?
|
Methylene blue activates metHb reductase. Methemoglobin is Hb with oxidized heme groups (Fe3+).
|
|
|
How do mature RBCs:
Produce ATP? Protect against hydrogen peroxide? |
Mature RBCs get ATP from anaerobic glycolysis. Lactic acid is the end product of RBC metabolism.
Pentose phosphate pathway produces NADPH in RBCs. NADPH and glutathione reductase reduce glutathione. Glutathione neutralizes hydrogen peroxide. |
|
|
What is:
1) Serum ferritin 2) Serum iron 3) TIBC 4) Iron saturation |
1) Serum ferritin correlates with ferritin stores in the macrophages. Ferritin is the soluble iron storage protein.
2) Serum iron represents iron bound to transferrin. 3) TIBC = serum iron + transferrin 4) Iron saturation = serum iron/TIBC |
|
|
What is the relationship of transferrin synthesis with ferritin stores in macrophages?
|
Decreased ferritin stores cause increased synthesis of transferrin.
|
|
|
Based on these iron studies, what is the pathology?
1) Increased serum ferritin, decreased serum iron, decrease TIBC 2) Decreased serum ferritin, increased TIBC, decreased iron saturation 3) Increased ferritin, decrease in tranferrin, increase in iron saturation |
1) Anemia of chronic disease. Hepcidin inhibits the release of iron.
2) Iron deficiency 3) Iron overload |
|
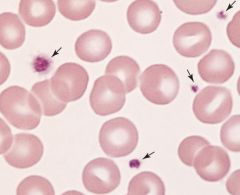
Describe these RBCs.
|
Normal RBCs. Area of pallor is Slightly less than half the total diameter of the RBC.
|
|
|
Describe the synthesis of protoporphyrin IX.
|
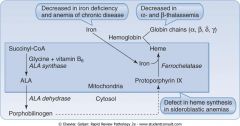
|
|
|
What are the four types of microcytic anemia? What are the RBCs microcytic?
|
Microcytic anemia: defects in the synthesis of Hb (heme + globin chains). Iron deficiency, sideroblastic anemia, thalassemia (alpha and beta), and anemia of chronic disease (ACD).
[Hb] determines the number of cell divisions a maturing RBC will undergo. A decrease in [Hb] will lead to an increase number of divisions resulting in a decreased MCV. |
|
|
What is the most common cause of microcytic (MCV < 80) anemia in men younger than 50? Women under 50? Men and women over 50?
|
The most common cause of microcytic anemia is iron deficiency. Men - peptic ulcer disease. Women - menorrhagia. M/W over 50 - polyps/colorectal cancer.
|
|
|
Patient has dysphagia for solids, achlorhydria, a swollen tongue, and koilonychia. Labs: decreased MCV, increased TIBC, and increased RDW. Diagnosis?
|
Iron deficiency. Plummer-Vinson syndrom: esophageal web, achlorhydria, glossitis, koilonychia (spoon nails).
|
|
|
Patient presents with diarrhea, abdominal pain, weight loss and fatigue. FBC shows a microcytic anemia. The patient is also eosinophilic. What caused this patients symptoms?
|
Necator americanus or ancyclostoma duodenale (hookworms - helminths) penetrate through the skin, travel to the lung, are coughed up, and then swallowed. Necator sucks blood from the intestinal wall causing iron deficiency. Eggs in stool can help diagnosis.
|
|
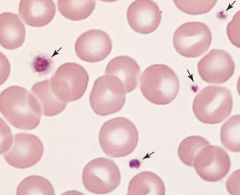
Need to enter diff pic. this is the wrong pic.
|
Iron deficiency: enlarged central area of pallor in the RBC.
|
|
|
Thalassemia is a quantitative problem of too few globins synthesized. What are the two types of thalassmia and what chromosomes does the defective arise from?
|
Alpha-thalassemia: alpha chain is synthesized by 2 gens on chromosome 16 (4 genes total). Four variants: silent carrier, alpha-thal, HbH (four beta chains), and Hb Barts.
Beta-thalassemia: one gene on chromosome 11. B-thal minor and B-thal major (Cooley's anemia). |
|
|
What would the Hb electrophoresis look like in alpha-thal trait?
|
Normal, because all Hb types require alpha-globin chain. The Hb concentration is decreased; however, the relative proportions of the normal Hbs remains the same.
|
|
|
What are the genetic abnormalities that cause beta-thalassemia? How does the Hb electrophoresis look like in beta-thal minor and major?
|
Mild anemia is caused by DNA splicing defect. Severe anemia is caused by DNA stop codons.
Beta-thal minor: decreased HbA, increased HbA2 and HbF. Beta-thal major: absent HbA, increased HbA2 and HbF. |
|
|
Name the causes of sideroblastic anemia.
|
Chronic alcoholism: alcohol is mitochondrial toxin; heme synthesis occurs in the mitochondria.
Pyridoxine (B6) deficiency: B6 is cofactor for aminolevulinic acid synthase. Often due to isoniazid. Lead poisoning: Pb denatures ferrochelatase, ALA dehydrase, and ribonuclease. |
|
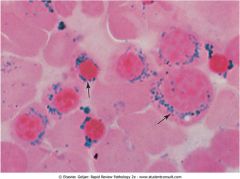
Bone marrow aspirate. What is the most common cause of this?
|
Sideroblastic anemia: defect in heme synthesis in the mitochondira is most commonly caused by chronic alcoholism. The picture shows ringed sideroblasts: blue iron granules around the nucleus of normoblasts which represent iron trapped within the mitochondria.
|
|
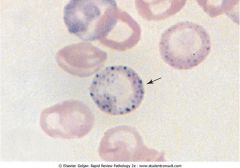
What is shown in these mature RBCs?
|
Sideroblastic anemia due to Pb poisoning causing basophilic stippling. Lead denatures ribonuclease, hence, he ribosomes persist in the cytoplasm.
|
|
|
Child presents with features that resemble encephalopathy. There is significant evidence of cerebral edema. Furthermore, there is marked demyelination and damage to neurons. How can lead poisoning lead to this condition?
|
Pb inhibits aminolevulinic acid dehydrase leading to a build up of ALA to toxic levels. ALA damages neurons, causes cerebral edema, and demyelination.
|
|
|
50 year old male presents with a foot drop. The patient is not diabetic. His labs show: increase in serum iron, increase in iron saturation, increase ferritin, and a decrease in TIBC. Diagnosis?
|
Iron overload. Most likely Pb poisoning causing peroneal nerve palsy.
|
|
|
Explain where and how B12 is absorbed.
|
Pepsin frees vitamin B12 from ingested proteins (pepsin requires H+). Free vit B12 is bound to R-binders (from salivary glands). Pancreatic enzymes in the duodenum cleave off the R-binders. Vit B12 binds to IF (from parietal cells). B12-IF is absorbed in the terminal ileum. B12 supplies last 6-9 years.
|
|
|
What is the pathogenesis of macrocytic anemia?
|
Impaired DNA synthesis (lack of B12 or folate) leads to a delay in nuclear maturation; a block in cell division leads to large, nucleated hematopoietic cells (megaloblasts). Cellular RNA and protein synthesis continue unabated; cytoplasmic volume continues to expand.
|
|
|
Name some causes of B12 deficiency.
|
Decreased intake: vegan diet, malnutrition.
Malabsorption: pernicious anemia, decrease gastric acid, Crohn's or celiac involving terminal ileum, bacterial overgrowth, diphyllobothrium latum (fish tapeworm). Increased utilization: pregnancy/lactation. |
|
|
Describe how each drug inhibits folate:
5-Fluorouracil Methotrexate, TMP-SMX Phenytoin Oral contraceptives Alcohol |
Inhibits thymidylate synthase.
Inhibit DHFR. Inhibits intestinal conjugase. OC: inhibit uptake of monoglutamate in jejunum. Alcohol: inhibits the release of folate from the liver. |
|
|
Draw out the relationship between B12, folate, and dUMP to dTMP.
|
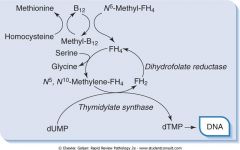
|
|
|
How does B12 cause peripheral neuropathy with sensorimotor dysfunction? Specifically what regions of the spinal cord does B12 deficiency effect?
|
B12 is involved in odd-chain FA metabolism. Odd-chains are broken down to propionyl-CoA and then to methylmalonyl CoA. Methylmalonyl CoA is converted to succinyl CoA via methylmalonyl CoA mutase and B12. B12 deficiency leads to an increase methylmalonyl CoA and corresponding acids which replace acetyl CoA in neuronal membranes, resulting in demyelination. Neurologic disease: posterior column dysfunction (vibratory sensation and proprioception) and later corticospinal tract (spasticity). Dementia.
|
|
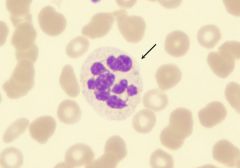
|
Hypersegmented neutrophils are excellent markers of folate and vitamin B12 deficiency.
|
|
|
Schillings test is used to determine the cause of _________. Explain the test.
|
Vit B12 deficiency.
Oral administration of radioactive B12. Reabsorption of B12 indicates dietary deficiency. Reabsorption of B12 after IF indicates pernicious anemia. Reabsorption of B12 after antibiotics indicates bacterial overgrowth. Reabsorption of B12 after administration of pancreatic enzymes indicate chronic pancreatitis. |
|
|
What are the causes of normocytic anemia with a corrected reticulocyte count of < 3%?
|
Blood loss < 1 week.
Early-stage iron deficiency. Early-stage ACD. Aplastic anemia. Renal disease (decrease EPO synthesis). |
|
|
Hemolytic anemias can due to intrinsic (defect within RBC) or extrinsic factors. Describe the two mechanisms (locations) of hemolysis.
|
Extravascular: phagocytosis by macrophages in the spleen; RBCs coated with IgG. Leads to an increase in serum unconjugated bilirubin.
Intravascular hemolysis: hemolysis occurs within blood vessels. This leads to a decrease in serum haptoglobin (combines with Hb). |
|
|
Patient presents with jaundice and abdominal pain radiating to the right scapula. Its discovered the patient has pigmented gallstones. Blood studies show a normocytic anemia. You explain to the patient they have a autosomal dominant disorder and that a splenectomy would be beneficial. What is the pathogenesis of this disease?
|
Hereditary spherocytosis (AD) is an intrinsic defect with extravascular hemolysis. Membrane protein defect results in the loss of RBC membrane and spherocyte formation: mutation in ankyrin (most common), or band 2, spectrin, or band 3. Increased permeability of spherocytes to sodium.
|
|
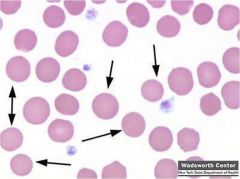
|
Hereditary spherocytosis (autosomal dominant). Defect in ankyrin leading to a loss in RBC membrane and spherocyte formation. No area of pallor.
|
|
|
What is the inheritance of:
Hereditary spherocytosis Sickle cell anemia Hereditary elliptocytosis Pyruvate kinase deficiency G6PD deficiency |
AD
AR AD AR X-link |
|
|
A normocytic anemia characterized by intravascular complement-mediated lysis of RBCs, neutrophils, and platelets occurring at night indicates what disease? How would you screen and confirm?
|
Paroxysmal nocturnal hemoglobinuria (PNH): acquired membrane defect in multipotent myeloid stem cells. Mutation causes loss of the anchor for decay accelerating factor which neutralizes complement attached to RBCs, neutrophils, and platelets.
Screen - sucrose hemolysis test. Confirm - Ham test (acidified serum test). |
|
|
What is the DNA mutation in sickle cell anemia?
|
Missense point mutation: substitution of valine for glutamic acid at sixth position of beta-globin chain.
|
|
|
Explain the threshold for sickling and what factors increase sickling in sickle cell anemia.
|
Sickling occurs when HbS molecules aggregate and polymerize into long needle-like fibers (boat-shaped). HbS concentration greater than 60% is the most important factor. HbAS trait doesn't sickle. Increase in deoxyhemoglobin increases the risk for sickling: acidosis, volume depletion, hypoxemia.
|
|
|
What is the danger of sickling in HbS? What endogenous substance decreases sickling?
|
Sickling increases adherence to endothelial cells in the microcirculation. Microvascular occlusions (vaso-occlusive crises) produce ischemic damage. HbF prevents sickling; first 5-6 months of life for an HbSS patient are protected. Hydroxyurea increases the synthesis of HbF.
|
|
|
What is the most common cause of death in adults in HbS patients? What is the most common presentation in infants?
|
Adults: acute chest syndrome - vaso-occlusion of pulmonary capillaries, chest pain, hypoxemia. Children have painful swelling of hands and feet due to bone infarcts.
|
|
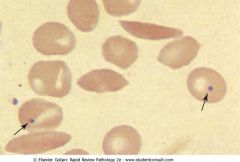
What infections is the patient susceptible to?
|
Peripheral blood with sickle cells and Howell-Jolly bodies (nuclear remnants). HbS patient undergo autosplenectomy and lose macrophage function; patients are susceptible to encapsulated bacteria: streptococcus pneumoniae, Salmonella paratyphi (osteomyelitis).
|
|
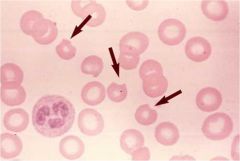
|
Heinz bodies are formed by damage to the hemoglobin component molecules, usually through oxidations, which causes the damaged molecules to precipitate and damages the cell membrane. Damaged cells are attacked by macrophages in the spleen, where the precipitate and damaged membrane are removed, leading to characteristic "bite cells".
Commonly occurs in G6PD |
|
|
Patient presents with fatigue and a decreased Hct. Labs come back: MCV - 90, elevated levels of 2,3-BPG, and peripheral blood smear shows echinocytes. Diagnosis?
|
Pyruvate kinase deficiency - autosomal recessive disorder. Path: intrinsic defect with extravascular hemolysis. Lack of ATP causes membrane damage and dehydration of the RBC, producing echinocytes.
|
|
|
Describe the difference between warm and cold type autoimmune hemolytic anemias.
|
Warm: RBCs coated by IgG are phagocytosed by splenic macrophages (extravascular). Spherocytes are produced if a small amount of membrane is removed.
Cold: IgM mediated hemolysis may be extravascular or intravascular depending on the degree of complement activaiton. |
|
|
What features are different between intravascular and extravascular hemolysis?
|
Extravascular: leads to an increase in serum unconjugated bilirubin, jaundice, and increased serum lactate dehydrogenase.
Intravascular: incresed plasma and urine Hb, hemosiderinuria, decreased serum haptoglobin, and increased serum LDH. |
|
|
Explain how the following drugs lead to hemolytic anemia:
Penicillin Quinidine Methyldopa |
Penicillin: IgG Abs against penicillin attached to RBC membrane.
Quinidine: immunocomplex (drug-IgM) depositis on the RBC causing intravascular hemolysis. Methyldope: drug alter Rh Ag on RBC causing autoantibodies against the Rh. |
|
|
Name some microangiopathic hemolytic anemias.
Name some macroagniopathic hemolytic anemias. |
Micro: HUS, TTP, DIC, HELLP.
Macro: aortic stenosis, prosthetic heart valves. |

