![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
22 Cards in this Set
- Front
- Back
- 3rd side (hint)
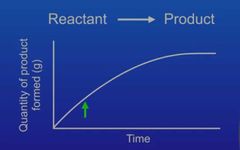
Describe the what happens in the quantity of product formed over time graph |
At the start the line is steep= fast reaction is taking place. Lot of reactant molecules are reacting and forming into product. The slope then gradually gets less steep due to the reaction slowing down. This is because most of the reactant molecules have turned into product. The line then becomes flat which indicates that the reaction has stopped (all of the reactant has turned into product)p.s gas is measured in cm3 |
Reactant>product |
|
|
What is the formula to find out the mean rate of reaction? |

Quantity of product formed/time taken |
|
|
|
30 grams of reactant was used in 10 seconds calculate the mean rate of reaction |
30/10= mean rate of reaction: 3g/s |
|
|
|
What is a tangent and what can it be used for |
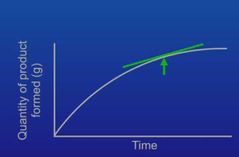
A tangent is a straight line that touches a curve and it's used for measuring the rate of a reaction |
|
|
|
How do you use the tangent to measure the rate of reaction at a specific point? |
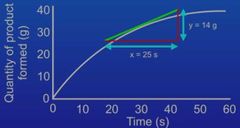
Firstly draw your tangent. Then construct a triangle from it. Then find the length (X axis) and width(y axis) of the triangle (in grams) then divide the y axis by the X axis. |
Straight line |
|
|
What is collision theory |
Collision theory States that in order for a chemical reaction to occur, the reacting particles must collide with each other. These collisions must have sufficient energy. The rate of reaction is determined by the frequency of collisions per second(number collisions per second) |
|
|
|
What is the relation between concentration and rate of reaction? |
As the concentration increases so does the rate of reaction.which means that the concentration is proportional to the rate of reaction. |
|
|
|
How to carry out the disappearing cross reaction |
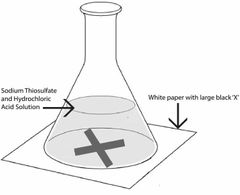
Sodium thiosulfate+hydrochloric acid makes sulfur which can make a solution cloudy. In this reaction, one of the products is sulfur. Firstly, put 10cm3 sodium thiosulfate and 10cm3 of hydrochloric acid solution into a conical flask and put a printed black cross underneath. Swirl the solution and start the timer. After some time the solution will turn cloudy. You then stop the timer when you can no longer see the cross. Repeat the experiment with lower concentrations of sodium thiosulfate solution. After completing the experiment , calculate the mean values for each concentration of sodium thiosulfate. Don't include any anomalous results when calculating the mean. |
Cloudy solution |
|

What are the negatives of the disappearing cross experiment |
Everyone has different eyesight so one person may see the cross for a longer period of time than someone with bad eyesight. This can be resolved however by using a larger cross. |
|
|

How do you carry out the hydrogen gas experiment |
Magnesium+hydrochloric acid makes magnesium chloride+hydrogen. This hydrogen gas allows us to measure the volume of hydrogen of gas produced. Firstly put 50cm3 hydrochloric acid into a conical flask then attach the flask to a bung and delivery tube. Then place delivery tube into container filled with water. Then place a measuring cylinder over the delivery tube. Add a 3 cm strip of magnesium to the hydrochloric acid and start the timer. The reaction produces hydrogen gas (which is trapped in measuring cylinder).Every 10secs measure the volume of hydrogen gas in the measuring cylinder until no gas is given off. Repeat the experiment using different concentrations of hydrochloric acid. |
|
|
|
What do both the disappearing cross and the hydrogen gas experiment show? |
They show that the greater the concentration the faster the reaction. |
|
|
|
What is the relation between the surface area and rate of reaction |
The rate increases when we increase the surface area of a solid. |
|
|
|
What is surface area to volume ratio? |
Smaller sized blocks have a larger surface area to volume ratio than larger blocks. This means that they have more particles on the surface which means more collisions per second. This increases the rate of reaction. |
|
|
|
What is the investigation of surface area to volume ratio |
Firstly put hydrochloric acid and calcium carbonate in a conical flask and put a marble chip in it And attach it to a bung and delivery tube (tube should be in container filled with water with a measuring cylinder on top.) The reaction should produce carbon dioxide gas. Repeat the experiment by putting more marble chips at a smaller size in the test tube . It can be hard to measure the hydrogen gas due to how rapid it is. |
|
|
|
What is the alternative investigation to the surface area to volume ratio experiment |
Place a conical flask containing hydrochloric acid with a marble chip inside and put it in top of a balance. Some of the mass is lost due to carbon dioxide escaping which can help us determine the rate of reaction. Putting cotton wool on top of the conical flask prevents acid from escaping. If it did escape, then we would get an anomalous result. However carbon dioxide can still escape through the cotton wool. |
|
|
|
What is activation energy? |
Is the minimum amount of energy required for particles to collide/react successfully |
|
|
|
What is the relation between temperature and rate of reaction? |
Increasing the rate of reaction increases the energy in particles. This makes the particles move faster which means more collisions.This means that more particles can overcome the activation energy barrier by colliding successfully. Which increases the rate of reaction. |
|
|
|
Why are catalysts useful |
They can speed up reactions at a low temperature which uses less energy and saves money. |
|
|
|
How do catalysts work? |
They increase the rate of reaction by choosing a different pathway for the reaction that has a lower activation energy. This means when a catalyst is present, the particles require less energy to cross the activation barrier. |
|
|
|
State a key fact about energy in reversible reactions |
If a reversible reaction is endothermic in one direction, then it's exothermic in the opposite direction. |
|
|
|
What is equilibrium |
It's when the forward and reverse reactions take place at the same rate |
|
|
|
What is Le chateliers principle? |
Le chateliers principle states that if you change the conditions of the reversible reaction at equilibrium then the system will counteract that change. |
|

