![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
102 Cards in this Set
- Front
- Back
|
What are the 4 states of matter?
|
Duh
1. Solid 2. Liquid 3. Gas 4. Plasma |
|
|
T/F
Matter occupies space. |
True
|
|
|
T/F
Each shell of the Bohr model of an atom has random energy level. |
False
Each shell has a Specific energy level |
|
|
What occurs when an atom loses an electron?
|
Ionization
|
|
|
What happens to the atom when it is ionized?
|
1. Loses Electron
2. Nucleus becomes + ion 3. Electron becomes - ion 4. Formation of ion pair |
|
|
Ionizing an atom requires sufficient energy to overcome the ___________ binding the electrons to the nucleus.
|
electrostatic force
|
|
|
T/F
Within a given atom, electrons in the outer shells are more tightly bound than the more distant outer shells |
False
The inner shells are more tightly bound than outer shells |
|
|
What effect on the binding energy does having a Large Atomic Number (high Z), which has more protons in its nucleus?
|
They bind electrons in any given shell more tightly than do smaller-Z elements
|
|
|
T/F
Non-ionizing radiation, such as visible light, infrared, microwave radiation, and radio waves have sufficient energy to remove bound electrons from their shells. |
False
They DO NOT have sufficient energy to remove bound electrons from their shells |
|
|
What term refers to the particles which are emitted from nuclei as a result of nuclear instability?
|
Radioactivity
|
|
|
Name 3 different types of radiation.
|
1. alpha (helium nucleu) two protons + two neutrons
2. beta (electron) 3. gamma (electromagnetic radiation) |
|
|
What type of radiation comes from a Helium nucleus (two protons & two neutrons)?
|
Alpha radiation
|
|
|
What type of radiation comes from electrons?
|
Beta radiation
|
|
|
What type of radiation comes from electromagnetic radiation?
|
Gamma radiation
|
|
|
What are examples of electromagnetic radiation?
|
gamma rays, xrays, visible light, infrared radiation (heat), microwaves, and radio waves
|
|
|
When using an xray machine, what percentage of incident energy is converted to heat and xray?
|
99% heat
1% xray |
|
|
What are the 3 main components of an xray machine?
|
Tube head
Arm Control panel |
|
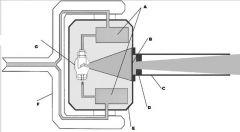
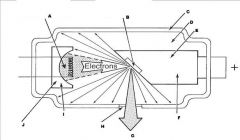
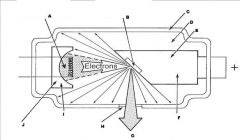
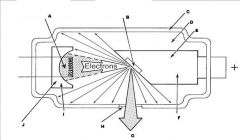
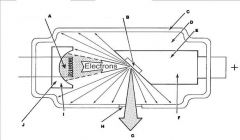
What part of the Tube Head is A?
|
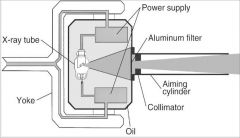
Power Sypply
|
|
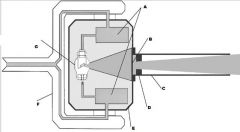
What part of the Tube Head is B?
|
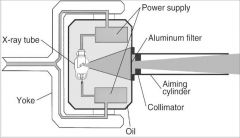
Aluminim Filter
|
|
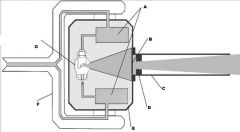
What part of the Tube Head is C?
|
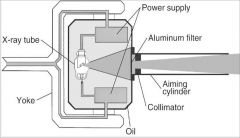
Aiming Cylinder
|
|
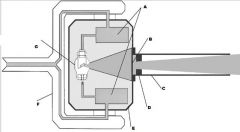
What part of the Tube Head is D?
|
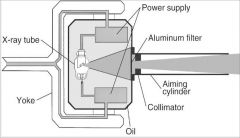
Collimator
|
|
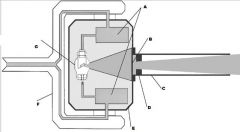
What part of the Tube Head is E?
|
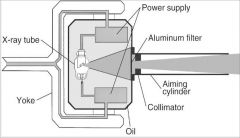
Oil
|
|
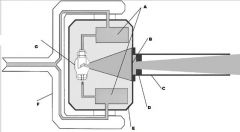
What part of the Tube Head is F?
|
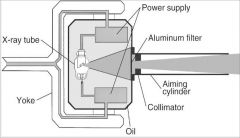
Yoke
|
|
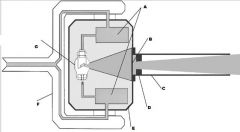
What part of the Tube Head is G?
|
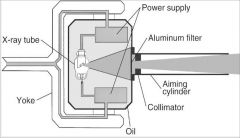
X-ray Tube
|
|
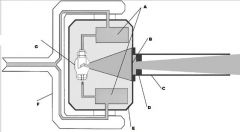
What part of the Tube Head is G?
|
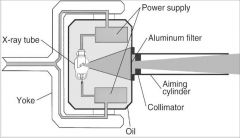
X-ray Tube
|
|
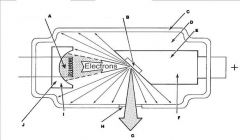
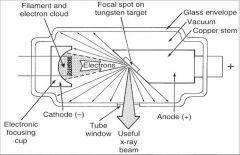
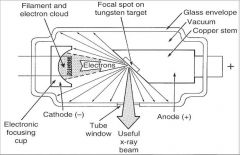
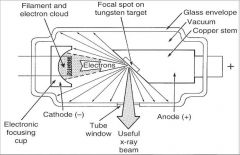
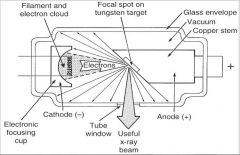
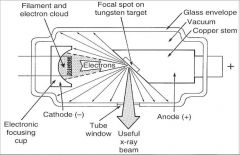
What part of an xray tube is A?
|
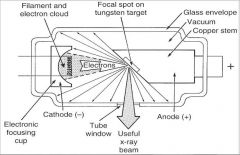
Filament and Electron Cloud
|
|
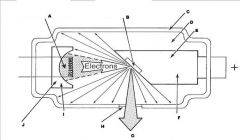
What part of an xray tube is B?
|
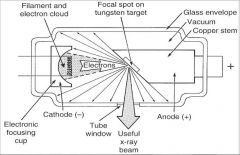
Focal spot on tungsten target
|
|
What part of an xray tube is C?
|
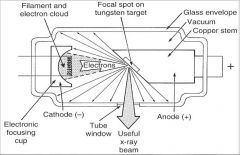
Glass Envelope
|
|
What part of an xray tube is D?
|
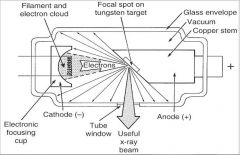
Vacuum
|
|
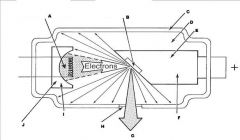
What part of an xray tube is E?
|
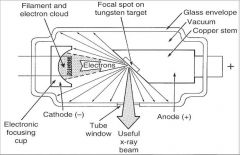
Copper stem
|
|
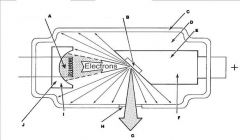
What part of an xray tube is F?
|
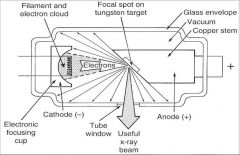
Anode (+)
|
|
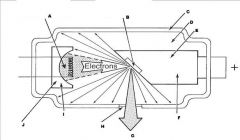
What part of an xray tube is G?
|
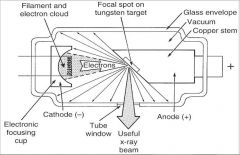
Useful xray beam
|
|
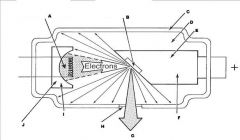
What part of an xray tube is H?
|
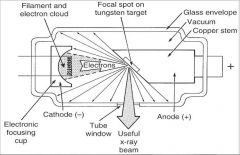
Tube Window
|
|
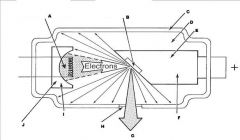
What part of an xray tube is I?
|
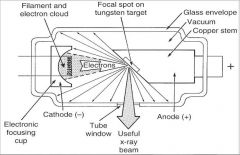
Cathode (-)
|
|
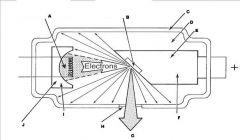
What part of an xray tube is J?
|
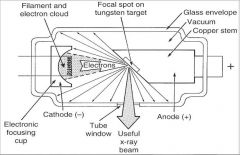
Electronic Focusing Cup
|
|
What components of the X-ray Tube compose the Cathode?
|
Cathode = Filament + Focusing Cup
|
|
What components of the X-ray Tube compose the Anode?
|
Tungsten Target Embedded in Copper Stem
|
|
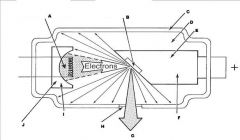
What are the major components of the X-ray tube?
|
Cathode (filament +focusing cup)
Anode (tungsten target embedded in copper stem) Casing (housing) Power supply |
|
|
What is the formula for Photon Energy?
|
E=hc/λ
|
|
|
In the formula E=hc/λ, what does E equal?
|
E= Photon energy
|
|
|
In the formula E=hc/λ, what does h equal?
|
h = Planck's constant
|
|
|
In the formula E=hc/λ, what does c equal?
|
c = speed of light
|
|
|
In the formula E=hc/λ, what does λ equal?
|
λ = wavelength
|
|
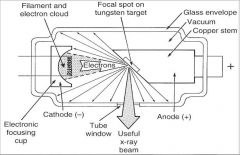
What is the Focal Spot?
|
The focal spot is the area on the target to which the focusing cup directs the electrons and from which xrays are produced.
|
|
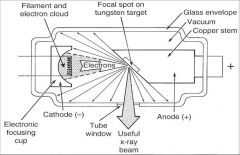
What happens to the sharpness of a radiographic image as the size of the focal spot decreases?
|
Sharpnes increases as size of focal spot decreases
|
|

Where are rotating Anodes used?
|
Used in medical CT
|
|
How are electrons generated in an xray tube?
|
Heat the cathode filament to generate electrons
|
|
How are the electrons generated by the heated cathode filament accelerated toward the anode?
|
A high-voltage potential between the anode and cathode is established to accelerate the electrons toward the anode.
|
|
What is the tube current?
|
Tube current is the flow of electrons through the tube
|
|
T/F
The mA setting on the filament current control refers to the current in the filament circuit. |
False
The mA setting on the filament current control actually refers to the tube current, typically about 10 mA. This is not the same as the current in the filament circuit. |
|
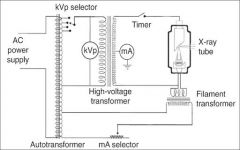
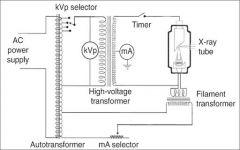
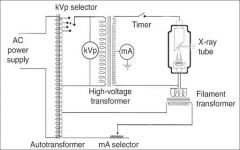
What amount of voltage is applied to the high voltage?
|
60,000 - 100,000 Volts (or 60-100kV)
|
|
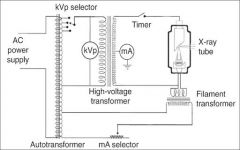
What does the timer do in the xray tube circuitry?
|
A timer is built into the high voltage circuit to control the duration of the xray exposure.
|
|
|
What is the passage of an electron near a nucleus, which results in electrons being deflected and decelerated generating continuous spectrum of energy?
|
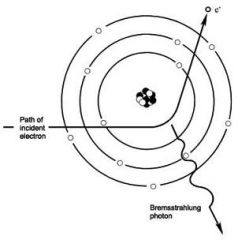
Bremsstrahlung radiation (breaking or decelerated radiation)
|
|
|
What are the properties of Characteristic Radiation?
|
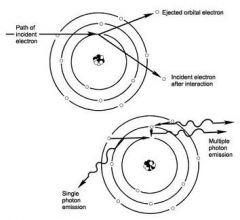
1. Incident electron ejects an inner electron from the tungsten target and an electron from an outer orbital is quickly attracted to the void in the deficient inner.
2. Subsequently, a photon is emitted with an energy equivalent to the difference in the two orbital binding energies. |
|
|
What property of Characteristic photons is because they represent the difference of the energy levels of electron orbital levels and hence are characteristic of the target atoms?
|
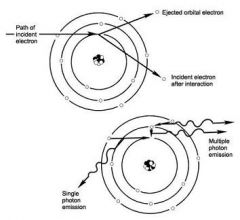
The energies of characteristic photons are discrete
|
|
|
What property of Characteristic photons is because they represent the difference of the energy levels of electron orbital levels and hence are characteristic of the target atoms?
|
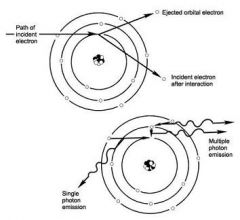
The energies of characteristic photons are discrete
|
|
|
What is the difference in keV between Bremsstrahlung radiation and Characteristic radiation?
|
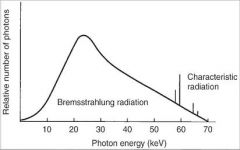
Characteristic radiation is higher
|
|
|
What are the 5 factors that control the x-ray beam?
|
1. Timer (beam exposure duration)
2. Exposure rate (mA) 3. Energy (kVp & filtration) 4. Shape (collimation) 5. Intensity (target-patient distance) |
|
|
What controls the number of photons generated in a radiograph?
|
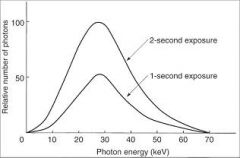
Exposure time controls the duration of the exposure and thus the number of photons generated
|
|
|
What happens the the photons generated when the exposure time is doubled?
|
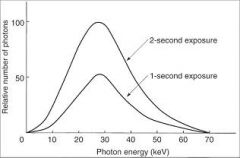
The number of photons generated at all energies in the x-ray emission spectrum is doubled.
|
|
|
What is the quantity of radiation produced by an x-ray tube directly proportional to?
|
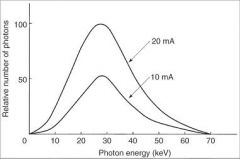
Tube Current mA
Time the tube is operated |
|
|
What will increase the potential difference between the cathode and the anode, thus increasing the energy of each electron when it strikes the target?
|
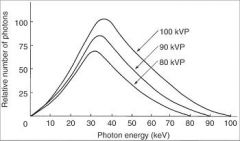
Increasing the Tube Voltage kVp
|
|
|
What 3 things will increase when Tube Voltage in increased?
|
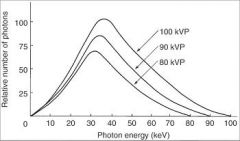
1. Number of photons generated
2. Mean Energy 3. Maximal Energy |
|
|
T/F
The higher the kVp and mean energy of the x-ray beam, the greater the penetrability of the beam through matter. |
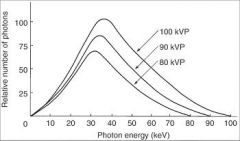
True
|
|
|
What photons should be removed from the beam to reduce patient dose?
|
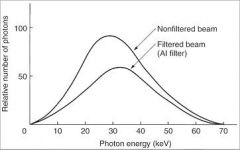
Low-energy photons
|
|
|
What preferentially removes many of the lower-energy photons with lesser effect on the higher-energy photons that are able to contribute to making an image?
|
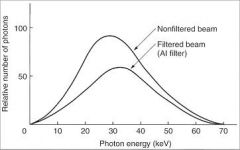
Aluminum Filter
|
|
|
What is a metallic barrier with an aperture in the middle used to reduce the size of the x-ray beam and thereby the volume of irradiated
|
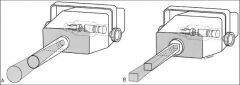
A collimator
|
|
|
What collimators are most frequently used in dentistry?
|
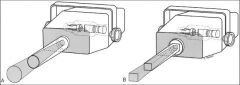
Round and Rectangular Collimators
|
|
|
T/F
Use of collimation improves image quality |
T
|
|
|
In collimation the x-ray beam is directed at the patient, the hard and soft tissues absorb about ___ of the photons and about ___ pass through the patient and reach the film
|
90%
10% |
|
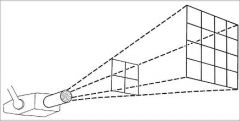
What is the formula for the Inverse Square Law?
|
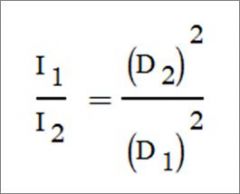
For a given beam the intensity is inversely proportional to the square of the distance from the source
|
|
|
What are 3 different interactions of X-rays with matter?
|
1. Coherent Scattering
2. Photoelectric 3. Compton Scattering |
|
|
What is Coherent Scattering?
|
1. Low-energy incident photon (<10 keV) passes near an outer electron
2. Photon causes electron to become momentarily excited at the same frequency 3. Photon ceases to exist 4. Excited electron returns to ground state & generates another x-ray photon w/the same energy as the incident beam 5. Secondary photon is emitted at an angle to the path of the incident photon |
|
|
Does Coherent scattering contribute to film fog?
|
Coherent scattering contributes little to film fog because the number of scattered photons is small and their energy is too low for many of them to reach the film or sensor
|
|
|
What percentage of the total number of interactions does coherent scattering account for in a dental exposure?
|
Coherent Scattering accounts for 7% of the total number of interactions in a dental exposure
|
|
|
What occurs when an incident photon gives up all of its energy to an inner electron ejected from the atom and an electron from a higher energy level fills the vacancy and emits a characteristic radiation?
|
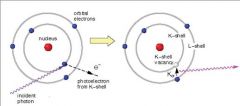
Photoelectric (radiation)
|
|
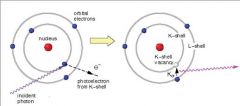
How does Photoelectric radiation manifest on dental radiographs?
|
Photoelectric radiation appears as a difference in optical density of the image.
|
|
|
What occurs when an incident photon interacts with an outer electron, producing a scattered photon of lower energy than the incident photon and a recoil electron ejected from the target atom?
|
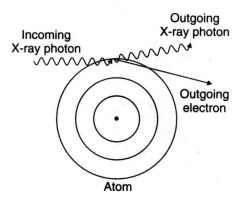
Compton Absorption
|
|
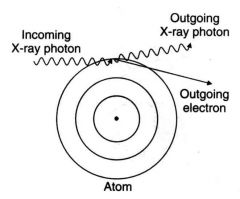
What percentage of x-ray interactions are compton?
|
49%
|
|
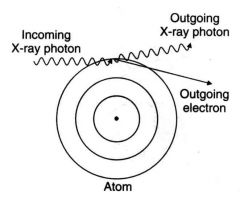
What effect does compton scattering have on an image?
|
Scattered photons darken and degrade the image while carrying no useful information. Ionization occurs
|
|
|
What does the absorption of the beam depend upon primarily?
|
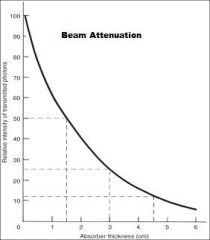
The Thickness & Density of the Absorber and the energy of the beam
|
|
|
What is a measure of beam energy describing the amount of an absorber that reduces the beam intensity by half?
|
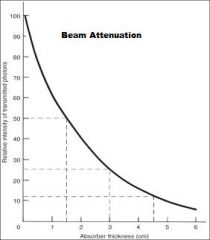
HVL (Half Value Layer)
|
|
|
What are the 2 systems for Disimetry?
|
Traditional and International Systems
|
|
|
In Dosimetry, what is the international system for measurements of Radiation?
|
SI
|
|
|
What is a measure of radiation quantity, the capacity of radiation to ionize air?
|
Exposure
|
|
|
What is the SI unit of exposure?
|
kerma (kinetic energy released in matter)
|
|
|
What does Kerma measure?
|
Kerma measures the kinetic energy transferred from photons to electrons and is expressed in units of dose (gray [Gy], where 1 Gy = 1joule/kg
|
|
|
What is a measure of the energy absorbed by any type of ionizing radiation per unit of mass of any type of matter?
|
Absorbed dose
|
|
|
What is the SI and Traditional units of measurement of Absorbed Dose?
|
Absorbed Dose
SI = Gy (Gy = 1joule/kg) Traditional = rad (radiation absorbed dose) note: 1 rad is equivalent to 100 ergs/g of absorber. One gray = 100 rads |
|
|
What is used to compare the biologic effects of different types of radiation on a tissue or organ?
|
HT (Equivalent Dose)
|
|
|
What is the equivalent dose formula?
|

HT =WR x DT
|
|

What does WR represent in the Equivalent Dose Formula?
|
WR represents the relative biologic effectiveness of different types of radiation
|
|

What does DT represent?
|
DT is the absorbed dose
|
|

What is the WR of alpha particles and xrays?
|
WR alpha particles = 20
WR x-rays = 1 |
|

What is the unit of equivalent dose?
|
HT is a sievert (Sv)
|
|
|
What is used to estimate the risk in humans by allowing the risk from exposure to one region of the body to be compared with the risk from exposure to another region?
|

Effective Dose
|
|

What does E represent?
|
E = effective dose
|
|

What does WT represent?
|
WT = tissue weighting
|
|

What is the unit of effective dose?
|
Sv
|
|
|
In radioactivity, what describes the decay rate of a sample of radioactive material?
|
Measurement of Radioactivity (A)
|
|
|
What is the SI unit for Radioactivity?
|
becquerel (Bq); 1 Bq = 1 dissintegration/second
|
|
|
What is the traditional unit of radioactivity?
|
Curie (Ci), which corresponds to the activity of 1 g of radium (3.7 x 1010 disintegrations/second)
|

