![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
Alpha Decay |
Emits a helium from nucleus. |
|
|
Beta Decay |
Emits an electron or from a nuetron |
|
|
Gamma Decay |
After other decay, nucleus releases gamma energy. |
|
|
Positron Emission |
Proton emits a positron and becomes a nuetron |
|
|
Who determined atoms released and absorbed fixed quanta of energy? |
Max Plank |
|
|
What principle did Werner Heisenberg create? |
The uncertainty principle: you cannot calculate the momentum and location of an electron at once. |
|
|
Principle quantum number (n) |
N increases, orbital increases and electron has higher energy levels and is less bound to nucleus, i.e. n=3 in the p orbital is the 3p orbital. |
|
|
Azimuthal quantum number (l) |
Values of 0 to n-l. Defines shape of the orbital. L=0=s, l=1=p, l=2=d, l=3=f orbitals. |
|
|
Magnetic quantum number (ml) |
Determines where orbitals are in relation to other orbitals. Range from -1 through 0 to +1. |
|
|
Spin quantum numbers (ms) |
Either +1/2 or -1/2, must be opposite to be in the same orbital. |
|
|
How many electrons can be in an orbital? |
No more than 2. |
|
|
Hund's Rule |
Most stable arrangement of electrons = maximum number of unpaired electrons |
|
|
Names of orbitals? |
S, p, d, f |
|
|
How many orbitals are increased with each letter? |
Two. S=1. P=3. D=6. F=7. |
|
|
Properties of metal |
Malleable, ductile, have luster, majority of elements, oxidize, form positive ions, conduct heat and electricity. |
|
|
Properties of transition metals/elements |
Refract light, several oxidation states, ionic solutions usually colored, contain actinides and lanthanides. |
|
|
Properties of Actinides and Lanthanides |
Uranium is only one found in nature, rest are man made. Called rare earth metals. |
|
|
Properties of metaloids |
Poor/fair conductors, brittle, can form alloys with metal. |
|
|
Non metal properties |
All gases, easily vaporized, brittle, insulate heat and electricity, hydrogen and helium take up 99% of the observable universe. |
|
|
Alkali metal properties |
Most reactive metals, react violently with water, creates basic solutions. |
|
|
Alkali earth metal properties |
Shiny, silvery white, occur in nature |
|
|
Halogen properties |
Crazy reactive, used in neon lights. |
|
|
Electronegativity |
How strong an atom pulls an electron. High ionization energy + high electron affinity = high electronegativity. Increases left to right, decreases top to bottom. |
|
|
Ionization energy |
How much energy to remove an electron from the atom in the gas phase. Increases left to right, decreases top to bottom. |
|
|
Isoelectronic |
Equal number of electrons |
|
|
Atomic radius |
Distance between nuclei. Distance decrease from left to right, increases top to bottom. Cation=smaller anion=larger radius. |
|
|
Ionization energy trends |
It takes less energy to remove am electron from a full orbital than a half full orbital. |
|
|
Electron affinity |
How much energy is released when an electron is added. Becomes more negative left to right same top to bottom. |
|
|
Periodic Group |
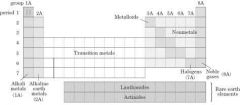
|
|
|
Periodic Trends |

|
|
|
Chemical Bonds |
Bonds between atoms/ions. Determine physical properties. Ionic, covalent, and metallic. |
|
|
Ionic Bonds |
Attraction between cations and anions; electron transfered. Usually between metals and non-metals. Electronegativity differences greater than 1.67. Strong, high melting points, solid. |
|
|
Covalent Bonds |
Shared electrons. Can be single (sigma), double (pi), or triple (also pi). More bonds=shorter distance between nuclei. |
|
|
Metallic Bonds |
Sea of electrons. |
|
|
VSEPR |
Electrons repel each other. Depends on central atom. Creates predictable shapes. |
|
|
Linear |
Two elements always linear. |

