![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
51 Cards in this Set
- Front
- Back
|
1-dimensional Schrodinger Equation?
|
Ĥψ = Eψ Ĥ = -ħ²/2m.d²/dx² + V(x) |
|
|
How do we interpret a wavefunction?
|
interpret as telling us the probability of the particle(s) being at that point in space: the square modulus of the wavefunction is proportional to the probability density |
|
|
eigenfunctions?
|
solutions to the Schrodinger equation |
|
|
eigenvalues?
|
corresponding energies E to solutions to the schrodinger equation |
|
|
for this course what do we need to work out the Hamiltionian for a system?
|
The kinetic energy operator, potential energy as a function of the coordinates
|
|
|
1 dimension operators?
|
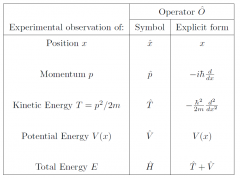
|
|
|
3 dimensions operators? |
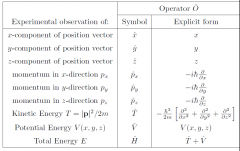
|
|
|
What are postulates?
|
basis of phisical laws that are not obvious in themselves, but when followed through describe nature as we observed it (with some approximations) |
|
|
Postulates of Quantum mechanics?
|
2)Each observable quantity (energy, position, momuntum, etc) is represented by an operator 3)if ψ is an eigen function of the operator for an observable, an attempt to measure the quantity will always give the corresponding eigenvalue, if it is not an eigenfunction then successive attempts to measure the quantity will give a random one of the eigenvalues |
|
|
expectation value of an operator?
|
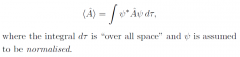
|
|
|
probability of observing eigenvalue an? and if
ψ = ψn |
=>|∫ψ*ψndτ|² ψ = ψn = ∫ψ*A(hat)ψ dτ =>A(hat)ψn = anψn |
|
|
Operator addition?
|
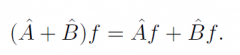
|
|
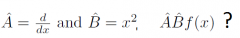
|
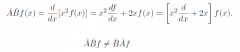
|
|
|
When are two operators equal?
|
d/dx exp(x²) = 2x exp(x²) but d/dx ≠ 2x because the operators would give different results on other functions |
|
|
Commutators?
|
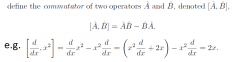
|
|
|
[B,A] compared to [A,B]
|
therefore if [A,B] = 0, [A,B]=[B,A] therefore the two operators commute |
|
|
Linear operators?
|
A is linear if A(c₁f₁+c₂f₂)=c₁Af₁+c₂Af₂ =>if Af=af =>A(cf) = cAf = caf= a(cf) =>any constant times f is also an eigenfunction with the same eigenvalue a |
|
|
Normalisation? |
∫ψ*ψdτ = ∫|ψ²|dτ = 1 allowing us to treat ψ² as a probability densitiy |
|
|
How to normalise?
|
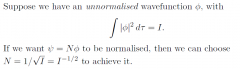
|
|
|
Harmonic Oscillator?
|
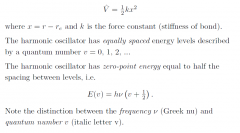
|
|
|
Classically forbidden?
|
wavefunctions can tunnel into region where V(x) > E which is not allowed in classical mechanics
|
|
|
Removing centre of mass motion?
|
for a vibrating diatomic molecule with two atoms of mass m₁ and m₂ -Translational motion of the molecule with mass M= m₁+m₂ -Vibrational motion of the nuclei as a single particle with reduced mass µ = (m₁m₂)/(m₁+m₂) kinetic energy operator T(hat) = -h(bar)²/2µ d²/dx² |
|
|
guessed functional form of harmonic oscillator?
|
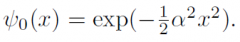
|
|
|
solve for the guessed functional form?
|

|
|
|
Reduced units?
|
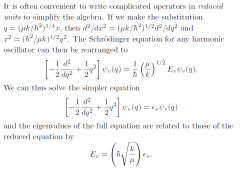
|
|
|
raising operator?
|
[H,A] = HA -AH consider H(Aψ) = (AH+[H,A])ψ = AHψ + Aψ =AEψ+Aψ = (E+1)(Aψ) A = raising operator - allows us to generate a new eigenfunction of H with a larger eigenvalue |
|
|
General energy level expression for harmonic osciator?
|
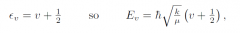
|
|
|
Exactly soluble problems in QM?
|

|
|
|
Approximate mechods in QM?
|
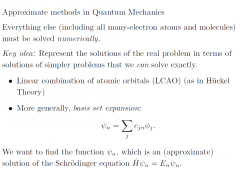
|
|
|
Basis set?
|
functions that we know before we start, chosen on physical grounds to give the flexibility we think is needed |
|
|
transpose?
|
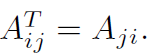
|
|
|
Kronecker delta notation?
|
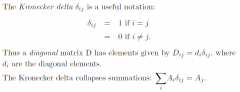
|
|
|
Dirac notation?
|
imply integration over all space and spin coordinates <m|n> is the overlap integral -If we write a state vector |n>, we imply that it is normalised |
|
|
expectation value?
|
<n|Ahat|n> abbreviated to <Ahat>n |
|
|
Hermitian operators?
|
=>swapping over the two functions merely changes a matrix into its own complex conjugate => operators corresponding to observable quantities (including the Hamiltonian) are always Hermitian |
|
|
Two important properties of Hermitian operators?
|
Eigenfunctions corresponding to different eigenvalues are orthogonal |
|
|
Variation principle?
|
The expectation value of the Hamiltonian calculated using an approximate wavefunction is always greater than or equal to the true ground state energy of the system |
|
|
linear variatinoal method?
|
write the trial wavefunction as a linear combination of basis functions, and seek of coefficients that minimize expectation value of H(hat) |
|
|
Functional variation approach?
|
trial wavefunction is written with parameters and the parameters are varied to find the lowest and best energy |
|
|
Heisenberg Uncertainty principle?
|
∆A∆B ≥ ½|<[Ahat,Bhat]>|
|
|
|
Conserved quantities?
|
-Orbital angular momentum (sometimes) -spin angular momentum (often) -Total angular momentum (always) use these to define quantum numbers |
|
|
magnitude of orbital angular momenum?
|
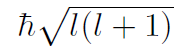
|
|
|
Symmetric and antisymmetric spin functions?
|
represent triplet and singlet states respectively
|
|
|
Pauli principle?
|
-the total wavefunction is always antisymmetric with respect to exchange of identical fermions and symmetric with respect to exchange of identical bosons |
|
|
Fermions?
|
Electrons and other particles with half-integer spins such as odd-mass nuclei |
|
|
Bosons? |
photons and other particles with integer spins such as even spins are bosons |
|
|
what space functions must a singlet spin function be paired with?
|
singlet = antisymmetric space = symmetric |
|
|
Hunds first rule?
|
when two atomic or molecular states arise from the same electron configuration, the one with higher spin multiplicity is lower in energy |
|
|
explain Hunds first rule?
|
conversely, two electrons with spins paired tend to pile together raising the energy |
|
|
Component of l in z direction?
|
hbar ml |
|
|
number of values of ml
|
for each l there are 2l +1 allowed values of ml 0, ±1,±2...±l |

