![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Equilibrium Constant |
Products / reactants |
K |
|
|
1 bar |
10^5 Pa |
|
|
|
Bar |
Unit for gas in the Kitchen formula |
|
|
|
760 torr |
1.01315 bar |
|
|
|
Endothermic Reaction Change in temperature |
T goes up and K does too Shifts right / reactant |
|
|
|
Inert gas |
No effect to equilibrium |
|
|
|
+delta S |
The products are more more ordered then reactants |
|
|
|
+delta heat |
Endothermic reaction |
|
|
|
Gibbs free energy |
G= dH -dST G= -RTln(K) |
|
|
|
Spontaneous |
-dG° & K larger then 1 |
|
|
|
Reaction is favored if? |
-dH and +dS |
|
|
|
Henderson -Hasselbach |
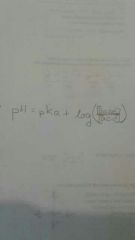
|
|
|
|
Q |
Reaction Quotient Evaluate with the concentration Not at equilibrium |
|
|
|
Q <K |
Equilibrium will move product/right |
|
|
|
Q>K |
Equilibrium moves reactants/left |
|
|
|
Volume increased |
Shifts left/ reactants |
|
|
|
Exothermic reaction and temp changes |
K decreases as T increases Shifts product favored |
|
|
|
Bronsted-Lowery Acid |
Proton donor |
|
|
|
Bronsted-Lowery Base |
Proton acceptor |
|
|
|
Weak acids make |
Strong bases |
|
|
|
Strong acids |
HCl H2SO4, sulfuric HBr HClO4, perchloric acid HNO3, nitric acid HI HClO, chloric acid |
7 |

