![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
267 Cards in this Set
- Front
- Back
|
Alveolar gas equation
|
PAO2 = PIO2 - 1.2 (PaCO2)
PIO2 = FIO2 (Pb - Ph2o) |
|
|
PaCO2 equation
|
PaCO2 = VCO2 x 0.863 / VA
VA = VE - VD VE = RR - Vt tidal volume |
|
|
Factors effecting O2 diffusion across the lungs
|
- Thickness of the membrane (increased thickness → impaired diffusion). Diseases: pulmonary edema, pulmonary fibrosis
- Surface area of the membrane. In disease parts may be removed/obstructed: pneumonectomy, emphysema - The diffusion rate of the specific gas (the more soluble the faster it diffuses). CO2 diffuses 20x faster than O2. - Pressure differences between the two membranes: because of different alveolar/arterial partial pressures O2 and CO2 diffuse in opposite directions |
|
|
Factors influencing the Hb saturation curve
|
Left shift (promotes O2 loading, occurs in lungs): reduced temperature and 2,3 DPG, increased carboxyhemoglobin and methoxyhemoglobin (have higher O2 affinity, so steal O2)
Right shift (promotes O2 dumping, occurs at metabolic sites): increased temp, 2,3 DPG, acidity (reduced pH, high CO2) |
|
|
CaO2
|
Arterial O2 content: sum of the O2 bound to hemoglobin and dissolved in the plasma
CaO2 = (Hb x 1.34 x SaO2) + (0.003 x PaO2) where SaO2 = oxygen saturation (bound/total binding sites on Hb available) PaO2 is the O2 molecules dissolved in plasma (not bound) |
|
|
4 radiographic densities
|
Air = black
Fat = medium gray Soft tissue = light grey Bone = white |
|
|
Silhouette sign
|
= loss of the normal borders between anatomical structures on an x-ray, particularly the lungs, heart, diaphragm, and mediastinum. The location of the sign (i.e. which borders are obscured) can tell location of the disease.
EX: - loss of of left heart border indicates abnormality in the lingula (left middle lobe). Can't be used for lateral lung disease b/c doesn't border - loss of right heart border = abnormality in the right middle lobe - loss of the diaphragmatic border = abnormality in the lower lobes |
|
|
3 causes of complete opacification of a hemithorax
|
- Atalectasis: collapsed lung. In obstructive atalectasis something (tumor, mucus plug, foreign body) completely blocks the main bronchus, air trapped gets absorbed just leaving soft tissue
- Pleural effusion: accumulation of excess fluid in the pleural space - Hemothorax: pleural space is filled with blood - Pneumonia (of the whole lung): air is replaced by pus. |
|
|
Differential for complete opacification of the hemithorax with shifting of the mediastinum
|
Shift to the opaque side: atalectasis (more space because of collapsed lung)
Shift away from opaque side: pleural effusion (increased volume pushes away) - No shift: pneumonia of the whole lung (rare) *Normal: 1/3 of the heart on the right, 2/3 on the left. |
|
|
Differential for pulmonary consolidation (and how to distinguish)
|
- Cancer (smoking history)
- Aspirated material (child, drowning) - Hemorrhage (trauma) - Pneumonia (fever, etc) Distinguish: mainly use history as it is difficult to differentiate radiographically. |
|
|
Atmospheric pressure
|
= the external pressure, will be the pressure inside the alveoli as long as long as the glottis is open.
- conventional value in pulm is 0 cm H2O |
|
|
Alveolar pressure
|
= the pressure inside the alveolus, should be atmospheric when the glottis is open
- exerts force on the lungs from the inside |
|
|
Intrapleural pressure
|
= the pressure surrounding the lungs within the pleural space. Composed of two pressures:
- Transpulmonary pressure: the pressure difference across the lungs (determines the size of the alveoli). Ptp = Palveolar - Ppleural - Transthoracic pressure: pressure difference across the thoracic wall. Ptt = Ppleural - Patm |
|
|
Recoil pressure
|
= the pressure with which an object (lung or chest wall) tries to regain it's desired shape.
- The inward elastic recoil of the lung opposes that of the chest wall (CW grows exponentially with pressure, lung grows logarithmically) |
|
|
Functional reserve capacity
|
= the volume of air at which the recoil of the lungs and chest wall are equal and opposite (airway and alveolar pressure is zero). Occurs at the end of passive respiration (is expiratory reserve + residual volumes)
|
|
|
Total lung capacity
|
= the volume of air in the lungs at maximal inflation.
- Cannot expand any further due to recoil of the lungs and chest wall. |
|
|
Residual Volume
|
= the volume of air in the lungs after maximal expiration.
- Cannot collapse further due to chest recoil. |
|
|
Lung compliance
|
= ∆V/∆P
- it is the slope between two points on the pressure volume curve of the lung. It is not linear, rather is greatest at moderate lung volumes and decreases at the extremes. - Lung demonstrates hysteresis, meaning compliance on inspiration differs from expiration for identical lung volumes. - Pulmonary compliance is a function of both the dynamic (compliance of the airways/getting air into lungs) and static compliance (stretchability of lung tissue itself) |
|
|
Systemic circulation
|
- functions to perfuse all of the body
- high arterial pressure (120/80) and high resistance - comes from the left ventricle and aorta - essential for life |
|
|
Pulmonary circulation
|
- functions to do gas exchange in the lung (terminal arterial branches are thinner/less smooth muscle than systemic) as well as perfuse the lungs
- matches perfusion to ventilation by shunting blood to well ventilated areas of the lung - carries 100% of cardiac output but at lower pressure (25/8) and low resistance from the right ventricle/pulmonary trunk - essential for life |
|
|
Bronchial circulation
|
- functions to perfuse the trachea and bronchioles (along the the pulmonary circulation). Blood flows into the pulmonary veins, contributing de-oxygenated blood to the left atria (not physiologically significant)
- hemoptysis from CF, malignancy, etc occurs predominantly in the bronchial circulation, so are embolized to treat - operates at high resistance and pressure (close to systemic) - receives blood from the left ventricle and aorta. - not essential for life. |
|
|
Factors that influence pulmonary vascular resistance
|
= generally accomplished by either recruiting more vessels or widening vessel diameter to protect pulmonary system from operating at too high pressure
Active factors: - Increase PVR: endothelin, alveolar hypoxemia, alveolar hypercapnia, sympathetic stimulation, NEpi/Epi, α-adrenergic agonists, PGF2α/PGE2, thromboxane, angiotensin, histamine, low pH - Decrease PVR: PGI2, NO, parasympathetic stimulation, acetylcholine, β agonists, PGE1, Bradykinin Passive factors: - Increase PVR: positive-pressure ventilation (increased Palv or Ppl), change in lung volume from FRC, increased interstitial pressure, increased blood viscosity - Decrease PVR: increased PAP, LAP, CO, pulmonary blood volume; gravity/body position |
|
|
Main factors that increase PVR
|
Active (drugs/chemicals):
- endothelin: protein that causes vasoconstriction - alveolar hypoxemia: induces vasoconstriction in capillaries in order to shunt blood to better ventilated ones - alveolar hypercapnia: induces vasoconstriction in the local vessels to shunt to better perfused areas Passive: - positive pressure ventilatoin: mechanical ventilation that often hyperinflates alveoli which compresses vessels and raises resistance, increasing PVR - Change in lung volume from FRC: increase or decrease in lung volume from FRC collapses or hyperexpands the alveoli causing increased resistance in the local vessels |
|
|
Main factors that decrease PVR
|
Active:
- PGI2 (prostacyclin): a prostaglandin that induces vasodilation and inhibits platelet aggregation - NO (nitric oxide): vasodilator Passive: (based on R= ΔP/Q or PVR= (mPAP - LAP)/CO) - Increased PAP (pulmonary arterial pressure): induces vessel recruitment and vasodilation **if this mechanism were absent it would instead increase pressure - Increased LAP (left atrial pressure) and CO (cardiac output): reduce PVR base on the equation |
|
|
Effect of gravity on perfusion in the lung
|
- Gravity creates a gradient of perfusion relative to the height of the heart. (greatest perfusion at the base)
- Blood pressure at the base of the lung, below the heart (helped by gravity), is greatest, which forces unused vessel to open and others to distend, resulting in reduced resistance and better perfusion. - Perfusion is so much better at the base that there is mere V/Q mismatch there. |
|
|
Alveolar/arterial/venous pressure zones
|
- due to hydrostatic pressure from gravity, the lung has it highest aterial and venous pressure at the base, while alveolar pressure is relatively constant in the lung (from Patm)
3 Zones: (not anatomical, solely dependent on relationship to gravity) - Top/Zone 1: PA>Pa>Pv, alveolar pressure exceeds arterial and venous pressure causing capillaries to collapse, limited/no perfusion (unless Pa/Pv modulate) - Middle/Zone 2: Pa>PA>Pv, arterial pressure is slightly greater than alveolar creating a small driving pressure (Pa-PA) allowing minimal perfusion. Base/Zone 3: Pa>Pv>PA, both arterial and venous pressures are greater than alveolar, so driving pressure (Pa-Pv) is very positive and vessels are fully open for perfusion |
|
|
Hypoxic vasoconstriction
|
= mechanism to reduce V/Q mismatch in the lung.
- capillaries to poorly ventilated alveoli vasoconstrict (in response to hypoxia there), shunting blood to better ventilated alveoli - this allows the lung to compensate for poor perfusion in disease states (atalectasis, walking pneumonia) to avoid hypoxia - this mechanism is also occurs in fetal lungs which are not ventilated at all. |
|
|
Pulmonary Vascular Resistance equation
|
PVR = (mPAP - LAP) / CO
NOT useful for determining what actually happens when you change these numbers. Only for static calculation Increasing any of these factors or increased blood volume leads to recruitment and distention of vessel, leading to reduction in PVR (except in disease states) |
|
|
Alveolar-arterial Gradient Equation
|
A-a grad = PAO2 - PaO2
PAO2 = FiO2(Patm - Ph2o) - PaCO2/ 0.8 = 150 - PaCO2/0.8 |
|
|
Indications for PFTs
|
- evaluate respiratory symptoms (especially cough and dyspnea)
- Assess abnormal lab and imaging studies - Monitor known diseases (and track how the patient is progressing and responding to treatment) - preoperative risk stratification (tentative) - research - benefit in healthy asymptomatic population uncertain (may be useful in helping smokers quit) |
|
|
Categories and types of PFTs available
|
Pressure-Flow:
- major: forced exhalation, spirometry, flow-volume loop - minor: airways resistance Pressure-volume: - major: lung volumes - minor: elasticity Respiratory muscles: - minor: inspiratory force, expiratory force Gas exchange: - major: diffusion, blood gases, pulse oximetry Exercise: - cardiopulmonary exercise test (CPET) Airway reactivity: - bronchodilator response - bonchial challenge |
|
|
Four lung volumes and capacities
|
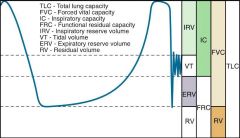
Volumes:
- tidal volume (VT): air inspired/expired during normal breathing (~500ml), includes deadspace air - Inspiratory reserve volume (IRV): max additional inspiratory volume above VT (~3000mL) - Expiratory reserve volume (ERV): max additional expiratory volume below VT (~1200mL) - Residual Volume (RV): volume remaining in lung after maximal forced expiration (~1200mL) Capacities: - Total lung capacity (TLC): total volume of lung (~5900mL), = IRV + VT+ ERV+ RV - Inspiratory Capacity (IC): maximal inspiration volume, = IRV + VT - Functional residual volume (FRC): residual volume in the lung during normal breathing = ERV + RV - Forced vital capacity: maximal expiratory volume: = IRV + VT + ERV |
|
|
Spirometry results
|
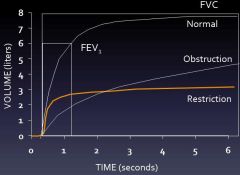
|
|
|
Flow-volume curve results: obstruction
|
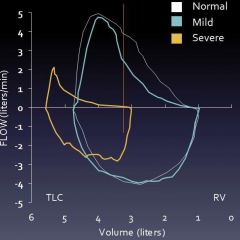
|
|
|
Flow volume curve results: restriction
|
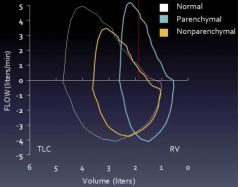
|
|
|
Flow volume curve: intra-thoracic obstruction
|
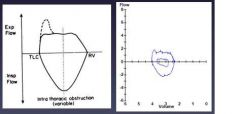
|
|
|
Flow volume curve: extra-thoracic obstruction
|
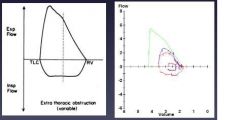
|
|
|
Gas dilution tests
|
= measures lung volumes by inhalation of nitrogen or helium for a specified period of time. Final dilution of the gas is used to calculate volume (Helium does not readily diffuse)
- this method is sensitive to errors due to leakage of gas, failure to measure gas in bullae (b/c He may not mix well in all parts of the lung) |
|
|
Body plethysmography
|
- most accurate measurement of lung capacities
- patient sits a box breathing through a shuttered mouthpiece - patient breathes against a closed shutter and pressure changes in the box are measured. - uses Boyle's law |
|
|
Diffusion capacity testing
|
= measures movement of gas across the semi-permeable membrane (gas uptake / driving gradient, (ml/min)/mmHg). Has 2 componentsL membrane (diffusion) and reactive (binding to hemoglobin)
- is a global test of gas transfer, measure things beyond the alveoli, must be normalized for Hb level - measure the slope between FACOfinal/exp (alveolar CO) and FACOinitial/insp |
|
|
Differential for changes in DLCO
|
Decreased DLCO:
- decreased surface area: emphysema, lung resection - decreased capillary volume: pulmonary vascular disease, valsalva maneuver - increased membrane thickness: pulmonary fibrosis - decreased hemoglobin: anemia, should be adjusted for [Hb] Increased DLCO: - increased capillary volume: exercise, early heart failure, obesity, Mueller maneuver, supine posture (so done when seated) - increased Hb: polycythemia - extravascular Hb: pulmonary hemorrhage |
|
|
Tests for bronchial reactivity
|
Bronchodilator response:
- patient given bronchodilator (usually albuterol) and PFTs are repeated after 15min. Response is significant if increased FVC or FEV1 by 12% AND 200mL. Never used to withhold bronchodilator treatment (relatively insensitive, with many false positives) Bronchial provocation: - patient given an irritant (usually methacholine or histmine) - used to evaluate for asthma when initial PFTs are ambigous/normal despite history - not used on patients with poor pulmonary function. - can also be done indirectly (triggering local bronchocontriction mediator release) with exercise, cold air, mannitor and hypertonic saline - interpretation: look for provocative dose that lowers FEV1 by 20% (lower the dose required, the worse disease) |
|
|
Restrictive vs Obstructive PFT results
|
Restrictive:
- rapid upstroke on spirograph - reduced FEV1 - Reduced FVC - Normal or increased FEV1/FVC - No late volume changes (plateau seen on spirograph) Obstructive disease: - slow inidital upstroke on spirograph - Reduced FEV1 - Reduced FVC (unless elevated from hyperinflation) - Reduced FEV1/FVC - Late volume changes on spirograph (no plateau) |
|
|
Intra-thoracic vs extrathoracic obstruction on PFTs
|
Intra-thoracic:
- expiratory plateau on FV loop - usually due to tracheal obstructions: tumors, traccheomalacia, tracheal inflammation - FEF50/FIF50 <1 Extra-thoracic - inspiratory plateau on FV - FEF50/FIF50 >1 |
|
|
Hyperventilation
|
= PaCO2 <35 (normal is 40). Tachypnea =/= hyperventilation (hyperventilation is EFFECTIVE increased respiration, may be slow/deep)
Causes: - physiologic: hypoxia, acidiosis (dumping CO2 to reduce acid), irritants, congestive heart failure. All lead to hypocapnia or increased respiratory rate which drop CO2. Sepsis, PE, pregnancy may often cause these - non-physiologic: pain, anxiety, psychogenic causes (will be normal in sleep). - Rx: aspirin, progesterone, β-agonists Consequences: - causes vasoconstriction and ischemia in the brain (clinical strategy during hemorrhage) may then have reperfusion injury when normalized, alkalosis causes neurotoxicity and increased neuronal excitability and seizures. - vasoconstriction in the heart leading to ischemia and arrhythmias - mucosal edema and smooth muscle contraction in the lungs (to slow breathing). This exacerbates people who are panicking Clinical presentation: dyspnea, Kussmaul breathing (slow, deep in acidosis), dizziness (no headache), visual changes, syncope, seizures, chest pain, arrhythmias, muscular weakness, parasthesias (particularly perioral in psychogenic), carpedal spasm, tetany Treatment: target underlying cause, brown paper bag, reassurance, sedation in severe cases |
|
|
Hypoventilation
|
= PaCO2 >45 (normal is 40)
Causes: - central (brain): neural defects affecting medullary center (nucleus of the tractus solitaries) that responds to and regulates respiratory rate. Ex: congenital central hypoventilation, hypothyroid/myxedema, drugs (sedatives, narcotics, etc) - muscular/PNS: defects in the conduction of central signals to the periphery or ability of the muscle tissue to respond to stimulation. Ex: ALS, myasthenia gravis, muscular dystrophy - pulmonary: obstructive or restrictive defects that prevent normal gas exchange. Ex: COPD, asthma Consequences: - primary: ↑PaCO2 & ↓pH, hypoxia - secondary: ↑HCO3 & ↓Cl- (kidney will compensate overtime), cerebral vasodilation, arousal from sleep (b/c normally breath less then anyway), Hb desturation, erythropoiesis, pulmonary vasoconstriction - clinical manifestations: headache, sleep disturbance, somnolence, cyanosis, polycythemia (↑% RBCs), pulmonary HTN, cor pulmonale (rt hrt failure) Treatment: depends on acuity. Most common respiratory acidosis is from drugs (correct or intubate). Chronic treat underlying disease. |
|
|
Nucleus of the tractus solitarius (NTS)
|
- respiratory pacemaker in the medulla that responds to and regulates respiratory rate
- recieves input from both the glossopharyngeal and vagus nerves - automaticity consists of: interval between discharges (determines RR) AND frequency of impulses during a discharge (determines degree of contraction and so depth of respiration/tidal volume) |
|
|
Signaling from the carotid body in chronic hypoventilation
|
- in chronic hypoxia/hypercapnia baroreceptors eventually become less sensitive and the PaCO2xMinute Venilation curve becomes less steep (loose responsiveness to changes in CO2)
- PaCO2 is normally the primary driver to breathe so these patients instead rely on hypoxia to trigger respiration - This is a problem when people are then given oxygen because they lose their drive to breath (b/c relying on hypoxia) |
|
|
Differentiating between different causes of hypoxia
|
Central: (can be consciously corrected)
- normal PFTs - normal muscle function tests - impaired hypoxic drive - impaired CO2 drive Neuromuscular: - ↓FEV1, ↓FEV, restrictive breathing pattern on PFTs - low pressures, forces, MVV - get low, shallow breathing in response to hypoxia or hypercapnia Lung disease - obstructive or restrictive PFTs - normal strength, ↓MVV - if chronic: not response to hypercapnia but response to hypoxia |
|
|
Mechanisms of respiratory drive
|
- Innate medullary pacemaker (nucleus of the tractus solitarius). Controls both respiratory rate (length of interval) and depth of respiration (impulses per discharge)
- Hypercapnia: (sensed primarily in the medulla). Responsds linearly with even slight increases in PaCO2 - Hypoxia: (sensed primarily in the carotid body), typically no response until PaO2<60mmHg (b/c Hb sat drops fast then) - Mechanoreceptors: irritant receptors (endo/exogenous irritants, vagus nerve, ↑RR, cough), stretch receptors in the lung (mediate Hering-Breuer reflexes, stronger on deflation), juxtapulmonary capillary receptors (detect vascular stretch and pressure (ex: CHF, thrombosis), congestion promotes ↑RR) |
|
|
CO2 accumulation and respiratory drive
|
- CO2 is the primary driver of respiration (aside from medullary pacemaker). Sensed mostly in the medulla
- PaCO2 builds up at 2.5mm Hg/min. Equivalent to inceased drive of ~3.5 L/min (normal is 40mmHg) - PaCO has a linear relationship to minute ventilation: slight changes in PaCO2 cause significant drive to breath (PCO2 ∝ Rate of CO2 production / minute ventilation) - In HYPOcapnia, the carotid body (hypoxia signaling) basically shuts down (overruled by low CO20 |
|
|
Central Alveolar hypoventilation
|
Causes:
- congenital defects (congenital central hypoventilation, Hirschsprung's) - conditions: Ondine's curse. hypothyroidism/myxedema, - drugs (sedatives, narcotics etc), post-sedation or anesthesia Presentation: - dyspnea absent - normal MVV, MIP, MEP, PFTs - ↑PCO2, ↑HCO3 - hypoxemia secondary to hypercapnea Therapy: - respiratory stimulants may be helpful (ex: progesterone) - diaphragmatic pacing - nocturnal ventilation |
|
|
Neuromuscular hypoventilation
|
Causes:
- Neuromuscular: myasthenia gravis, MS, ALS, muscular dystrophy - Chest wall: kyphoscoliosis Presentation: - dyspnea, orthopnea (dyspnea when lying flat) - ↓MVV, ↓MIP/MEP, ∓restrictive PFTs - normal drive, hypoxia due to ↓FRC, poor cough - Cor pulmonale as a terminal event Treatment: - Stimulants NOT helpful - treat underlying condition - nocturnal ventilation |
|
|
Pulmonary hypoventilation
|
Causes:
- obstructive: COPD, bronchiectais - Restrictive: Pulmonary fibrosis Presentation: - PFTs reveal physiology consistent with disease state - respiratory drive likely impaired if chronic (hypercarbic>hypoxic) - Hypoxia due to underlying disease and hypercarbia leading to cor pulmonale as a terminal event Treatment: - treat underlying disease - CAREFUL oxygen supplementation - Nocturnal ventilation |
|
|
Mechanisms of respiratory drive
|
- Innate medullary pacemaker (nucleus of the tractus solitarius). Controls both respiratory rate (length of interval) and depth of respiration (impulses per discharge)
- Hypercapnia: (sensed primarily in the medulla). Responsds linearly with even slight increases in PaCO2 - Hypoxia: (sensed primarily in the carotid body), typically no response until PaO2<60mmHg (b/c Hb sat drops fast then) - Mechanoreceptors: irritant receptors (endo/exogenous irritants, vagus nerve, ↑RR, cough), stretch receptors in the lung (mediate Hering-Breuer reflexes, stronger on deflation), juxtapulmonary capillary receptors (detect vascular stretch and pressure (ex: CHF, thrombosis), congestion promotes ↑RR) |
|
|
CO2 accumulation and respiratory drive
|
- CO2 is the primary driver of respiration (aside from medullary pacemaker). Sensed mostly in the medulla
- PaCO2 builds up at 2.5mm Hg/min. Equivalent to inceased drive of ~3.5 L/min (normal is 40mmHg) - PaCO has a linear relationship to minute ventilation: slight changes in PaCO2 cause significant drive to breath (PCO2 ∝ Rate of CO2 production / minute ventilation) - In HYPOcapnia, the carotid body (hypoxia signaling) basically shuts down (overruled by low CO20 |
|
|
Central Alveolar hypoventilation
|
Causes:
- congenital defects (congenital central hypoventilation, Hirschsprung's) - conditions: Ondine's curse. hypothyroidism/myxedema, - drugs (sedatives, narcotics etc), post-sedation or anesthesia Presentation: - dyspnea absent - normal MVV, MIP, MEP, PFTs - ↑PCO2, ↑HCO3 - hypoxemia secondary to hypercapnea Therapy: - respiratory stimulants may be helpful (ex: progesterone) - diaphragmatic pacing - nocturnal ventilation |
|
|
Neuromuscular hypoventilation
|
Causes:
- Neuromuscular: myasthenia gravis, MS, ALS, muscular dystrophy - Chest wall: kyphoscoliosis Presentation: - dyspnea, orthopnea (dyspnea when lying flat) - ↓MVV, ↓MIP/MEP, ∓restrictive PFTs - normal drive, hypoxia due to ↓FRC, poor cough - Cor pulmonale as a terminal event Treatment: - Stimulants NOT helpful - treat underlying condition - nocturnal ventilation |
|
|
Pulmonary hypoventilation
|
Causes:
- obstructive: COPD, bronchiectais - Restrictive: Pulmonary fibrosis Presentation: - PFTs reveal physiology consistent with disease state - respiratory drive likely impaired if chronic (hypercarbic>hypoxic) - Hypoxia due to underlying disease and hypercarbia leading to cor pulmonale as a terminal event Treatment: - treat underlying disease - CAREFUL oxygen supplementation - Nocturnal ventilation |
|
|
A-a gradient
|
= PAO₂ - PaO₂
PAO₂ = FiO₂ x (Patm - PH₂O) - PaCO₂/R PAO₂ = 0.21 x (760 - 47) - PaO₂ / 0.8 Normal is 7-12, may vary with age. Increases with increasing FiO₂ ABG is given as pH/PaCO₂/PaO₂ |
|
|
Physiologic causes of Hypoxemia
|
Most cases of acute respiratory failure are due to more than one mechanism
- Hypoventilation - V/Q mismatch - Shunting (right to left) - Impaired Diffusion - Altitude induced |
|
|
Hypoventilation induced hypoxemia
|
Definition: reduced O2 in the alveoli causing reduced O₂ diffusing into the pulmonary capillary. PaCO₂ > 45mmHg
Characteristics: A-a gradient is usually normal (due to rapid response to hypercapnia of increased RR), usually corrects with small amounts of O₂ Mechanisms: - CNS depression: drug overdose, CNS lesions impacting the respiratory center or mechanoreceptors - neuromuscular: PNS lesions (ALS, Guillain-barre), muscle dysfunction (myasthenia gravis, polymyositis), chest wall dysfunction (kyphoscoliosis) - obesity-hypoventilation syndrome |
|
|
V/Q mismatch induced hypoxia
|
Definition: imbalance between ventilation and perfusion in the lung resulting in ineffective oxygenation (varied in different regions of the lung)
Characteristics: increased A-a gradient, may respond to increased FiO₂, usually normal PaCO₂ (except when severe) Mechanisms: - Ventilation with inadequate perfusion: pulmonary embolism - Perfusion with inadequate ventilation: pneumonia, pulmonary edema (CHF), acute lung injury (ALI/ARDS), atalectasis, pulmonary fibrosis, COPD |
|
|
Shunt induced hypoxia
|
Definition: return of blood to the left heart without being oxygenated (extreme V/Q mismatch)
Characteristics: increased A-a gradient, No response to increased FiO₂, usually normal CO₂ Mechanisms: - Anatomic (complete alveolar bypass): intracardiac shunts, pulmonary arteriovenous malformations, hepatopulmonary syndrome - Physiologic (pefusion of non-ventilated alveoli): atalectasis, pneumonia, ALI/ARDS |
|
|
Diffusion limited hypoxia
|
Definition: ineffective diffusion of O₂ across the alveolus into the pulmonary capillary
Characteristics: increased A-a gradient, usually responds to FiO₂, exercise-induced hypoxemia (faster moving blood so less diffusion) Mechanisms: - alveolar inflammation of fibrosis: interstitial lung disease, pulmonary fibrosis |
|
|
Acute respiratory failure
|
Definition: severe (acute) impairment of ability to perform adequate gas exchange in the respiratory system
Causes: - Normal CXR: CNS event (stroke, drug overdose, injury), neuromuscular disorders, airway obstruction (COPD, asthma), alveoli (pulmonary embolism) - Abnormal CXR: ALI/ARDS, aspiration, pneumonia, hydrostatic (cardiogenic) pulmonary edema, obstructive lung disease, pulmonary embolism, pneumothorax |
|
|
Acute lung injury (ALI) and Acute respiratory distress syndrome (ARDS)
|
Dx criteria: acute onset after at risk diagnosis, bilateral infiltrates on CXR, PaO₂/FiO₂ ≤ 300 (ALI) or PaO₂/FiO₂ ≤ 200 (ARDS), no left atrial hypertension (not evidence of LVF)
Gas exchange abnormalities: - Alveoli flooded with edematous fluid (shunting and V/Q mismatch, increased capillary leak) - Surfactant deficiency (decreased production and function) - "Stiff" lungs from diffuse alveolar damage and pulmonary edema (increasing respiratory load and worsens ventilation, decreased lung compliance) |
|
|
At risk diagnoses for ARDS
|
Direct Lung Injury
- Aspiration of gastric contents: 36-44% - Pulmonary contusion: 18-25% - Pneumonia/sepsis: 25-40% Indirect Lung Iniury - Non-pulmonary sepsis: 25-40% - Abdominal trauma: 18% - Multiple fractures: 10-48% - Hypertransfusion: 21-34% |
|
|
Management of ARDS
|
- usually requires ICU stay
- treat underlying cause of ARDS - restore oxygenation: PaO₂ 55-60mmHg, O₂ sat > 88-90% - Intubation and mechanical ventilation may be necessary (low tidal volume hastens weaning and improves survival--high volumes causes injury) - mortality rate is still 30-40% |
|
|
Indication, objectives, complications for mechanical ventilation
|
Indications
- Assurance of airway patency: depressed consciousness, dysfunction of upper airway, inability to cough or clear secretions - Restore oxygenation (usually can maintain PaO₂ without intubation) - Maintain ventilation and removal of CO₂ (improves the work of breathing) - Intubation is a clinical decision Ojectives - improve gas exchange: improve oxygenation, remove CO₂ - relieve respiratory fatigue - avoid complications - provide support while underlying cause of respiratory failure is addressed Complications - right mainstem intubation during intubation - aspiration - ventilator associate pneumonia - tracheal injury/stenosis - patient discomfort - barotrauma: injury related to positive pressure ventilation |
|
|
Asthma definition
|
= Chronic inflammatory disorder of airways (including smooth muscle hypertrophy/spasm, airway edema and remodeling, inflammation and increased mucous production)
- Many cellular elements/infiltrates involved (basophils, eosinophils**, neutrophils) - Recurrent symptoms, especially at night and early morning - Widespread and variable obstruction (usually diffuse inflammation through the conducting airways) - Often reversible spontaneously or with treatment (though reversibility may be incomplete) - Inflammation increases hyper-responsiveness and can result from specific triggers (hence variety, continuity) - On CXR should look normal (excluding changes/remodeling from chronic disease) |
|
|
COPD definition
|
- Airflow limitation that is not fully reversible (and is minimally responsive to bronchodilators)
- Airflow limitation is usually progressive (more so than normal aging) and associated with an abnormal inflammatory response to noxious particles and gases - Mixture of small airways disease and parenchymal destruction: Relative proportions vary from person to person, all smokers have inflammation but COPD only in susceptible persons (10-15% of smokers) - Variable natural history but generally progressive - Risk factors: mostly smoking, also air pollution (domestic fires especially) |
|
|
COPD subtype definitions
|
Chronic bronchitis:
- chronic productive cough for 3 consecutive months for 2 consecutive years --problem is increased airway resistance Emphysema - abnormal enlargement of airspaces distal to terminal bronchioles accompanied by destruction of alveolar walls (and capillaries) and without obvious fibrosis --problem is decreased lung recoil - on CXR: see large, flattened lungs with bolus changes |
|
|
Therapy Asthma vs COPD
|
Asthma:
- eliminate or avoid triggers when possible - for mild cases: use of a short acting β-agonist - for more severe: inhaled corticosteriods (variable doses), inhaled corticosteroids + long acting β-agonist (leukotriene receptor blockers, methyl xanthines, IgE antibodies). Avoid chronic oral steroids if possible COPD - avoid/eliminate any triggers: SMOKE, air pollution - mild/short term: short-acting β-agonist or anti-cholinergic (not effective for maintenance therapy) - chronic: possibly inhaled corticosteroids, quality of life modifications (smoking cessation, exercise, diet), home oxygen only if hypoxic (to prevent cor pulmonale, doesn't really improve symptoms) |
|
|
Interstitial Lung Disease
|
= a heterogeneous group of lung disorders predominantly affecting the lung parenchyma by infiltration of cellular or non-cellular material. Results in an impairment of gas exchange.
- may also affect the alveolar spaces, blood vessels, and distal airways - results in 100K hospital admissions annually, 15% of patient seen by pulmonologists. Higher incidence in specific populations - Generally occurs as a result of uncontrolled inflammation and tissue remodeling (due to genetics, GERD, viruses, diet, exposures, etc) leading to fibrosis and scaring of the lung - No real classification by can stratify based on known or unknown cause (ex: connective tissue disease vs. sarcoidosis) |
|
|
Clinical presentation of patients with ILD
|
Symptoms:
- cough and dyspnea on exertion - usually develop over months to years, except acute interstitial pneumonitis which may emerge over a few weeks and be rapidly fatal Physical Exam: - End respiratory crackles (velcro) - +/- digital clubbing - disease specific findings PFTs - restrictive pattern: infiltration of parenchyma leads to decreased lung compliance and lung volumes - low DLCO: infiltrates thicken the alveolar capillary membrane impairing gas exchange Radiographic findings: - CXR: bilateral reticular infiltrates are common - HRCT (varies by disease): common patterns of abnormality (reticular, honeycombing, ground glass, consolidation, cysts, nodules, traction bronchiectasis), anatomic distribution (diffuse, upper/lower, central/peripheral), associated findings (mediastinal lymphadenopathy, dilated esophagus) Histologic appearance: - Usual Intersitial Pneumonia (IUP) pattern |
|
|
Idiopathic pulmonary fibrosis
|
Epi:
- M>F, 25-30/100,000. Higher incidence if >75 Prognosis: 50% survival after 3.5 years Treatment: - no steroid or immunosuppression (harmful) - lung transplant - oxygen supplementation - pulmonary rehab - other (enrollment in research) |
|
|
Nonspecific interstitial Pneumonia (NSIP)
|
- interstitial lung disease with varying degrees of inflammation and fibrosis within the alveolar walls, usually temporally uniform (lymphocytic infiltrates are everywhere)
- Often confused with Usual Interstitial Pneumonia (UIP) because of similar distribution - On HRCT: reticular infiltrates, peripheral and basilar predominance, with ground glass infiltrates and no honeycombing. - Epi: M<F (associated with CT disorders, HSP), mean onset 49 - Often chronic onset, fevers sometimes seen - treatment: good results with steroids, some require immunosuppressants. Better prognosis than IUP |
|
|
Bronchiolitis obilterans Organizing Pneumonia (BOOP)
|
- aka: Cryptogenic organizing pneumonia (COP)
- ILD characterized by fibrous plugs obstructing airways to alveoli. - Seen as a consequence of infection or inhalation injury (associated with CTD, drug induced) or idiopathic (called COP) - fairly non-specific histology (often misdiagnosed as pneumonia), on CT can show multiple patchy consolidations +/- ground glass, can be localized or diffuse - Responsive to steroids with days to weeks (for resolution on CT). Treat longer to prevent relapse |
|
|
Desquamative Intersitial Pneumonia (DIP)
|
- ILD associated with smoking (>90%) (respiratory bronchiolitis ILD is also)
- characterized by increased, pigmented alveolar macrophages that fill alveolar spaces. Chronic onset, clubbing often seen Tests: normal CXR 1/5 cases, CT will have ground glass opacifications, most will have restrictive PFTs and decreased DLCO Epi: M>F, mean age 45 Treatment: smoking cessation, unclear if steroids are beneficial. Mortality is 20-30% mean survival is 12 years. |
|
|
Hypersensitivity pneumonitis
|
- ILD due to inflammatory reaction to inhaled antigen. Generally occurs around small airways.
- Characterized by bronchiolocentric lymphoplasmacytic infiltration and poorly formed non-necrotizing granulomas and giant cells - 4 stages: acute, subacute, chronic, endstage fibrosis - Common triggers: microbial agents (bacteria, fungi, amoebae, atypical mycobateria --hot tub), animal proteins (bird antigens, farmer's lung), low and high molecular weight chemicals Tests: CXR may be normal, CT 8% may be normal or subtle, HRCT can have any ILD pattern but in chronic tends to spare bases (also fibrosis/honeycombing) Treatment: avoidance, steroids won't work if you don't stop the exposure |
|
|
Types of Occupational lung diseases
|
- asbestosis
- coal workers pneomoconiosis (simple: radiographic abnormalities only, OR complicated: progressive massive fibrosis) - silicosis - Berylliosis |
|
|
Cause of Drug-induced ILD
|
- antibiotics: Nitrofurantoin (given for chronic UTIs)
- Anti-inflammatory agents: methotrexate, cyclophosphamide - Cardiac drugs: Amiodarone (can cause NSIP, BOOP, HP responses) - Chemotherapeutic agents: bleomycin, busulfan - recreation and illicit drugs (talc) |
|
|
Pneumoconiosis
|
= a constellation of disorders defined by interstitial lung fibrosis due to duct accumulation and foreign body immune response.
3 major classes: - coal worker's pneumoconiosis - asbestosis - silicosis |
|
|
Coal Worker's pneumoconiosis (or anthracosis)
|
= fibrotic condition which results from significant exposure to inhaled carbon (coal dust).
Pathology: coal particles progressively accumulate in lungs (not cleared) and are engulfed by alveolar macrophages, stimulating them to release enzymes, cytokines, oxygen radicals, and fibroblastic growth factors. This promotes chronic inflammation and fibrosis, and formation of nodular lesions Histology: aggregations carbon-laden macrophages show up as black, granular areas. Large nodules can be necrotic and cavity forming. |
|
|
Asbestosis
|
= diffuse fibrosis in the lung parenchyma due to chronic exposure to asbestos.
- Asbestos fibers: family of hydrated silicates with multiple forms (20-100μm long, 2μm wide). Serpentine are mostly harmless (can dissolve), amphibole can cause mesothelioma Pathogenesis: macrophages attempt to phagocytize fibers, releasing fibrogenic cytokines leading to fibrosis Histology: fibers are long/straigh, gold in appearance due to coating with iron and protein. "golden corkscrew w/ drumsticks". Lungs have interstitial inflammatory infiltrate (similar to alveolitis) with multinucleated macrophages - At risk populations: mining industry, asbestos installers (roofing material, insulation, break lines) - Associated disorders: bronchogenic carcinoma, pleural plaques (benign), mesothelioma |
|
|
Silicosis
|
- subtype of pneumoconiosis caused by inhalation of crystaline silica
- Pathogenesis: small silica particles travel to the alveolar space, are engulfed by macrophages, react to form silica hydroxide free radicals which kill macrophages and release particles. Progressive macrophage lysis leads to activation and release of inflammatory/fibrotic mediators Histo: areas of nodular fibrosis (+/- black patches of carbon), only see silica particles (2-3μm) under polarized light At risk occupations (10-15yrs exposure): mining, ore processing (esp quartz), stone cutting/polishing, sandblasting, work with abrasives |
|
|
Immunologic reaction in hypersensitivity pneumonitis
|
- ILD resulting from hypersensitivity to ORGANIC antigens: animal/vegetable proteins (farmer's lung, bird fancier's lung [bird feces, feathers, serum]), bacterial products, spores of thermophilic bacteria (hot tubs), spores of fungi
Initial reaction: Type III IgG immune complex mediated Chronic/severe reaction: Type IV, cell-mediated, leading to giant cell formation and granuloma formation Histo: lymphocyte/macrophage infiltration of prebronchial tissue and alveolar septa. Prominent lymphocytes and multi-nucleated giant cells, few neutrophils interstitially. Alveolitis progresses to fibrosis Clinical presentation: fever, chills, tachypnea, dyspnea, cough, chest tightness, malaise, weight loss (if chronic), rales but no wheezing/bronchoconstriction. Should improve when removed from antigen (unless chronic damage) - this differs from asthma (type I, IgE mediated) |
|
|
Sarcoidosis
|
= multiorgan granulomatosis caused by immune dysregulation in susceptible individuals presumable after certain environmental exposures
- Pathogenesis: not known, thought to be cell-mediated (T helper cell) response to antigen triggering lymphocytic infiltration. Multinucleated giant cells have wreath-like arrangement of nuclei - Histo: well formed, non-necrotizing granuloma (nodular aggregate of macrophagestes) with rim of Th CD4s in lungs and lymph nodes Clinical presentation: often asymptomatic, CXR may show lymphadenopathy in mediastinum, decreased peripheral blood Th CD4s Most frequently effects: lymphnodes (esp. hilar and mediastinal), lungs (interstitial fibrosis), liver and spleen (usually asymptomatic), heart (more severe), skin |
|
|
Honey comb lung
|
= radiographic finding of ILD
- looks like gaps and reticular spaces just under the pleura of the lung (associated with cobblestoning on the pleural surface) - results from chronic fibrosis and restructuring of the lung parenchyma - Occurs in restrictive diseases and prevents patients from fully expanding lungs - Common causes: IPF, bullous emphysema, diffuse interstitial fibrosis, pneumoconiosis, chronic TB, sarcoidosis |
|
|
Idiopathy pulmonary fibrosis (IPF)
|
= chronic progressive, fibrosing interstitial lung disease of unknown cause (limited to the lungs)
- typically affects M>F, 50-70. - Associated with smoking (>20p/y), GERD, exposure to metal dust, wood dust, solvents. Possibly underlying viral cause (EMV, HHV). Possible genetic predisposition Clinical presentation: progressive dyspnea (insidious onset), non-productive cough (in spasms), digital clubbing, diminished breath sounds, bilateral "velco" crackles - diagnosis is of exclusion (since idiopathic): exclude known causes, have UIP pattern of HRCT (reticulation, traction bronchiectasis, air trapping), histologically dense fibrosis (frequent honeycombing), temporal heterogeneity of lung tissues (health and unhealthy tissue adjacent), lack of granulomas or inflammation |
|
|
Acute respiratory distress syndrome
|
- condition characterized by inflammation in the lung parenchyma leading to impaired gas exchange with associated systemic release of inflammatory mediators, possibly leading to multiple organ failure.
- Diagnostic criteria: bilateral infiltrates of CXR after at-risk diagnosis, PaO2/FiO2 >200, nor left atrial hypertension or evidence of CHF - Caused by direct or indirect lung injury: gastric aspiration, pulmonary contusion, exacerbation of existing pneumonia or sepsis, other trauma Histo: evidence of pneumonia and diffuse alveolar damage. Neutrophils and lymphocyte migrate to inflamed tissue and amplify damge |
|
|
Distribution of Adrenergic Receptors
|
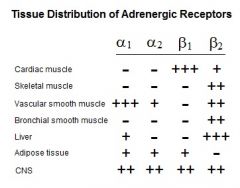
|
|
|
Epinephrine
|
Mechanism: preferentially activates β receptors (vasodilation) but also α receptors (dominant at high dose, vasoconstriction). Rapidly metabolized by MAO (T1/2 <1min)
Functional Effects: induces profound bronchodilation, ↑ cardiac output, ↑ blood pressure (↑ HR, ↑ strength of contraction, ↑ vasoconstriction (α receptors)), at low doses vasodilation (β receptors) Therapeutic potential: Emergency medicine for cardiac arrest, acute bronchospasm, shock. Must by IV (degraded orally) |
|
|
Dobutamine (Dobutrex)
|
Mechanism: selective β₁ receptor agonist
Functional effects: ↑ cardiac contractility, ↑ HR Indications: EM for shock and cardiac arrest, short-term treatment for heart failure. Sometimes used in stress tests for cardiac abnormalities |
|
|
Sympathomimetic drugs
|
- drugs that mimic the action of the sympathetic neurotransmitters NE and Epi. Made by phenylalanine substitutions
Direct acting: act on adrenoreceptors (α/β) Indirect acting: displace catecholamines or inhibiting reuptake. - effect of drugs depends on the selectivity of α/β receptors and their distribution in the tissue (allows for selective effects) |
|
|
Albuterol (also salmeterol, tertbutaline, pirbuterol)
|
- Mechanism: selective β₂ agonist (selectively skeletal muscle, bronchial SM, not adipose or cardiac). Relatively poor substrate for MAO so T1/2 is several hours
- Functional effect: relaxes bronchial smooth muscle with little effect on heart rate - Side effects: tachycadia, skeletal muscle tremor - Indications: (oral/inhaled) treatment/prevention of bronchospasms (asthma) - Contraindicated by cardiac disease and diabetes (due to liver action releasing glucose) |
|
|
Phenylephrine
|
- mechanism: selective α₁ agonist (high effect on vascular smooth muscle, moderate CNS). T1/2 of several hours
- Functional effect: potent vasoconstrictor therefore decreases the volume of the nasal mucosa reducing airflow resistance - Indications: nasal decongestant (oral or spray) - Contraindications: hypertension, MAOI use (sustained increase in BP) |
|
|
Clonidine
|
- Mechanism: selective α₂ agonist, binds mostly presynaptic α₂ receptors providing feedback to slow catecholamine release
- Functional effects: (oral or patch) suppresses sympathetic output in the CNS, decreasing overall tone, peripheral resistance, HR and BP Indications: HTN (less popular), glaucoma eyedrop (vasoconstricts b/c high receptor population), ADHD - Side effects: cotton mouth and sedation |
|
|
Phenelzine (tranylcypromine, selegine)
|
- MAO inhibitor: inhibits catcholamine degradation by monoamine oxidase propagating existing neural impulse (indirect action)
- Indications: (oral,T1/2 24hrs) depression, parkinson's disease (enhances dopaminergic tone, prolonging action) - Contraindications: other sympathomimetics or rich foods (wine, beer, cheese, chocolate, etc which are normally degraded by gut MAO) which may cause potentially fatal hypertensive crisis |
|
|
Methylphenidate, amphetamine
|
- binds reuptake transporters (NET) and vesicular transporters (VMAT) in the presynaptic terminal causing unregulated and prolonged non-vesicular catecholamine release (not release in bursts via vesicles)
- Functional effect: CNS stimulant effecting several systems (NE, DA, 5-HT), enhances sympathetic tone and increases focus) - Indications: ADHD and narcolepsy - Side effects: GI upset from decreased motility, mild HTN, tachycardia, insomnia. (amphetamine causes pyschosis [identical to schizophrenia], addition so limited use) |
|
|
Pseudoephedrine, phenylpropanolamine
|
- mechanism: binds to reuptake (NET) and vesicular transporters (VMAT) causing reverse catecholamine transport and release. Does not effectively cross BBB so few CNS effects.
- Functional effect: respiratory mucosa vasoconstriction, bronchial relaxation, increased heart rate and contractility - Indications: nasal decongestant (cold, allergies, sinusitis) - Side effects: high dose may cause hemorrhagic stroke |
|
|
Sympatholytic agents
|
- α/β adrenergic antagonists.
- commonly used for HTN (with ACE inhibitor/channel bl/ocker/diuretic), cardiac disease, angina, cardiac arrythmias - Side effects: fatigue, sleep disturbances, impaired athletic performance (lower HR), weight gain (lipolysis) |
|
|
Propranolol
|
- non-selective β receptor antagonist (blocks 1 and 2 receptors). Typically oral, T1/2 of several hours
- Functional effects: decreases HR, strength of contraction (so decreases stress on the heart, can stabilize arrythmias), reduces BP (blocks β1 in kidney effecting renin/angiotensin) - Indications: HTN, heart disease, angina, arrhythmias, muscle tremor - contraindications: asthma (inhibtion of B2 in lung causing bronchoconstriction) - used off label by muscians, competitive shooters, surgeons to reduce tremors |
|
|
Metoprolol
|
- selective β1 antagonist, making it cardio-selective (some effects also in CNS and adipose). Given orally, T1/2 of several hours
- Functional effect: decreases HR and strength of cardiac contraction, decreases BP (effects kidney renin/angiotensin system) - Indications: heart disease, angina, arhythmias (preferred for patients with asthma b/c no lung β2 stimulation) |
|
|
Phentolamine
|
- non- selective α adrenergic receptor antagonist. Given orally, t1/2 of several hours
- Functional effect: block pre and post-synaptic α receptors reducing overall sympathetic output, induces severe hypotension (blocks tonic vasoconstriction, rapidly dialating) - Indications: pheochomocytoma (adrenal medulla tumor causing massive E release and sympathetic over activation causing cardiac, HTN problems), severe hypertensive crisis (ex: MAOI uses who eats rich foods) |
|
|
Prazosin
|
- selective α1 antagonist. Given orally, T1/2 several hours
- Functional effects: reduces BP (by blocking vascular SM constriction) - indications: HTN, benign prostatic hyperplasia (targets α1 receptors on prostate, preferred is Tamsulosin which doesn't cause hypotension) - Side effects: postural hypotension and fainting upon standing (normally there would be a sympathetic spike on postural change to vasoconstrict and prevent BP drop in the brain--inhibited) |
|
|
Yohimbine
|
- selective α2 receptor antagonist. Prepared from bark of African tree, herbal or prescription forms. Taken orally, T1/2 of several hours
- Functional effect: inhibits CNS α2 receptors preventing presynaptic feedback, resulting in upregulation of endogenous E, increasing BP and flow to genitals - Indications: erectile dysfunction - Side effects: HTN, sleep disturbances - Contraindications: uses with sympathomimetics and MAOIs |
|
|
Reserpine
|
- indirectly acting sympatholytic: binds to and blocks vesicular monoamine transporter (VMAT) preventing vesicular packaging and release of catecholamines (taken orally, T1/2 of 36hrs)
- Functional effect: total shut-down of sympathetic nervous system (no release of catecholamines) - Indications: refractory Raynaud's Syndrome, HTN (not currently used b/c of side effects) - Side effects: severe CNS effects including depression and impaired cognition |
|
|
Asthma (disease and pathogenesis)
|
= chronic abnormal inflammatory state of the conducting airways characterized by reversible hyper-responsiveness, variable obstruction, and episodic symptoms of the airway (wheeze, cough, chest tightness, SOB)
- linked to environmental and genetic factors (pollutants, allergens, smoke, 100's of genes) Pathogenesis: - Normal: naive CD4s differentiate into Th1s in response to irritants in the airway results in recruitment of macrophages and low-level IgG response - Asthma: dominant differentiation of naive CD4's into Th2 lymphocytes in response to irritants resulting in cytokine release and recruitment of eosinophils, mast cells and plasma cells which release factors causing inflammation and constriction |
|
|
Physiologic targets for asthma therapies
|
Inflammation and reactive mediators: (steroids and other immune-modulators)
- Mast cells: Activated by allergen IgE, non-specific activators, adenosine. Release of pro-inflammatory polypeptides (cyto/chemo-kines), lipid mediators (LTs, PGs), and granules (histamine, heparin, TNF, etc) - other immune response cells Bronchial Smooth muscle tone - Constriction: M1-ACh, H1-histimine, Cys-LT: receptor activation cause Ca release and muscle contraction. - Parasympathetic nerve activity (Vagal Sensory Contrictor Reflex): M1-muscarinic receptors in the airway respond to air flow (exercise-induced), cold, smoke, etc inducing reflexive release of ACh causing constriction of bronchial SM (more ACh at night -->nocturnal asthma) - Relaxation (β agonists): β2 receptor activation cause cAMP release and relaxation (cAMP deactivated by phosphodiesterase) |
|
|
Advantages/Disadvantages of aerosol drug delivery
|
Types: metered dose inhaler (+/- spacer), nebulizer
Advantages: - reduced drug dosages - reduced systemic distribution/effects - Overall reduced systemic side effects Disadvantages: - inefficient: as much as 90% is deposited in the mouth (only a small fraction makes it to target) - acquired skill to use inhaler: need to time inhalation/pump, otherwise don't get proper treatment - irritation: from dry-dose inhalers - limited utility for youngest patients: babies can't manage timed inhalation |
|
|
β adrenergic receptor agonists for asthma/COPD treatment
|
- Albuterol (short-acting), Salmeterol (long-acting)
- β2 selective, targeting bronchial SM: simulated GPCR β2 receptors to release cAMP and relax SM/bronchodilate - Aerosol and oral forms - concerns: tolerance (leading to continued self-medication and crisis), increased risk of exacerbation with long acting - side effects: cardiac stimulation, tremors, etc (especially oral) |
|
|
Corticosteroids for Asthma/COPD therapy
|
- Fluticasone (flovent)
- Mechanism: binds to and activates nuclear receptors to modulate expression of genes involved in inflammation (and some that aren't), overall cause immunosuppression - increase lipocortin and IkB expression which blocks production of many lipid mediators (via inhibition of PLA2 which makes arachadonic acid), cytokines and chemokines - Problems: local (candidiasis) and systemic side effects (stress-like adrenal gland suppression) with both oral and aerosol forms. - Various doses to match disease severity (aerosol forms are much less than tablet) |
|
|
Cromolyn Sodium for asthma/COPD therapy
|
- alternative therapy choice
= membrane stabilize: inhibits mast-cell activation by stimulants and degranulation, reducing the inflammatory cascade - Aerosol form only, though distributed systemically via pulm absorption. Short half-life so have to medicate immediately before exposure - Individualized efficacy (good for those for whom it works, entirely ineffective in others) - few side effects or risks |
|
|
Muscarinic receptor antagonists in asthma therapy
|
- ipratopium
- alternative therapy, targets the parasympathetic vagal nerve reflex - mechanism: antagonizes bronchiole SM M1-muscarinic receptors preventing activation (which triggers ACh release and bronchoconstriction) - Aerosol only (charged so does not cross the membrane well) - Individualized efficacy but very good for some (esp. exercise and nocturnal asthma) - Very few side-effects due to limited absorption |
|
|
Leukotriene modifiers in asthma therapy
|
- inhibits the formation of leukotrienes later in the production pathway than steroids (which inhibit phospholipase A2 via lipocortin)
- Zilueton: inhibits 5-LPO, which normally converts essential fatty acids to LTs - Montelukast (singulair): inhibits leukotriene receptors to prevent action - Currently tablet only, may be aersol soon - good efficacy but individualized and variable - Side effects: montelukast: few, generally well tolerate, zileuton: elevates hepatic enzymes, numerous drug interactions (warfarin, theophylline, etc) |
|
|
Methylxanthines in Asthma therapy
|
- Theophylline
- Mechanism: not entirely understood, believed to antagonize adenosine receptors (which activate mast cells) causing bronchodilation (unlikely to be phosphodiesterase inhibitor, due to low therapeutic doses) Major limitations: low therapeutic index (no separation between therapeutic and side-effect curves), high toxicity, many food/drug interactions (metabolized by P450's and competes for binding with plasma proteins) - Caffeine is very similar to theophylline but 35% less potent |
|
|
Omalizumab in asthma therapy
|
- mechanism: IgE antibody which prevents it from binding receptor: prevents mast cell activation and production of inflammatory mediators
- Infusion only - can be very efficacious but only for allergen-based asthma - side effect: anaphylaxis |
|
|
Matching asthma drug/dose with disease severity
|
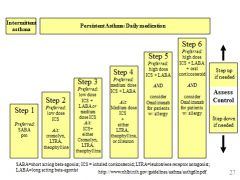
|
|
|
Drug treatment vs disease severity in COPD
|
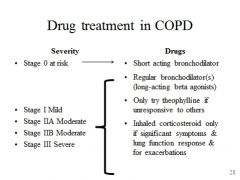
Therapy only really helpful if the COPD is triggering bronchoconstriction (generally poor response)
- Only O2 therapy prolongs survival |
|
|
Unique respiratory physiology of children
|
- Larger tongue: can cause obstruction of airways if loss of tone
- obligate nose-breathers: increase or persistence of congestion will lead to breathing difficulty - higher (C1 at 7yrs vs C4-5) more anterior glottis opening: high vocal cords make intubation more difficult - larger, more floppy, more angled epilottis (also larynx is cone shaped w/ cricoid rings at narrowest vs. vocal cords in adults): edema in the cricoids (Croup) or epiglottis can significantly obstruct airway - large occiput (anterior head) and lax next support: airway closure on incorrect head positioning - ribs more horzontal (vs anterioinferiorly slanted): more perpendicular, less curved diaphragm: weaker - increased lung compliance, less cartilage, decreased rib ossification: laryngeal breaking (vocal cords close on inhalation--grunting, respiratory distress), Hering-Breuer reflex (inspiration before full exhalation) - Infants: thicker respiratory epithelium, slower mucosal clearance, poor collateral ventilation: increased risk of collapsed airway due to obstruction |
|
|
Acute laryngotracheitis: ("Croup")
|
- an extrathoracic obstruction resulting in an inspiratory plateau on FV curve (trachea collapse although alveoli stay open)
= inflammation of the cricoids (larynx/trachea; may extend to bronchi--add bronchitis to name) - most common 6mo-4yrs, peak 18-24mo (younger kids have maternal antibodies/less pleural pressure, older kids have bigger airways) - 75% caused by parainfluenza, other viruses: RSV, influenza, adeno, herpes (tend to be more severe/protracted) - Clinical features: rhinorrhea, sore throat, mild fever. Progresses to barking cough, hoarsness, inspiratory stridor +/- fever, worsening symptoms at night, increased HR/RR, nasal flaring, retractions, cyanosis, biphasic stridor or silence - may see Steeple sign on CXR (closed trachea) but don't usually get test: clinical diagnosis Management: - usually resolves spontaneously, hydration, antipyretics, cold humidified air - severe cases: corticosteroids (oral dexamethasone), nebulized epinephrine (doesn't alter natural course of obstruction--rebound obstruction possible/worse) |
|
|
Epiglottitis
|
- extrathoracic obstruction in children (inspiratory plateau)
= bacterial cellulitis of the supraglottic structures -- A medical emergency (can fully occlude the airway) - can occur in children at any age, usually <5 - mostly H. flu B (now rare due to vaccination, 50% will have HiB elsewhere), also β-hemolytic strep (A, B, C), Staph aureus Clinical features (often misdiagnosed as Croup, onset typically abrupt w/ early morbidity) - v. sore throat w/ choking sensation, difficulty swallowing (due to pain), drooling, respiratory distress, anxiety, stridor, dyspnea, high fever, muffled voice, may show tripod position, thumb sign on CXR - Management: Emergent intubation, supportive care afterward, antibiotic therapy (3rd gen cephalosporin), rifampin prophylaxis for close contacts, corticosteriods?, epinephrine not recommended |
|
|
Foreign Body aspiration
|
- aspiration of a foreign body: depending on size may obstruct supraglottic airway, trachea or right bronchus (usually, more vertical)
- intrathoracic obstruction: cause expiratory plateau, airway collapse on expiration Presentation (may be known or unknown) - sudden coughing or wheezing (or may be insidious/overlooked), often persistent -may cause persistent/recurrent pneumonia - severe cases: cyanosis, drooling, stridor, difficultly vocalizing, seizures, bradycardia, cardiopulmonary arrest - On exam: decreased breath sounds, delayed air entry, wheezing, asymmetric breath sounds - lung/lobe hyperinflation/mediastinal shift or atalectasis (due to lack of collateral ventilation) on CXR, may not see the object if radio-opaque Common causes: peanuts, hot dogs, popcorn, coins, candy, small toys |
|
|
Bronchiolitis in children
|
= inflammation of the bronchioles (intrathoracic obstruction)
- Clinically: tachypnea, chest retractions, prolonged expiration (apnea in young infants), cough, wheezing (polyphonic), crackles, dehydration, decreased activity in child <2yo, +/- conjunctivitis/otitis/pharyngitis - imaging: hyperinflation, atalectasis, consolidation/pneumonia, peribronchial thickening - major cause of hospitalization in infants <1yp, esp 2-6mo - Pathology: virus leads to accumulation of lymphocytes and neutrophils (which release mediators) causing edema and airway narrowing. Necrosis of the epithelium leads to cellular debris contributing to obstruction - Commonly caused by RSV: (respiratory/droplet transmission), 3-4 day incubation, nearly everyone gets by age 2, peak incidence 2mo, usually just URI in healthy infants (25-40% progresses), 3-4 month season in the winter Risk factors: day care settings, Fall/winter, male gender, malnutrition, prematurity, crowding, etc - Management: mostly outpatient, fluids, prophylactic antibodies, hold feeding, humidified O2, glove/gown/handwashing (highly transmissible) |
|
|
Childhood wheezing disorders
|
transient early wheezing:
- associated decreased lung function (prematurity, maternal smoking) Non-atopic (viral) wheezing: - associated with viral URI (RSV, rhinovirus) - thought that inflammation lowers susceptibility to irritants Atopic wheezing: "asthma" - classically begins before age 6 - symptoms: atopy (allergen sensitization), airway hyper-responsiveness (type I), increased IgE - attacks last up to several hours, consist of chest tightness, SOB, wheezing, cough - 50% subsides in adolescence and reappears in adulthood |
|
|
Environmental effects on lung development
|
- respiratory system continues to develop during childhood
Events that increase risks for asthma and allergies: - increased IgE (already had exposure) - sensitization to Alternaria (pumpkin mold) - being an obese female - maternal prenatal smokin - other triggers: smoke/fumes, cold air, stress, exercise, drugs Events that decrease risk: exposure to other children and animals |
|
|
Clinical features of cystic fibrosis
|
Upper respiratory: chronic sinusitis, nasal polyps
Lower respiratory: cough, sputum, chronic bronchitis or pneumonia - GI: pancreatic insufficiency (malnutrition), meconium ileus, intestinal obstruction, rectal prolapse, liver dysfunction (biliary obstruction, cirrhosis), diabetes mellitus Reproductive: late onset of puberty, infertility (absence of vas deferens, chronic disease state) Skin: increased salt in sweat, digital clubbing, prolonged infant jaundice |
|
|
Classes of CFTR mutations
|
Class I: premature stop codon
Class I: abnormal protein trafficking (ΔF508) Class III: defective regulation (loss of activation by ATP/cAMP) Class IV: reduced chloride transport Class V: splicing defects (reduced production of normal protein) Class VI: accelerated turnover |
|
|
Pathopysiology of respiratory disease in CF
|
- reduced Cl- secretion and increased Na absorption lead to depletion of airway surface liquid, thickened adherent mucus, and ciliary dysfunction.
- obstruction leads to infection which leads to inflammation leading to obstruction (from neutrophil detritus/DNA)... - Progressive cycle leads to progressive, irreversible structural changes and lung damage (bronchiectasis) - after pulmonary exacerbations lung function does not return to 100%, leading to progressive loss of function with each exacerbation |
|
|
Complications of CF
|
- Malabsorption and weight loss due pancreatic insufficiency and chronic disease state
- Lung disease (from chronic bronchitis and pneumonia): loss of function, atalectasis, bronchietasis, pneumothorax, hemptypsis, respiratory failure |
|
|
CF therapies targeting CFTR protein
|
- Ivacaftor ("Kalydeco"): targets class III mutations (5%), acts to open the ion channel and stimulate Cl- transport
- PTC 124 ("Ataluran"): targets class I mutations (~10%), induces ribosome to allow read-through of premature stop codon - VX-809: for class II mutations, uses small molecules to correct folding and recognition errors and allow for proper trafficking to PM |
|
|
Treatment strategies in CF
|
- minimize malabsorption and prevent weight loss (maximize calorie intake, take supplemental enzymes)
- target errors in CFTR protein/protein maturation - augment airway clearance: dornase alpha (clear neutrophil debris), hypertonic saline (cough simulator, moisten mucus), bronchodilators, physical percussion/vibration/postural drainage (Positive Expiratory Pressure, the Vest, breathing techniques, exercise) - Antibacterial therapy: infection control and prophylaxis against exacerbations (aerosols and systemic) - reduce inflammation: steroids, cox inhibitors, macrolides - replace damaged lungs: transplantation |
|
|
Embryonic stages of lung development
|
Embryonic: 4-7 weeks
- the respiratory diverticulum buds off of the laryngotracheal groove of gut tube (where Nkx2.1 is expressed). Septation occurs concurrently separating the trachea from the esophagus - the diverticulum elongates into a tracheal portion and bifrucates for to form the bronchopulmonary segments of the pulmonary tree. Lung buds begin to fill the pleural cavaties Pseudoglandular (8-16): - period of major formation/growth of the duct systems (before terminal respiratory components) - pulmonary arterial system beings to form w/ elongating vessels paralleling duct formation - histologically lung resembles salivary gland (hence the name) Canalicular Stage (17-26w): - formation of the respiratory bronchioles due to budding of ducts formed in previous stage - intensive ingrowth of vessels and association of capillaries with bronchiole walls Terminal Sac Stage (26-term) - alveoli bud from respiratory bronchioles - alveolar epithelium differentiates into type I and II (surfactant) cells (dramatically increases premie survival) Postnatal: - ~90% of alveoli are formed, mostly through septation of existing alveoli sacs creating collateral ventilation pathways |
|
|
Tracheoesophageal fistulas
|
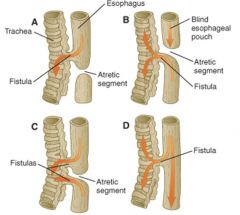
- occur do to incomplete septation of the trachea and esophagus (1/3-4000 births)
- Normally: respiratory diverticulum grows outof laryngotracheal groove and mesoderm grows in from the sides partitioning them (via Nkx 2.1, BMP-4, mutations cause high incidence of TEF), epithelia of the tubes then grows over making the tubes distinct with mesoderm layer in between - clinically manifest as choking or regurgitation - complications may persist after surgical repair: tracheomalacia, abnormal peristalsis, respiratory complications (commonly due to GERD) |
|
|
Branching morphogenesis in lung formation
|
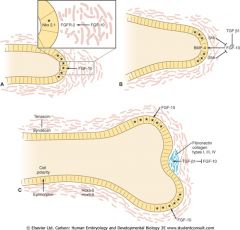
= iterative process of lung bud growth, elongation, and dichotomous subdivision of the terminal respiratory tree units
- Mesenchymal cells surround the lung bud epithelium induce outgrowth and bifrucation of the epithelium - FGF-10 produced by mesenchymal cells at the tip of the bud, cause it to grow toward the source of the FGF-10. Apical epithelial proliferation promoted by expression of Nkx2.1 - FGF-10 initiates epithelial secretion of BMP-4 which inhibits proliferation of apical epithelial cells, and SHH (sonic hedgehog) which stimulates mesenchyme proliferation while inhibiting FGF-10. Mesenchyme also secretes factors that promote synthesis of extracellular matrix (fibronectin, collagen, etc) - when cell proliferation at the tip is reduced and cells are bounding by ECM, lateral mesenchymal cells secrete FGF-10 (where [SHH] is low) creating two new budding sites laterally - Mesoderm determines endoderm growth and branching while endoderm signals mesoderm differentiation into SM wall (induced by BMP-4, SHH) |
|
|
Role of FGF-10 in respiratory development
|
- FGF-10 is produced by mesenchymal cells at the tip of the budding bronchiole.
- FGF-10 is a chemoattractant for endoderm and is a major factor in branching - When knocked-out the organism does not develop lungs but does have a normal trachea: FGF-10 is important for growth of the pulmonary tree but no the initial growth and septation of the trachea. |
|
|
NKx2.01 in respiratory development
|
- in knockouts trachea and esophagus do not form but hypomorphic lungs still bud.
- Nkx: responsible for septation of the trachea and eosophagus, also marks the formation of the thyroid gland - Prometes epithelial wall production and the first step in branching morphogenesis |
|
|
Major microbial pathogens in community acquired pneumonia
|
Top 4 pathogens:
- streptococcus pneumoniae: 2/3 of bacteremic pneumonia, risk factors: infant, elderly, underlying disease (HIV, diabetes, splenic dysfunction, cirrhosis, defective antibodies), African American or Native American - Mycoplasma pneumoniae: higher incidence age 5-20, respiratory droplet spread, closed populations at higher risk (military, schools) - Legionella pneumonophila: exists in low levels in water sources (amoebas & protozoa can be hosts), outbreaks linked to contaminated water, no P2P transmission documented - Chlamydia pneumoniae: 1/4 of pneumonia in school age children (50% seropositive by adulthood), P2P transmission by respiratory droplet, no seasonal variety, can carry asymptomatically, re-infect Other bacterial agents: H. influenza, S. aureus, group A strep, klebsiella Viral agents: influenza, RSV, adenovirus, parainfluenza |
|
|
Common clinical features of CAP
|
Typical Disease:
- present with classic symptoms: cough (+/- purulent sputum, hemotypsis), pleuritic chest pain (on inspiration), fevers/sweating, chills, dyspnea, confusion (elderly), headache, fatigue - pathogen is usually S. pneumoniae (but may be H. flu or other bacteria). Usually detectable on gram stain, culture on standard media - CXR showing lobar infiltrates and elevate WBC Atypical Disease: - Mild URI with dry cough and dyspnea - pathogens include mycoplasma, legionella, chlamydia, and viruses. Gram stain/standard media culture not helpfu - CXR showing patchy infiltrates - usually more mild cases, except for legionella |
|
|
Diagnostic tools for CAP
|
- CXR: lobar or patchy infiltrates, pleural effusions
- sputum gram stains: helpful in typical disease presentations. S. pneumo appears as gram + cocci in pairs/chains "lancet" - blood cultures: (preferred to sputum, since difficult to get), gold standard for S. pneumo diagnosis - selective media culture: to atypical disease presentations - Urinary antigen test: for Legionella - Direct Fluorescent Antibody (DFA) of sputum - Cold agglutinins: can occur in mycoplasma pneumo when patient develops IgM to own RBCs. Autoantibodies cluster at 4⁰C - Antigen and nucleic acid tests: becoming available for respiratory viruses and atypical pathogens |
|
|
Diagnostic criteria for S. pneumococcal pneumonia
|
= Leukocytosis (elevated WBC), positive CXR, plus one of:
- positive sputum or brochioalveolar lavage culture - positive blood culture - gram stain with gram+, lancet-shaped cocci growin in pairs or chains When cultured on blood agar S. pneumo produces green tinged colonies due to α hemolysis. Grown with optochin disk (which S. pneumo is sensitive to, resulting in inhibition zone). May also identify with catalase testing (strep is negative), solubility in bile acids (S. pneumo is soluble), using an rRNA probe |
|
|
S. Pneumo virulence factors
|
- capsular polysaccharide: prevents phagocytosis of bacteria
- Techoic acid and Peptidoglycan: stimulates inflammatory cytokines - Pneumolysin: cytotoxic for phagocytic and epithelial cells - Choline-binding proteins: allow bacteria to bind to surfaces - autolysin: releases peptidioglycan - Pneumococcal surface protein A: inhibits complement activation - IgA protease: counteracts mucosal defense mechanism |
|
|
Available pneumococcal vaccines
|
Polysaccharide vaccine: (23 valent polysaccharide)
- derived from the polysaccharide capsule of bacteria (23 capsule serotypes chosen for virulence frequency) - B-cells process it and differentiate into plasma cells and produce antibodies to it. Does not include memory B cells. - T cells not involved: in infants/young children T-independent immune function is not fully developed so cannot be vaccinated with polysaccharides - intended for elderly, older children, adults with risk factors Protein Conjugate: (originally 7 valent, now 13 in 2010) - composed of polysaccharide plus carrier protein. - polysaccharide stimulates B-cell antibody production, carrier protein is present to T-cells which then enables production of stronger antibodies and memory B-cells - suitable for infants and young children b/c of T-cell activation - approved for adults but not in common use b/c of no recommendations |
|
|
Predisposing factors for pneumonia
|
Immunodeficiencies
- congenital: defective immunity (T cell, B cell, neutrophil, macrophage) - acquired: AIDS, transplant, cancer, steroids Lowered host pulmonary resistance: extremes of age (infants, elderly) or impaired function of the respiratory system (direct pathogens, altered anatomy, congestion, etc) Chronic lung diseases: CHF, COPD, diabetes Decreased or absent splenic function: sickle cell disease, post-splenectomy |
|
|
Conditions that impair respiratory clearance
|
- direct pathogen effects (eg. viral disruption of the epithelium)
- Altered anatomy (COPD, obstruction by tumor) - loss or suppression of cough reflex (CVA, neuromuscular disorders, drugs (EtOH) - Mucociliary interruption: immotile cilia syndrome, smoke - interference with alveolar macrophages: pulmonary alveolar proteinosis, tobacco smoke, iatrogenic causes (surgery) - pulmonary congestion and edema (eg. CHF) - Accumulation of secretion (eg. CF, COPD) |
|
|
Focal Pneumonia
|
ex: bronchopneumonia, lobar pneumonia, bronchiolar pneumonia
- Patchy consolidation that may affect one lobe or multiple. Distribution is often bilateral and basal, since secretions tend to gravitate to lower lobes - can be extensive and eventually coalesce to resemble lobar ("confluent lobar pneumonia") - orgnanisms: staph aureus, strep, hemophilus, pseudomonas, legionella, gram negative - inflammation is neutrophil driven: invade alveolar space |
|
|
Pulmonary defense mechanism
|
Anatomic and mechanical:
- mucociliary blanket (ciliated epithelium + mucus): line nose and upper respiratory tract. Traps fomites and then is transported by ciliary action to back of throat for swallowing or clearance - glottis: prevents gastric aspiration Filtering and clearance: - filtering: nasal & pharyngeal clearance, branching of tracheobronchial tree - clearance: mucociliar escalator, alveolar clearance Immune system: - innate alveolar macrophages: found in alveolar spaces, phagocytose small particles that escape the mucociliary membrane - innate neuotrophils: recuited to the lung by cytokines in response to irritants - innate proteins (lysozyme, lactoferrin, etc) - Adaptive: bronchus associated lymphoid tissue (BALT), B and T cells produce immunoglobulins |
|
|
Classification schemes for pneumonia
|
Pathogenesis:
- exogenous (TB, mycob, legionella, yersinia, anthrax) vs endogenous source (s. pneumo, H flu, S. aureus, anerobes, enteric gram -) - inhalation v. aspiration v. bacteremia - primary vs. secondary (lung secondarily involved, distant from initial infection--bacteremia or infected thromboemboli, usually S. aureus, Salmonella, enteric G-) Epidemiology: community acquired (normal oropharyngeal flora, exogenous organisms) vs. nosocomial (gram -s and hospital associated pathogens--S. aureus) Anatomic distribution: focal (broncho) vs. lobar Time course: acute (most baterial, PMN inflammation, 5-10days) vs. chronic (fungi, mycobact, also nocardia, actinomyces) Biologic agent: various |
|
|
Lobar pneumonia
|
- inflammation and consolidation involves the majority or entire lobe of the lung--delimited by pleura or fissure, though often has pleural involvement (pleuritis or empyema)
- Organisms: S. pneumo (classic), K. pneumo, S. aureus 4 stages: - congestion/engorgement - red hepatization - grey hepatization - resolution/organization |
|
|
4 stages of lobar pneumonia
|
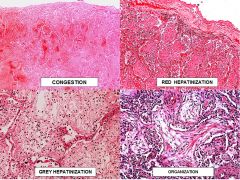
Congestion/Engorgement:
- external: heavy, crepitant lungs - internal: frothy blood filled exudate - Path: engorged capillaries, swollen alveolar epithelium, airspaces filled with edema fluid, RBCs, desquamated epithelium Red Hepatization: - external: heavy (sinks in water) non-crepitant lungs - internal: dark red/reddish brown, dry/granular consistency due to airspace fibrin deposition - Path: airspaces filled with organized fibrin containing RBCs, PMNs, leukocytes, and desquamated epithelium Grey hepatization: - external: dense, friable, grey to white - internal: moister than red hepatization, turbid fluid exudates - path: alveolar exudate w/ PMNs, most RBCs lysed and less obvious organized fibrin (starting to break down) Resolution/organization: spontaneous resolution or treatment success - resorption of inflammatory exudate and restitution of of normal underlying structures - lung remodeling occasionally occurs: organizing pneumonia, interstitial fibrosis and scarring |
|
|
Complications of Pneumonia
|
Abcess formation: tissue destruction and necrosis (+/- cavitation)
- often polymicrobial: S. aureus, S. pyogenes, Pseudomonas - pathogenesis: aspiration, bacterial, septic embolism, tumor Pleural Involvement: pleuritis and adhesions, empyema Organization/fibrosis Bacterial dissemination: pericarditis, endocarditis, meningitis, sepsis |
|
|
Aspiration pneumonia
|
- occurs in debilitated patients, unconscious patients, during repeated vomiting
- Aspirated materials: gastric contents, oral flora - Pathology secondary to toxic (gastric) effects and polymicrobial infection (oral flora) - Location of pneumonia related to anatomy - often results in severe necrotizing bronchopneumonia |
|
|
Atypical Pneumonia
|
General pathology: interstitial lymphocytic involvement preceded by alveolar PMNs
Pathogens: faculative or obligate intracellular bacteria and viruses, difficult to culture with standard medias Mycoplasma: - 9% of CAP, affects younger age ("walking pneumo") - focal pneumonia, Path: peribronchiolar, interstitial leukocytes and luminal PMNs Chlamydial Pneumonias: - C. trachomatis: infants (acquired during birth), Path: nodular foci of interstitial lymphocytes -C. Pneumoniae: 6-12% of adults, often mild or asymptomatic, no pathology from humans - C. psittaci: zoonosis from birds, early PMNs followed by lymphocytes Coxiella burnetii (Q fever) - exogenous: inhalation of aerosols of birth produces of sheep, cattle, goats, cats, rabbits - often asymptomatic, focal pneumonia w/ interstitial and alveolar infiltrates of macrophages Others: rickettsiae, legionella, viruses (influenzia A & B, parainfluenza, coronavirus) |
|
|
Viral Pneumonia
|
- difficult to detect: may require EM and immunohistochemistry, often precedes bacterial pneumonia
- General Path: diffuse alveolar damage, necrosis Pathogens: - Adenovirus: exudates with necrosis and hemorrhage, intranuclear inclusions with blurred nuclear membrane "smudge cell" - cytomegalovirus: immunocompromised patients, various path: intraalveolar hemorrhage & edema, interstitial pneumonitis, cytomegalic cells (enlarged, intranuclear and cytoplasmic inclusions) - Herpes simplex: secondary to aspiration in infected oral secretions, often involves tracheobronchial tree, Path: necrosis % hemorrhage, intranuclear inclusions (Type A: eosinophilic w/ halo, Type B: homogeneous, glass) - Measles: part of systemic disease, Path: interstitial pneumonitis, multinucleated giant cells (intranuclear inclusions w/ halo, cytoplasmic incldusions) - PCP: a fungus, associated w/ T cell dysfunction (AIDS, transplant, cancer, steriods), cysts visible on silver stain |
|
|
TB global epidemiology
|
New cases: 8.8mil in 2010, >95% in developing countries, Asia/Africa (80% in 22 countries), 1.4mil HIV+
Detection rates: global 63%, <50% in Africa, big problem in controlling TB. Effective point of care Testing would save >500,000/year Affected populations: those suffering barriers to care (migrant workers, prisoners, minorities, refugees), women (700K/yr, also stigma, infertility, orphans), poor nutrition (25%), HIV (25%), also tobacco/alcohol use, diabetes Death Rate: ~1.5mil/yr (98% in developing world), ~350K due to TB/HIV |
|
|
Possible outcomes of TB exposure
|
No infection: 1/2-2/3 of exposed persons are not infected due to innate/sterilizing immunity. No T-cell priming so no immune sensitivity, negative TST
Progressive disease: (<5%) - after initial infection progress immediately to disease (high bacterial load) - Risk factors: HIV, <5yo, immune-suppressed, other conditiosn (diabetes, renal failure, malignancy, chemo, gastrectomy, silicosis, fibrotic changes on CXR), malnutrition (<90% ideal BW), recent infection (<2y), substance abuse - can have atypical findings of CXR (does not follow reactivation pattern) Reactivation of disease (5-10%) - present with upper lobe cavitary disease - initial infection: alveolar macrophages and dendritic cells are infected causing increased bacterial load until adaptive immunity controls. Some organisms persist in macrophages/granulomas, allowing reactivation Latent Infection (90%) - as above, after initial infection some organisms persist as macrophages/granulomas but don't reactivate. - positive PPD from initial immune activation (though some revert to negativity after transient period, probably dependent on resolution of infection) - may become reinfected depending on prevailing host immunity |
|
|
Diagnostic Methods for latent TB infection
|
Tuberculin skin test (TST)
- uses purified protein derivative to stimulate delayed Type IV hypersensitivity response - positive criteria: ≥5mm (HIV, TB contact, +CXR, immunosuppressed/transplant), ≥10mm (>5yrs from high prevalence country, IV drugs, healthcare workers, at-risk disease states, Mycobacteria lab workers, <4yo or children around high-risk adults), ≥ 15mm everyone else - limitations: false + (cross reaction with BCG and NTM), false - (anergy in immunosuppressed), requires two visits - preferred over IGRA in children (no data/recommendations), serial testings (HCW) IFN-gamma release assay (IGRA): - in-vitro T-cell based assay measuring INF-γ in whole blood/peripheral blood mononuclear cells (sensitized T-cells produce it in response to TB specific antigens). Quantify INF-γ with ELISA (QFT) or ELISPOT (TSPOT) - Preferred over TST for BCG vaccinated, people who won't return for read |
|
|
Diagnostic methods for Active TB infection
|
Sputum AFB Smear:
- not specific for TB, indicative of mycobacteria infection. Sensitivity can be low to (~27% in patients with HIV) - stains: fluorochrome (most sensitive), carbol-fuchsin (cheaper) - graded 0-4+ based on # of organisms seen (need 5x10^3 to see anything) Nucleic acid amplification test (NAAT): - done on sputum after positive AFB - 99% sensitive/specificity for TB Culture - more sensitive than AFB - TB is slow growing (24hr doubling time), takes weeks to culture - Broth (faster) or solid media (cheaper) - identify species with DNA probes for different mycobateria - perform drug susceptibility testing on positive culture (broth or solid media, still takes a week w/ best tech) GeneXpert: - real-time PCR on patient sputum, results in <2hrsm but REALLY expensive - will scan for Rifampin resistance mutations if first test is positive (good marker for multi-drug resistance) - high sensitivity and specificity for AFB+ samples, slightly less for AFB- Gambian Pouched Rat; - detect TB in sputum - sensitivity 87%, specificity 93% |
|
|
Clinical Manifestations of TB
|
Big 4: cough, fever, night sweats, weight loss
Others: - pulm: pleuritic chest pain, hemotypsis - systemic: chills, appetite loss, easy fatigability HIV patients: frequently atypical presentation, delaying diagnosis - disseminiated/extrapulmonary disease is common - CXR findings are often atypical: basically anything, lower lobe interstitial infiltrates, adenopathy |
|
|
Short course therapy for TB
|
- RIPE for susceptible TB. Use directly observed therapy (DOT) when possible to improve adherence (often a problem, PubHealth issue)
- Use combination of 4 drugs initially, go down to 2 after susceptibility testing and 2 mo culture (never use/add single drug: resistance). Treatment lasts 6-9 months: 4 drugs/2mo, 2 drug/4 or 7mo if 2mo culture is + Rifampin: - inhibits DNA-dependent RNA polymerase (4 subunits/4 genes), bactericidal - resistance: single mutations in rpo B gene (81 bp) can cause resistance, scanned for by GeneXpert - side effects: Hepatitis, orange secretions, GI upset, bleeding, flu-like symptoms, rash, many drug interactions (induction of P450-3A: methadone, coumadin, OCP, azoles, steroids, protease inhibitors, etc) Isoniazid: - interferes with mycolic acid synthesis - side effects: hepatitis, peripheral neuropathy (give pyridozine to prevent), CNS effects Pyrazinamide: - unknown mechanism, may interfere with fatty acid synthetase I - side effects: hepatitis, GI upset, hyperuricemia, arthralgias Ethambutol - disrupts cell wall synthesis - side effects: optic neuritis |
|
|
Drug resistant TB
|
Result from poor TB control programs and inappropriate Rx. Two types:
- MDR -TB: resistance to atleast isoniazid and rifampin - XDR-TB: resistance to INH, RIF and fluoroquinolone and injectable antibiotic (capreomycin, amikacin/kanamycin). Therapy: - MDR-TB: longer treatment (6-24mo), more costly regimens, drug toxicity, second-line drugs (kanamycin, capreomycin, fluoroquinolones, streptomycin, cycloserine, enthionamid, etc) - XDR-TB: often untreatable, especially in HIV+ patients |
|
|
Immune Reconsitution Inflammatory Syndrome (IRIS)
|
- paradoxical response in HIV+ TB patients that are restarted on anti-retrovirals (or HIV+ with other infection)
- rapid increase of CD4 triggers inflammatory response to the secondary infection causing other symptoms (fever, tissue damage, pus collection) - Recommendation is to start ARV during TB therapy (improves survival). If CD4<50 start immediately, otherwise wait 2mo to reduce IRIS - may resolve on own, generally treat through w/ steroid or non-steroidal anti-inflammtories w/o stopping antibiotics or ARVs |
|
|
Treatment for latent TB infection
|
- daily INH for 9mo (self administered), <50% completion rate
- Rifampin for 4mo - 3mo course of rifapentine + INH (DOT administered 1/week): higher adverse reactions but not inferior to straight INH (probs due to better compliance). Can't use for HIV+ (drug interactions) or children/pregnant women, presumed infected w/ MDR resistant strain |
|
|
Define granulomatous inflammation
|
- chronic inflammatory response of activated T-cell immunity and macropages
- macrophages (from blood recruited monocytes) and T lymphocytes then cluster and form a granuloma (central macrophages with rim of lymphocytes) - There are a number of causes: infectious (mycobaterial, fungal other), autoimmune (saroidosis, Wegner's, hypersensitivity pneumonitis), Other (foreign body, pneumoconiosis) - inflammation may be necrotizing/caseous (myocbacteria, Fungal, Wegner's) or non-necrotizing (sarcoidosis, Beryllosis, foreign body) |
|
|
Primary vs Secondary pulmonary TB
|
Primary
- results from aspiration of M. tuberculosis contaminated droplets - Immune response: T-cell mediated and macrophage response which results in formation of necrotizing granulomas (spherical macrophages w/ rim of lymphocytes, central necrosis, may see acid fast bacilli in necrotic region "red snappers") - Ghon complex: granuloma in the periphery (Ghon focus) plus hilar or bronchopulmonary granulmona indicating lymph involvement. May have endobronchial, cavirary, or advanced patterns Secondary/reactivated - reactivation of latent TB infection, usually in disabled, elderly, immune compromised - tends to affect the apices of the lungs (high O2 tension preferred by TB) |
|
|
Miliary TB
|
= disseminated TB infection (liver, lungs, spleen--well perfused organs esp), named for millet seeds that resemble diffuse yellow, round lesions in lung
- results from the erosion of TB into a pulmonary vein, and subsequent systemic distribution (or lymph spread, different organs involved) - prognosis depends on organs involved, ex: adrenal gland involvement may lead to adrenal insufficiency, most patient will have hepatospenomegaly |
|
|
Morphologic appearance and clinical course of TB in immune compromised patients
|
- TB is an AIDS defining illness
- Immunocompromised patients have higher TB incidence, more severe infection, atypical features, often multidrug resistant strains - these patients can also contract MAC: an environmentally acquired mycobateria that is often aggressive and disseminated in AIDS patients. In non-AIDS may have pre-existing lung disease. There are different anti-mycobaterial drugs (don't respond to TB drugs), but difficult to eradicate. Often present as ill-defined lesions in the lung |
|
|
Granulomatous disease caused by fungal organisms
|
- can be primary or secondary reactivation, resemble TB. Typically in immunocompromised patients
- causative fungi are dimorphic: mycelia (mold) at room temp, yeast bud morphology at body temp "mold in cold, yeast in heat" Diseases: - cryptococcocosis: Cryptococcus neofromans, encapsulated yeast (narrow base budding, on path spheres w/ white space (capsule), present in soil and bird droppings, immunocompromised patients only - histoplasmosis: Histoplasma capsulatum: unencapsulated yeast (narrow base budding), infects alveolar macrophages (form histoplasmomas: walled off/calcified, necrotic lesion), can cause fibrosis, pneumonia and disseminated disease, Ohio and Mississippi river valleys, normal and compromised patients - Coccidioidomycosis: Coccodioides immitis (multiple budding), spherules on path w/ endospores inside, multiple body cocci, southwestern US, normal & compromised patients - Blastomycosis: Blastomyces dermatidis (broad base budding), can cause pulm, skin and bone lesions, southeast/south central US, normal and compromised patients |
|
|
Non-infectious cause of pulmonary granulomatosis
|
Wegner's granulomatosis
- necrotizing granulomatous inflammation: lung cavitation, necrotizing granulomas, alveolar hemorrhage w/ capillaritis, medium/small vessel vasculitis - ELK manifestation: ENT/lung/kindney, saddle nose deformity - treat with immunosuppression (have to differentiate from TB, that would be bad...), evaluate disease activity with ANCA (IgG antibody against antineutrophilic primary granules and monocyte lysosomes--2 staining patterns) and systemic vasculitis, variable clinical outcome Foreign Body granuloma: multiple nuclei spread throughout, if near aiways--aspiration, if near vessels--IVDU - cause diffuse pulmonary emboli from non-clot material in vessels (talc from IV drugs, indwelling catheters, silicon from breast implants) - lipoid pneumonia: from lipid inhalation, causes lipid droplets Beryllosis: due to acute and chronic exposure to beryllium (old fluorescent bulbs, mining, industry) - hypersensitivity reaction producting non-necrotizing granuloma, similar in appearance to sarcoidosis Silicosis |
|
|
Epidemiology of sarcoidosis
|
- African americans: 3x more common that caucasians, more severe disease, F>M, onset later in life >40
- US overall: incidence usually before 50, peak 20-39 - Variable incidence between countries, highest in scandiavian countries (40/100K /yr) |
|
|
Pathobiology of sarcoidosis
|
- multisystem disorder of unknown etiology
- characterized as interstitial lung disease, but may also effect airways. Forms non-necrotizing granulomas which tend to follow lymphatics (but can also travel in airways) - disease is associated with Class II MHC's: specific local signal susceptibility vs protection - Granulomas are formed when an APC picks up an unknown antigen, interacts with Th CD4, releasing cytokines (incl. TNF) initial granuloma formation (not well understood) |
|
|
Clinical Presentations of Sardoidosis
|
- highly variable disease without a single classic presentation
- Most often incidental finding on CXR or on skin, or symptoms including cough, dyspnea, chest pain, fever, eye and skin findings, fatigue, weight loss - presentation can be significant in determining prognosis: limited to lungs is better than outside chest, cardiac involvement is most life-threatening, hypercalcemia and nephrosclerosis suffer more severe disease course - Classically restrictive PFTs but may also be normal or even obstructive due to airway and parenchyma involment - Can effect every organ of the body: intrathoracic and lung are most common (95%, incl airway cobblestoning), also skin (incl. erythema nodosa, lupus pernio), eye (lacrimal, pulpil), lymph, and spleen lesions, Bell's palsy - Lofgren's triad: arthritis, erythema nodosum, hilar adenopathy. Correlates to good prognosis, usually spontaneous resolution - Heerfordt's syndrome: parotid swelling, uveitis, Bell's palsy, fever |
|
|
Treatment of sarcoidosis
|
- the majority of sarcoid cases resolve on their own without treatment.
- cardiac involvement is severe and often requires a pacemaker and and automatic defibrillator in case of heart block - Generally if pulmonary symptoms are worsening treatment is immune suppression: glucocoriticoids (do not change natural history of the disease) and anti-TNFs (interferes with granuloma pathogenesis), severe pulmonary failure can be treated with lung transplant |
|
|
Pathogenesis of hypersensitivity pneumonitis
|
= a hypersensitivity (Type III, IV) reaction to inhaled organic antigens causing non-necrotizing granuloma formation.
- there are many known antigens including avian proteins (Bird-fancier's lung), wheat and soy proteins, inseticides, mold (farmer's lung), fungi, bacteria (hot tub lung) - Disease is CD8 mediated, in which CD8 immune response causes cytokine release inducing inflammation or direct tissue injury. - Granulomas appear similar to sarcoidosis (CD4 mediated) but are less defined, usually peribronchial with prominent giant cells |
|
|
Clinical presentation of hypersensitivity pneumonitis
|
- patients are usually non-smokers (95%)
- CXR is often normal, may show mid/upper zone predominance of central lobular ground glass micro nodular opacities with air trapping Acute (most common): occurs 4-6 hrs after exposure, resolves without treatment if exposure removed. Non-specific symptoms (cough, dyspnea, fevers, chills, myalgias, tachypnea, tachycardia, fine crackles). Tend to be more severe on re-exposure Subacute: more gradual due to repeated low dose exposures, similar symptoms as acute which resolve without intervention within 1 day after exposure removal Chronic progressive (least common, 5%): very subtly onset of productive cough, dyspnea on exertion, fatigue, weightloss. Symptoms do not resolve with removal of antigen (due to progressive fibrosis) |
|
|
Wegener's granulomatosis (granulomatosis with polyangitis)
|
= disease of unknown etiology leading to necrotizing granulomas and vasculitis
- Symptoms: classically ELK, but also involves skin (saddle nose deformity), eyes, nose, mucus mucosa, heart, nervous system - Diagnosis is made based on clinical symptoms, CXR/CT (non-specific pattern of nodular opacities), p-ANCA serology, biopsy - Treatment consists of immune suppression (cyclophosphamide, glucocorticoids (not sole therapy), ritixumab, azathioprine, methotrexate), plasma exchange for renal failure - with treatment 85-90% remission, with relapse up to 50% depending on patient's involvement, without treatment almost always fatal in 2 years |
|
|
Influenza virus
|
- negative sense RNA virus with a segmented genome allowing for subtyping (for susceptibility/treatment)
- 3 types A, B, C with A being the most severe (within A there are different isoforms) Clinically: high fever, severe myalgias, headache, chills, cough, dyspnea, GI upset, nausea - may lead to primary viral pneumonia or secondary bacterial pneumonia, complications can be fatal - also associated with otitis media, Reye's syndrome (affects brain, liver causing hypoglycemia), myopericarditis, encephalitis |
|
|
Parainfluenza virus
|
- single stranded RNA virus, with 4 serotypes (Pflu 1 most common, related to mumps)
- Clinically: (20% of ped respiratory infections) laryngotracheobronchitis (Croup: subglottic edema, barking cough, low grade fevers), coryza-like illness and other URIs that precede croup, bronchioiltis (second most common cause after RSV), infrequently pnumonia |
|
|
Respiratory syncytial virus
|
- single stranded RNA enveloped virus, with two subgroups based on glyoproteins for viral attachment
- cause the formation of large syncytium (multinucleate fusion cell) Clinically: - most common cause of severe lower respiratory tract infection in infants, cause 90% of bronchiolitis, 40% of bronchopneumonia, can also cause croup (10%) - particularly a concern for premature infants who are particularly susceptible to respiratory infections, older children have less severe infections like bronchitis |
|
|
Human metapneumovirus
|
- RNA virus of the paramyxovirdae family
- seasonal virus spread by close contact - causes illnesses similar to RSV (bronchiolotis, bronchopneumonia, croup) - co-infection with RSV can cause severe bronchiolitis or pneumomia |
|
|
Adenovirus
|
- DNA virus, with 47 serotypes
- half of infections are asymptomatic (nearly every adult as antibodies) - infected epithelia cells undergo necrosis, slough off, potentially leading to viremia and infection of other organs Clinically: (URI + conjunctivits) - in infants: pharyngitis, otitis media, pneumonia, diarrhea - in children: URI, pneumonia, pharyngoconjuctival fever, diarrhea - Adults and immunocompromised people have different courses |
|
|
Rhinovirus and Coronavirus
|
Rhino: single stranded RNA virus, cause of the common cold
Corona: single stranded RNA virus, caused SARS outbreak in 2003 (Severe Acute Respiratory Syndrome) |
|
|
Structure of the influenza virus
|
- negative sense RNA virus with a segmented genome
- Viral RNA is surrounded by a lipid bilayer with several coating proteins (coded for by each segment of the genome). - Segment 4 is hemagluttinin (a fusion protein, important for binding host cell receptors for viral entry) - segment 6 is neuraminidase (enzyme critical for release of newly packaged virus form cell, allows for propigation between cells) - M2 protein (Influenza A only) forms an ion channel that is important for resistance Subtyping (H#N#): depends on the various hemaglutinin (15+) and neruaminidase (9 types) variants present Pandemics occur when influenza A develops a new hemaglutinin subtype which no-one had antibodies to (occurs due to antigenic shift, from reassortment during transmission through different viral vectors, e.g. birds). Epidemics of influenza A and B arise when minor changes to H or N occur allowing the virus to escape from circulating antibodies (often point mutations in surface proteins due to genetic drift) |
|
|
Influenza vaccine
|
- trivalent vaccine usually containing A H3N2, H1N1, and a B strain
- injection usually contains inactivated or subunit viral proteins, nasal spray contains live attenuated virus (provides better mucosal immunity, recommended for health, non-pregnant people) - recommended >6mo, contraindicated by egg allergy, Guilain-Barre syndrome - Efficacy depends on age and immunocompromised status (can't develop immune memory). Under 65 70-90% efficacy, >65 30-70%, >65 in hospital/nursing home only 30-40% efficacy due to decreased immune response with age - new vaccine for the elderly has higher dose of H antigen, produces better antibody response - Vaccine still reduces hospitalizations and death due to influenza in the elderly population |
|
|
Anti-virals for viral respiratory infections
|
Amantadine/Rimantadine: influenza A prophylaxis, block viral uncoating preventing replication. No protection against influenza B, should be taken in 48h of symptoms, commonly GI and CNS side effects, resistant influenza strains emerging
Oseltamivir (Tamiflu): inflluenza treatment/prophylaxis, neuraminidase inhibitor, oral dosing Zanamivir (Relenza): influenza treatment/prophylaxis, poor bioavailability (inhaled only) Peramivir: influenza treatment, neuraminidase inhibitor, compassionate use only Ribavirin: RSV treatment, mechanism unknown (GTP depletion?), aerosol for infants (can cause bronchospasm), oral for transplant patient, both need supportive care Cidofovir: disseminated adenovirus, nucleoside analogue, not recommended for pulmonary disease Immunotherapy: disseminated adenovirus, intravenous immunoglobulins, not for isolated pulm disease |
|
|
Mechanisms of transmission of respiratory viruses
|
Influenza: viral shedding occurs before symptoms appear, release for 5-7d, spread by direct transmission (into mucus membranes), airborne (aerosolized droplets), indirect (via a surface). Important to vaccinate HCWs to prevent transmission
Parainfluenza: direct, aerosolized, indirect transmission RSV: droplet nuclei, direct contact Adenovirus: droplet nuclei, fecal-oral route, can survive prolonged periods in environment. Some subtypes can be vaccinated (only military does this) Transmission prevention: vaccination, isolation of infected patients (masks, contact), hand washing, avoiding contacts when sick, prophylactic antivirals for patients with severe pulmonary complications |
|
|
H1N1 epidemics of 1918 and 2009
|
1918: decreased life expectancy by 10 years, killed more people than WWI
- lead to rapid viral pneumonia (rather than predisposing secondary bacterial) causing damage and ARDS, leading to multi system failure and rapid death 2009: abnormal mortality rates (highest in 18-60y rather than elderly/infants) |
|
|
Indications for lung transplant
|
= end stage lung disease with 1-2y life expectancy
Idiopathic Pulmonary Fibrosis (46%) Emphysema Cystic Fibrosis Other (eg. sarcoidosis) |
|
|
Guidelines for recipient selection for lung transplantation
|
a. clinically/physiologically severe lung disease
b. no effective medical or surgical treatment other than transplant c. adequate cardiac function, no significant CAD d. Absence of major organ dysfunction e. No history of cancer in the last 5 years f. acceptable nutrition status g. no history of tobacco use in the past 6mo h. BMI <30 i. acceptable bone mineral density j. ambulatory with rehabilitation potential k. satisfactory psychosocial profile and support system - selection is then based on the lung allocation score Relative contraindication: advancing age (increasing mortality >45) |
|
|
Pathophysiology of acute lung rejection
|
Acute rejection
- frequent complication early after transplant (70%) - radiographs show non-specific interstitial infiltrate, diagnosed with transbronchial lung biopsy (show lymphocytic infiltrate) - Treated with 3 day steroid pulse Chronic rejection: - frequently complication, technically the result of all transplants, limits long term survivial - symptoms: dyspnea, cough, recurrent infections - radiographs show hyperinflation and gas trapping, poor diagnostic sensitivity on biopsy (20%) - Bronchiolitis obliterans syndrome (terminal bronchioles fill with scar tissue preventing air intake), progression scored by decline in FEV1 (BOS1 is 20% decline) - Treatment is immunosuppression, generally poor response (ultimately fails, would have to re-transplant) |
|
|
Common complications of lung transplant
|
- acute and chronic rejections
- bronchiolitis (BOS is major cause of death after first year) - cytomegalovirus infection - other infection (major cause of death in first year, remains significant complication) - lymphoma - other malignancies - Coronary artery vasculopathy - hypertension - renal failure - hyperlipidemia - diabetes |
|
|
Lung donors
|
- most are brain dead, rarely donor after cardiac death
- cause of death mostly intracranial bleed (aneurysm, subarachnoid hemorrhage), closed or open head trauma Criteria: (rare that all criteria are met perfectly) Age <60 minimal smoking history, especially if >50 no history of cancer HIV and Hep C negative, Hep B is okay if recipient is vaccinated PaO2 >300mmHg on 100FiO2 Clear CXR, bronchoscopy, visual inspection |
|
|
4 pillars of medical ethics
|
Beneficence: the doing of active goodness, refers to moral obligation to act for the benefit of others (treatment must do good)
Non-maleficence: do not harm, most decisions are risk/benefit analysis Autonomy: the ability of rational individuals to make informed decisions without coercion. (After transplant what should the patient be allowed to do?) Social justice: fairness, implies degree of limited resources (how should they be distributed?) Ethical problems arise when these pillars conflict with eachother |
|
|
Legal rules for transplantation in the US
|
Definition of dead: "an individual who has sustained irreversible cessation of circulatory and respiratory functions or irreversible cessation of all functions of the entire brain including brainstem"
Donation decisions: - Uniform Anatomical Gift Act (UAGA), 1968: legality of donating organs for transplant/research/education, ultimate decision is family's 2006 UAGA modification: neither the attending physician or physician who declared death may participate in organ harvest or transplant. Helps avoid conflicts of interest, formalized ability of donors to decide prior to death - National Organ transplant Act, 1986: outlawed sale of organs, established Task Force on Organ Transplantation, Organ Procurement & Transplantation Network (OPTN), and Scientific Registry of Transplant Recipients (SRTR) Donor Identification: use an opt-in system with 38.2% sign up rate Fair allocation: OPTN contracts with UNOS (United Network of Organ Sharing) to manage the waitlist, separated into geographic regions Quality Assurance: Advisory Committee on Organ Transplant (ACOT), 25 member committee appointed by the Heath & Human Services Secretary |
|
|
Ethical dilemmas in lung transplant
|
- performing lung transplant in older people who derive less benefit?
- favor listing patients with diseases such as CF that will get many years from their organs? - limit transplant for patients with self-inflicted disease (emphysema)? - approach to patients with less chance for good outcomes due to societal constraints (lack of social support)? - single vs double lung transplant to help one or two people? - Separation of treating and organ recovery physician needed to keep public trust but may endanger harvest options? |
|
|
Attempts to address ethical problems of transplant
|
organ allocation:
- originally had a need-blind system that valued social justice over other pillars, allowed gaming the system with early listing. - now use the Lung Allocation Score (LAS): allocation is score based on medical urgency and transplant benefit/survival. Goal is to distribute organs over as broad a geographic area as possible. Resulted in decrease in deaths on wait list but lightly decreased survival (transplanting sicker patients) Eligibility for transplant: only 1/10 patients referred get on the list. Each center has specific criteria, most consider age, CV history, cancer, social support, smoking |
|
|
Special circumstances in transplantation
|
Donation after cardiac death: (0.24% of donors) made under circumstances of cardiac death after withdrawal of life support (ONLY controlled situations)
- Living Related (lobar) Lung transplantation: in selecct cases it is possibly for parents to each donate a lobe to a child with end-stage disease. Some loss of function for parent, benefit over cadaver? -Multi-listing? (wealthy) patients are able to list at centers that have the shortest list, multi list themselves as long as the can get to the site within an hour. - Questionable use of high-risk donors: benefit of transplant even if organ isn't perfect? |
|
|
pulmonary edema
|
= extravascular fluid in the lungs
- pulmonary edema is not a specific disease but rather the end result of other diseases so treatment is of the underlying cause 2 Mechanisms: - cardiogenic: failure of the left hear to adequately remove blood from the pulmonary circulation - non-cardiogenic: injury to the lung parenchyma or vasculature - can lead to impaired gas exchange and may cause respiratory failure - symptoms: dyspnea, cough, excessive sweating, anxiety - chronic may be associate with signs of fluid overload (left ventricular failure); peripheral edema, raised jugular venous pressure, hepatomegaly, inspiratory crackles, 3 heart sound |
|
|
Hydrostatic pulmonary edema
|
- main cause of pulm edema
= due to increased capillary hydrostatic pressure second to elevated pulm venous pressure. Fluid is low-protein concentration, and due to CHF, MI, renal failure or IV fluid resuscitation Progression: fluid accumulates in perihilar interstitium (lowest pressure, high compliance, limited lymph drainage), then alveolar interstitium (active lymph), then aveolar spaces (affects lung function and oxygenation). Fluid will have basal and perihylar distribution due to gravity Changes that occur: increase lymph flow, decreased lung plasma protein concentration, increased microvascular pressure, increased airway resistance Clinical findings: Kerley's B lines (thickening of interlobular septa d/t engorged lymphatics), CV findings (S3 gallop, murmurs, LV hypertrophy, JVD, leg edema), base crackles, CXR (bilateral opacities, perihylar/basalar distribution, increased interstitial markings), Path (alveoli filled with pink fluid, little cellular infiltrate, swollen lymph) |
|
|
Starlings law and mechanisms of pulmonary edema
|
4 edema mechanisms can cause development of pulmonary edema based on starling's law, Jv = Kf [(Pc-Pi) - σ(πc - πi) - Qlymph:
1. increased hydrostratic pressure gradient (Pc-Pi). Normally Pc>Pi favoring fluid movement out. Can increase the interstitial pressure some to prevent edema. 2. Decreased oncotic pressure gradient (πc - πi); increases flow to interstitium where [protien] is higher. Some ability to prevent by reducing πi and increasing πc. 3. Decreased σ (reflection coefficient: pore size, capillary leak), increased Kf (but transport coefficient, steady state leakiness). No mechanisms to correct 4. Decrease lymph flow. Normally postive and can be increased to accomodate more flow and prevent edema |
|
|
Permeability Edema
|
= typically acute due to disruption of the pulmonary capillary membrane causing fluid and protein link into the interstitium. Classified as ALI/ARDS depending on extent of damage/loss of function (PaO3/FiO2 of 300,200)
Pathophys: injury to endothelium → loss of barrier, dysfunctional type 1/2 pneumocytes→ macrophages release mediators→ lymphocytic infiltration→ edema and inflammation of interstitium→ altered gas exchange 3 etiologies: - direct injury: pneumonia, aspiration, inhalation, near drowning, toxin exposure - indirect: sepsis, major trauma, pancreatitis, transfusion (anything resulting in inflammatory cascade), toxin exposure Clinical findings: CXR (bilateral fluid accumulation, may be patchy/asymmetric depending on damage), path (areas consumed with fluid and infiltrate, hyaline membrane formation, adjacent areas are normal), no CV findings |
|
|
Mechanisms of edema resolution
|
These mechanisms begin to be overwhelmed around 24-25mmHg capillary pressure, without oncotic mechanism this occurs at 11mmHg (in endothelial damage)
- reductions in the interstitial oncotic pressure via protein sieving - increased interstitial hydrostatic pressure, minimizing incoming flow - increased plasma oncotic pressure: favors retention of capillary fluid - increased lymphatic removal of fluid Additionally: - alveolar epithelium can pump fluid out of alveolar space with Na/K ATPase transport. Not effective in endothelial injury - paracellular transport from the alveolus to the capillaries - alveolar macrophages phagocytose insoluble material |
|
|
Pulmonary hypertension
|
= elevated pressure in the pulmonary artery (>25 mmHg)
- measured using right heart cath inserted via subclavian vein - non-necessarily a disease, rather the manifestation or pathologic result of another disease state: pulmonary vascular disease, LVF, endstage lung disease - acutely may be due to rapid occlusion of pulmonary circulation: pulmonary embolism, ARDS (diffuse vasoconstriction due to hypoxia) |
|
|
WHO classification of pulmonary hypertension
|
Group 1: idiopathic PAH, heritable PAH, drug induced, associated with other systemic disease (congenital, HIV, etc). mPAP >25mmHg, pulmonary wedge pressure <15mmHg (PCWP, no back fluid from left heart). No significant obstructive/restrictive, left heart, or thromboemolic disease.
Group 1': pulmonary veno-occlusive disease (PVOD), pulmonary hemangiomatosis (PCH), mPAP >25, PCWP <15, no other associated conditions Group 2: PAH due to heart disease (valvular disease, sytolic/diastolic dysfunction). mPAP >25mmHg, PCWP > 15 (due to back up of fluid from the dysfunctional heart) Group 3: PAH due to underlying lung diseases (anything causing chronic hypoxia induced shunting, eg. COPD, ILD, sleep-disordered breathing, chronic high altitude) Group 4: chronic thromboembolic pulmonary hypertension (CTEPH) (from PE that become endothelialized, subacute pulmonary embolic disease). Only PAH completely curable: endarterectomy to remove embolus Group 5: PAH w/ unclear or multifactorial causes, e.g.: myeloproliferative diseases, traumatic or autosplenectomy, sarcoidosis, end stage renal disease, hyper/hypothyroidism, glycogen storage diseases |
|
|
Chronic thromboembolic pulmonary hypertension
|
= endothelialization of PE rather than normal clearance (w/in 30 days) causing progressive remodeling, hypertrophy, narrowing of vessel lumen and ↑PVR/rt heart pressure
- This is the only form of PAH that is surgically curable (besides transplant) - incidence is 3.8% of patients w/ PE. Risk factors: younger age, larger emboli, idiopathic venous thromboembolism, history of previous PE - diagnosed w/ V/Q scan showing discrete areas of significant V/Q mismatch |
|
|
Diagnosing PAH
|
Rt heart catheter: directly measurer mPAP
CXR: rt heart enlargement, engorgement of pulmonary vasculature EKG: increased rt heart strength, large R and V1 suggests increased mass of rt ventricle Doppler echo: septal deviation to the left ventricle, tricuspid regurgitation (due to dilation) |
|
|
Idiopathic pulmonary hypertension
|
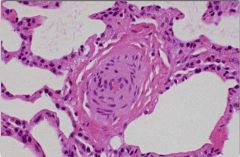
= WHO group 1 PAH, affecting 4-6/million, F>M, 20-30's, median survival 2.8y before treatment
Symptoms: initial (non-specific, delay in diagnosis): progressive dyspnea on exertion, chest pain, palpitations, fatigue, dizziness, syncope w/ significant exertion, coughing. Later (Rt hrt failure): limited performance (class I -IV), edema, syncope w/ minimal exertion, ascites PE: loud pulmonic valve closure (P2) due to increased back pressure, tricuspid valve regurgitation (L. sternal border), right ventricular heave, ascites, JVD, peripheral edema Pathology: proliferation of vascular wall associated with vascular remodeling (all components: intima, adventitia, SM), plexiform lesions (disorganized conglomeration of vascular cells, fibrin leading to occlusion of distal pulmonary arteries) Natural history: pre-sypmtomatic/compensated by rt. heart hypertrophy (↑PVR, ↑PAP, NL CO), symptomatic/decompensating (↑PVR, ↑PAP, ↓ CO), declining/decompensated (↑PVR, ↓PAP, cor pulmonale) |
|
|
Therapeutic options for PAH
|
All major drugs are targeted at treating vascular abnormalities due to underlying disease
Endothelin receptor antagonists (e.g. Bosentan): inhibits endothelin binding which normally causes vasoconstriction and SM proliferation Oral PDE-5 inhibitors (e.g. Sildenafil): prevent phosphodiesterase from degrading cGMP, increasing NO vasodilation and (moderate) anti-proliferation effects Prostacyclin analogues (e.g. Esoprostenol, "Flolan"): analogue of prostacyclin which increase cAMP in vascular epithelium promoting vasodilation and inhibiting vascular component proliferation and platelet aggregation Supplemental drugs: warfarin (prevent emboli), diuretics, supplemental O2, digoxin for CHF, ionotropes for CTEPH, calcium channel blockers for patients with maintained vasoreactivity |
|
|
Pathophysiology of VTE
|
DVT
- majority of DVT formation is related to endothelial cell activation, causing activation of the extrinsic/tissue activation factor clotting cascade - vessel wall damage in 1/50, more often tissue factor expression endothelial surface thought to be causative - Venous thrombi are primarily fibrin (unlike arterial which are mostly platelet) which entrap RBCs forming red clots - platelet aggregation is no seen at the site of thrombus attachment suggesting cascade activation precedes platelet activation, though they are eventually activated, usually downstream. Anti-platelets are not appropriate treatment PE - 90% start in lower extremities and travel to the lungs - PE causes increased PVR, forcing the RV to work harder, causing septal deviation and interfering w/ LV function, causing decreased stroke volume and CO. (isn't just a problem of blood flow to the LV) |
|
|
Risk factors for VTE
|
- men, African Americans, older age (incidence doubles every 10yrs)
- after illness or trauma: malignancies, end-stage heart failure, COPD, spinal cord injury, major trauma, neurosurgery, gynecology (delivery), other surgery, hip fractures and replacements - smoking, obesity (>10kg), OCP use, estrogen therapy Virchow's triad: - vessel wall damage: exposes underlying collagen, promoting clot formation. Ex: surgery, central line (esp. femoral), vein harvest or ligation, prior unresolved DVT - venous stasis: non-flowing blood clots. Ex: volume overload (CHF, cor pulmonale, renal failure), age >40, immobilization, varicose veins (?), MI, stroke, paralysis, anesthesia, spinal injury - hypercoagulable state: predisposition to clot. Ex: cancer, high estrogen state (pregnancy, OCP, obesity), sepsis, blood transfusions, inflammatory disease, heparin-induced thrombocytopenia, genetic disorders (AT111 deficiency, Protein C/S deficiency, antiphospholipid AB, homocystinuria, Factor V Leiden mutation) - Risk assessed w/ Wells Clinical prediction rule for DVT |
|
|
Wells Clinical Prediction Rule for DVT and PE
|
DVT: All one point except alternative diagnosis (-2)
- active cancer (Rx w/in 6mo, palliation) - paralysis, paresis, immobilization of lower extremity - bedridden >3 days because of surgery (within 4 weeks) - localized tenderness along distribution of deep veins (sign of DVT) - Entire leg swollen (sign of DVT) - Unilateral swelling of >3cm - Unilateral pitting edems - collateral superficial veins - (-2) Alternative diagnosis as likely as or more likely than DVT Interpretation: >= 3 (high risk, 75% will have DVT), 1-2 pts (moderate risk, 17%), <1 (low risk, 3%) PE: - (3): clinical symptoms of DVT - (3): other diagnosis less likely than PE - (1.5): HR > 100 bpm - (1.5) immobilization or surgery within past 4 weeks - (1.5) Previous DVT or PE - (1) Hemotypsis - (1) Malignancy Interpretation: >6 (high risk, 78.4%), 2-6 (moderate, 27.8% get more tests), <2 (low, 3.4%) |
|
|
DVT prophylaxis
|
Mechanical (intermittent compression devices: uses air sleeves on legs to aid venous return for patient otherwise immobilized (or contraindicated for anti-coagulation). Only effective during continous use (reduced DVT incidence from 15% to 6.9% or 4% when combined w/ LDUH)
Garment: compression socks Medical: - Anti-coagulation with LDUH, LMWH subQ, Factor Xa inhibitors. - all increase bleed risk 4-6% (caution for patients w/ spinal/epidural anesthesia--catastrophic bleed consequences), reduce DVT incidence by 60-70% - tends to be under-utilized |
|
|
DVT diagnosis
|
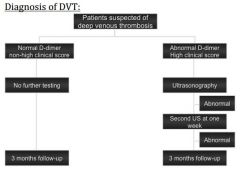
Given clinical suspicion of DVT (Well's Protocol) get D-dimer test (looks for breakdown products of fibrin clots, very sensitive, non-specific). If abnormal get ultrasonography to find the clot. Repeat after 1 week if normal (may skip if entire leg is imaged)
If patient has a non-high Well's score and normal D-dimer no follow up is needed |
|
|
Pulmonary Embolism diagnosis
|
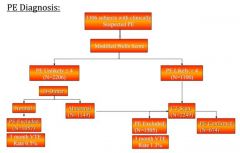
Christopher Study:
- stratify patients w/ Well's score as likely (>4) or unlikely (<= 4) - for unlikely get D-dimer. If normale no further testing - for PE unlikely/D-dimer abnormal and PE likely get CT (89-95% specific for PE, not indicated in renal failure or pregnancy due to contrast) - positive D-dimer is only a clot 40-66%, many other disease states may elevate. Uses monoclonal antibodies for D-dimers on ELISA - V/Q scan: uses radioisotopes to measure V/Q mismatch, need to be supported by clinical prediction scores |
|
|
Treatment for DVT
|
Acute: depends on location of the thrombus (proximal: iliac, common femoral or distal: thigh above knee)
- catheter therapy: (proximal DVT) mechanically break up clot w/ radiologically guided catheter with local thrombolytics (systemic not indicated to prevent further DVT) - Anticoagulation (distal DVT): heparin is best option, coumadin (delay in efficacy so can't be used alone, but start for maintenance). Stop anti-coagulation when INR >2, and begin ambulation (embolism 1-2%) and compression stockings to prevent post-thrombotic syndrome Long Term anti-coagulation: - provoked DVT: cease risky behavior (OCP, smoking, surgery <3mo) - malignancy: treat with anticoagulants for 3 months or until remission - unprovoked (stage IV cancer, factor V leiden): life-long anti-coagulation unless contraindicated. Idiopathic DVT is almost 50% of clots, high recurrence in people w/ hypercoagulable states |
|
|
Treatment for PE
|
Anti-coagulation:
- Short term (usually resolves in 2 weeks): unfractionated heparin, LMEH - Long-term: coumadin unless contraindicated Thrombolytics: - really only in cases of massive PE with hypotension/shock used w/ anti-coags - other indications: rt heart dysfunction, recurrent PE, prevention of PAH - no demonstrated benefit of thrombolytics over anti-coags (clot resolution, recurrence, mortalitly, complications). - higher risk of bleeding than anti-coags (22% vs 8 heparin) - E.g. Alteplase, given by peripheral IV over 2hrs, improves RV function in 40% vs 17% heparin alone |
|
|
Prototype anti-coagulant, anti-platelet,and thrombolytic drugs
|
Anti-coagulants:
- Warfarin: oral, inhibits vitamin K which is necessary for synthesis of pro-coagulation factors - Heparin (unfractionated, LMW, synthetic): parenteral, inhibits active thrombin and factor XA as a cofactor for antithombin-3 Direct thrombin inhibitors: Lepirudin & Bivalirudin (IV polypeptides), Argatroban (IV, sm. molecule), Dabigatran (oral) Antiplatelet drugs: - aspirin: inhibits thromboxane synthase - clopidogrel "plavix": oral, irreversible ADP receptor antagonist on platelets - abciximab: IV, antibody to glycoprotein 3b/3a preventing fibrinogen binding/activation Thrombolytics: - streptokinase: binds plasminogen to form "proactivator complex" that autocatalytically produces plasmin - Altepalse: recombinant human plasminogen activator (clot specific) |
|
|
Warfarin: mechanism, indications, delivery
|
Mechanism:
- in vivo inhibition of vitamin K epoxide reductase, which limits vit-k dependent synthesis (carboxylation) of factors 2,7,9,10 in the liver Use: thrombus prophylaxis, reduction (long term) Indications: DVT, cerebral vascular thrombosis, A. fib, rheumatic heart disease, valvular heart disease, MI, unstable angina. Contraindicated by pregnancy Adminstration: oral, long lag time before onset of action and prolonged activation after termination, narrow therapeutic window - Effects monitored with prothrombin time, INR 2-3 min - Adverse effects: excessive bleeding (treat OD w/ vit K or plasma transfusion), drug interactions (P450 inhibitors, estrogen), possible rebound effect after termination |
|
|
Heparin: mechanism, indications, delivery
|
Mechanism: acts as a cofactor for antithrombin-3 (protease inhibitor), complex then inhibits active thrombin and Factor Xa
Formulations: - unfractionated: neg. charged animal polysaccharide, requires monitoring w/ partial thromboplastin time (PTT), unpredictable due to plasma protein binding - LMWH: Factor Xa specific, better bioavailability, more predictable active, lower bleed risk, no monitoring - Synthetic: high factor Xa specificity, longer half life (less frequent dosing), fewer adverse effects than UFH Uses: immediate thrombus prophylaxis, breakdown, can be used w/ thrombolytics, fetal safe, in vivo/vitro Indications: DVT, PE, MI, surgical prophylaxis (w/ anti-platelets), maintain patency of intravascular cannulas Adverse effects: excessive bleeding (treat OD w/ protamine sulfate--+ charge binds it), heparin induced thrombocytopenia (HIT) |
|
|
Direct thrombin inhibitors: mechanism, indications, delivery
|
Mechanism: poly-peptides or small molecules directly bind thrombin preventing fibrinogen cleave into fibrin
Indications: patients with heparin induced thrombocytopenia, thromboemolism prophylaxis, Afib - IV and oral preparations |
|
|
Antiplatelet drugs: mechanisms, indications, side effects
|
Aspirin:
- irrreversible COX inhibitor resulting in decreased thromboxane production, inhibits platelet aggregation - delivery: oral, use low dose to inhibit platelets but not endothelial cells (so don't inhibit prostacyclin which inhibits platelet aggregation) - Adverse effects: GI bleeds Abciximab - mechanism: monoclonal antibody to glycoprotein 2b/3a (platelet receptor for fibrin) prevents fibrinogen binding so inhibits platelet activation - IV administration, immediate activity - Adverse effects: possible allergic rxn Clopidegrel: - mechanism: irreversible antagonist of ADP receptors blocking glycoprotein 2b/3a activation, inhibiting platelet activation - oral administration - Adverse effects: thrombotic thrombocytopenic purpura (TTP: blood clots is small vessels, low platelets) |
|
|
Thrombolytic drugs: mechanism, indications, adminstration
|
General: indicated in EM for clot lysis: MI, extensive DVT, acute/massive PE, acute ischmeic stroke, throbotic occlusion, intravascular cannulas/catheters
- Adverse Effects: Bleeds (treat with aminocaproic acid--binds plasminogen and plasmin to suppress fibrin degradation) Streptokinase: (inexpensive) - mechanism: non-enzymatic plasminogen activator promoting auto-catalysis to plasmin which cleaves fibrin - adverse effects: systemic proteolysis of fibrinogen, elicits anti-bacterial antibodies Alteplase: (expensive, short half-life) - mechanism: recombinant human tissue plasminogen activator, clot specific (kinetically active when bound to fibrin, concentrates in clot region) |
|
|
Central Sleep apnea
|
= cessation of airflow during sleep due to loss of respiratory effort.
Mechanism: - at onset of sleep respiratory drive drops and apneic threshold (low PaCO2 such that medulla holds breath to restore CO2) rapidly increases above the PaCo2. There is then transition period of apnea as the PaCO2 climbs to new level Problems: - Higher CO2 threshold because of chronic hypercapnia (so longer period of apnea)→ hypoxia → arousal - Loop gain (inappropriate response to stimulus: over efficient chemoreceptors, muscles, lungs): body compensates too much → high PaCO2 → hyperventilation → arousal - Delayed feedback: in heart failure slow blood flow to medulla results in signal lag → prolonged apnea → high PaCO2→ hyperventilation → arousal |
|
|
Cheyne-Stokes Respiration
|
= constant cycle of hyper- and hypoventilation due to loop gain or delayed feedback mechanisms of apnea
- Result is cyclical periods of waxing and waning respiraton - often due to underlying heart failure causing delayed feedback |
|
|
Obstructive sleep apnea
|
Pathogenesis:
- abnormal pharyngeal collapsibility (retropalatal or retrolingual) due to pharyngeal narrowing and increased respiratory resistance. During inspiration negative pressure closes upper airways (muscles relaxed during sleep so not kept open) Risk factors: - obesity, age>50, male gender, large neck (>17m, >16w), overbite, short mandible, thick tongue (mouth tumors, amyloidosis, trisomy 21, acromegaly), hypertrophied lymph in pharynx, nasal packing or trauma, URI Signs/symptoms: "Stop, Bang": snoring, somnolence, observed apneas, pressure (hypertension), presence of risk factors Consequences: - decreased pleural pressure, pulling walls of the heart out causing increased cardiac strain, potential LHF - chronic hypoxia and hypercapnia are associated with spontaneous nocturnal death - increased risk for PAH, cor pulmonale due to vasoconstriction - continual arousal can cause cerebral dysfunction, excessive daytime somnolence, intellectual deterioration, behavioral disorders |
|
|
Treatment of obstructive vs central sleep apnea
|
Obstructive:
- Nasal Continuous Positive Airway Pressure (CPAP): basically stents hypopharynx and prevents collapse due to negative pressure. Best treatment but 30% non-adherence - Surgery: removal of upper airway to create space: uvular, palatal pharyngoplasty (UPPP), painful and may not work - mandibular enhancement devices: hold tongue down, advance lower jaw Central: - Medications: direct respiratory stimulants, meds that alkalinize urine inducing metabolic acidosis - treat underlying disorder: CHF, afterload reduction - CPAP - ? Inspired CO2 for loop gain problems (prevents CO2 from getting too low) |
|
|
Pickwickian syndrome/Obesity-hypoventilation
|
= clinical syndrome in which morbidly obese people are unable to breathe deeply or quickly enough, resulting in low oxygen saturation, due to chronic sleep apnea
- Presentation is morbidly obesity, alveolar hypoventilation, hypersomnolence during the day. Hard to reverse, usually progress to chronic hypoxemia, cynaosis, eventually cor pulmonale. |
|
|
Fluoroquinolones: mechanism, toxicities, coverage
|
Mechanism: inhibit DNA replication by inhibiting topoisomerase II and IV which uncoil and recoil DNA.
Toxicities: GI upset, CNS (dizziness, headache, confusion/agitation, seizures), rash/itching, hypersensitivity, photosensitivity, disruption of bone/joint/cartilage development, tendon rupture, heptotoxicity, dysglycemia Spectrum: - Cipro/Levo: enterococci, neisseria, H. flu, M. catarrhalis, pseudomonas (decreasing), atypical pneumona (C. pneumo, mycoplasma, legionella), salmonella, shigella, Y. pestes - Levo only "respiratory fluoroquinolone. 3rd gen": strep pneumo, anerobes, mycobacteria |
|
|
Macrolides: mechanism, toxicities, coverage
|
E.g: erythromycin, clarithromycin, azithromycin
Mechanism: inhibit bacterial protein synthesis by binding 50S ribosome and dislocating tRNA during elongation Toxicities: phlebitis, pain at infusion site (avoid with central IV), GI upset (cramps, nausea, emesis, diarrhea due to increase motility), during pregnancy chronic cholestatic hepatitis, associated w/ transient sensorineural hearing loss and polyventricular tachycardia Spectrum: - gram + (s. pyogenes, s. pneumo, s. aureus, corynebacterium diptheria), gram - (legionella, B. pertussis, Camypylobater jejuni, B. henselae, H. ducreyi, M. catarrhalis), mycoplasma, chlamydia Indications: pneumonia (typical, atypical), skin and soft tissue infections, CAP, pertussis, diphtheria, chlamydia, patients w/ beta-lactam allergy, MAC prophylaxis w/ azithromycin in AIDS |
|
|
Tetracyclic derivatives: mechanism, toxicities, coverage
|
E.g. doxycycline, minocycline
Mechanism: bind 30S ribosome preventing binding od tRNA during elongation Toxicities: GI upset (nausea, vomiting, diarrhea, esophagitis), teratogenic (fetal teeth and bone deformity, discoloration), photosensitivity Spectrum: anaerobes, mycoplasma, chlamydia, rickettsiae Indications: STDs (chlamydia), tick-bourne diseases (Rickettsiae, Rocky mountain spotted fever, Lyme disease), Zoonoses, vibrio cholera, CAP |
|
|
Direct vs indirect acting parasympathomimetics
|
- both mimic the action of the parasympathetic nervous system "rest and digest"
- direct: activating mucarinic (cholinergic) receptors to increase tone at the effector junction - indirect: irreversible or reversible inhibitors of AChase, so proling ACh at neural junction and potentiate the effects of normally releaseed ACh |
|
|
Direct acting cholinergic agonists
|
Parasympathomimetics:
Synthetic: Bethanecol (Urecholine) - Mechanism: activates muscarinic receptors to increase smooth muscle tone - indications: urinary bladder atony - contraindications: intestinal or bladder obstruction, prostatic hyperplasia: can generate enough SM force to rupture the bladder Naturally occurring: Pilocarpine (Isopto Carpine, Ocusert) - mechanism: activates muscarinic receptors. In the eye this facilitates contriction of the iris sphincter and ciliary muscle (widens angle of eye) to promote drainage of the aqueous humor Indications: glaucoma |
|
|
Indirect acting cholinomimetics
|
Reversible: Physostigmine (Antilirium), Neostigmine (Prostigmin)
Mechanism: targets active site of AChE (to prevent ACh degradation and prolong signaling) and either competitively binds (Edrophonium) or undergoes reversible carbamylation (spontaneously degrades covalent bond) Indications: glaucoma, antidote for OD of anti-muscarinics (causing muscle paralysis), myasthenia gravis (diagnosis and treatment) Irreversible: organophosphates mechanism: serves as AChE substrate and irreversibly binds (enzyme cleavage leaves serine residue in binding pocket), very lipophilic (can cross all barriers) Indications: insecticides (accidental poisoning), glaucoma, military nerve gas Overdose: too much parasympathetic action SLUDGE (salivation, lacrimation, urination, diaphoresis, gastrointestinal, emesis). Treated with cholinesterase reactivators (Pralidoxime) reactivate AChE by attacking phosphate bond in the binding pocket |
|
|
Effect of parasympathetic simulation on target organs
|
Veins, arterioles: dilation
Heart: decreased HR (@ SA node) & force Bronchi: contraction Iris: radial (none), sphincter contraction Cilary muscle: contraction GI tract: contaction Urinary sphincter: relaxation (trigone), increase urge to urinate salivary glands: increase secretion **indirect acting cholinergic agonists will stimulate para and sympathetic b/c both use ACh, but para is dominant because it occurs twice (ganglion and end effector organ) vs sympathetic which is only pre-ganglionic junctions |
|
|
Cholinergic Antagonists
|
- inhibit parasympathetic activity by competitively binding muscarinic receptors and preventing ACh signaling
- little effect on ganglion and skeletal muscle cholinergic receptors - structurally similar to ACh but rigid (fused rings) so cannot conformational changes to activate receptors once in the binding pocket - effectively have sympathetic effects because removing opposing parasympathetic impulse |
|
|
Cholinergic antagonist drugs
|
Atropine:
- indications: acute MI w/ sinus bradycardia (blocks SA nodes, increasing HR which prevents ventricular pacemaking), slit-lamp eye exam (block contraction of iris sphinter and clilary muscles→dilation and paralysis of accomodation), organophosphate/AChE/parasympathetic poisoning (antagonizes high level ACh) - Side effect: decreased bladder motor activity (problem in prostatic hypertrophy), decreased exocrine glad secretions (dry eyes, mouth, skin), decreased GI motility (constipation) Ipratropium: Indications: asthma, COPD (inhibits vagal bronchoconstriction signaling). Not as effective as Beta 2 agonists Scopolamine: - indications: motion sickness prophylaxis (crosses CNS readily) - side effects: at high dose can cause hallucinations, disorientation, coma, death |
|
|
Differential diagnosis for cavitary lesions
|
C: cancer/neoplasm (squamous cell carcinoma, metastasis)
A: autoimmune/vasculitis V: vascular (septic emboli) I: infection (mycobaterial, fungal, other) T: trauma Y: young (bronchogenic cyst) |
|
|
Mechanisms for pleural effusion
|
A change in the starling forces:
- increasing hydrostatic pressure (as in volume overload in CHF) causing extra fluid flow into pleural space (normally already is) - decreasing oncotic pressure (as in low protein states like cirrhosis & nephritic syndrome) causing decreased fluid retention in circulation Increase in permiability - anything that disrupts the capillary membrane: pneumonia/infection, malignancy, serositis Decreased lymphatic clearance (ex. malignancy blocking channels) Impaired lung re-expansion: decrease in pleural pressure (atalectasis, trapped lung) **95% of pleural effusions are due to CHF, malignancy, infection, PE, post-surgical (thoracotomy, sternotomy) |
|
|
Clinical presentation of pulmonary effusion
|
Symptoms:
- dyspnea: increase pressure causing inefficiency of respiratory muscles (diaphragm) - cough: fluid distorts the lung - pleuritic chest pain (worsens w/ respiratory effort): due to stimulation of somatic nerves in parietal pleura Physical exam: decreased/absent breath sounds and dullness over effused area (usually in base of lung, occasionally focal/lobular) PFTS: - slightly reduced lung volumes due to fluid occupying space - minimal effect on gas exchange due to changes to maintain V/Q match - reduced compliance of respiratory system (may increase respiratory effort) Radiographic: - blunting of costophrenic angle if >300ml Fluid - U shaped meniscus sign (large effusions, loose borders of adjacent structures) - free flowing liquid settles in the bases |
|
|
Clinical presentation of pneumothorax
|
Symptoms:
- dyspnea: due to pressure and tension on respiratory muscles - pleuritic chest pain: to somatic nerves on parietal pleura Physical exam: - decreased/absent breath sounds on affected side, HYPER-RESONANCE on percussion, increased expansion of the hemithorax PFTs: - decreased lung volumes (collapsed) - hypoxemia due to lower V/Q ratio Radiography - visible thin white line (vertical) = visceral pleura of collapsed lung, also not the absence of lung markings lateral to line. - expansion of the chest wall on the side involved - mediastinal/tracheal shift to contralateral side in tension, or ipsilateral side otherwise (mores space) |
|
|
Determining transudative vs. exudative pleural effusions
|
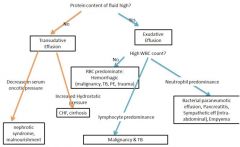
First: thoracentesis (unless you know why fluid is there, take it out. Especially: unilateral, bilateral but unequal, febrile, evidence of pleurisy)
Analyze fluid with: total protein, LDH (lactate), glucose, cell count and differential, gram stain and culture, cytology, pH Transudative effusion: alteration of systemic factors that regulate pleural fluid, local capillary membrane is intact Exudative effusions: occur when local lung disease or associated lung structures allows fluid accumulation. Ex: capillary damage, lymph obstruction. Capillary barrier function is disrupted so high protein content. |
|
|
Light Criteria for pleural effusion
|
95% sensitivity for detecting exudative effusions (exudative effusions meet 1+ criteria, tansudative meet none):
- pleural fluid/serum total protein ratio >0.5 - Pleural fluid/serum LDH ratio >0.6 - pleural fluid LDH >2/3 upper limit |
|
|
Different types of exudative pleural effusion
|
Paraneumonic effusions: complication of pneumonia, usually culture negative. 3 stages: exudative (inflammation/capillary leak), fibrinopurulent (loculations of fluid), organized (scaring/pleural rind: inelastic membrane on lung surface, restrictive). Progressive stages require more aggressive treatment: abx only, tube thoracostomy, pleural peel/surgical decortication
Empyema = pleural infection: gross pus removed on throracentesis or organisms on gram stain or cultures. WBC typically >100k, glucose <60 Malignant: hematogenous metastases, cancer erosion through lung, occlusion of lymph, cytology usually positive (60-80%), typically reccurs (advanced cancer), requires pleurodesis Hemothorax: pleural fluid >50% serum hematocrit, usually malignancy, TB, PE, trauma Chylothorax: (not exactly exudative) turbid or chalky white pleural effusion with high lipid content due to non/traumatic disruption of the thoracic duct |
|
|
Mechanisms of pneumothorax/air entrance into the pleural space
|
Spontaneous (w/o preceding trauma/cause): in patient without (primary spontaneous) or with lung disease (secondary spontaneous) rupture of cytic lung structures (blebs) allows air leak
Traumatic Event: penetrating trauma (stab, bullet), iatrogenic (unintended consequence of diagnostic/therapeutic procedure) Tension (medical emergency--decompress): inspired air in the pleural space (from either above method) is trapped (can't get into lung or outside on expiration) creating high pressure and collapsing lung and circulatory system |
|
|
Management of pneumothorax
|
Small pneumothorax: (<30% of hemithorax)
- in the absence of underlying disease: observe, x-ray and provide supportive care including supplemental O2 to displace the N2 in pneumothorax (from atmospheric air) Large pneumothorax: place chest tube w/ one-way valve (Heimlich) Repeated pneumothorax: seal off blebs via pleuodesis (artificially obliterate the pleural space w/ sterile talc powder--iritates, fibroses together) Tension pneumothorax: use large gauge needle (18+) in 2nd intercostal space to decompress emergently, replace with chest tube. |
|
|
Clinical manifestations of lung cancer
|
Small Cell symptoms: persistent cough, hemotypsis, swelling of face/neck, SOB, wheezing, repeated pneumonia or bronchitis, hoarsness, fatigue, weight loss
NSCLC: cough, dyspnea, chest pain, hemotypsis, pneumonitis, weight loss, generalized weakness, anorexia, fever, anemia Presentation based on location of disease: - dysphagia due to esophageal obstruction from nodal involvement/mediastinal invasion - hoarsness, diaphragm dysfunction: encasement of nerves at mediastinum/hilar lymph - Horner's syndrome (ptosis, myosis, anhydrosis): apex tumors (Pancoast) causing sympathetic nerve paralysis - nerve root compression: invasion of the spine from centralapex tumors - Pancoast syndrome (neuritic arm pain, arm/hand muscle atrophy): apex tumors paralyze the brachial plexus - pleural effusion: obstruction of lymph - superior vena cava syndrome (periorbital/facial edema, headache): mediastinal tumor occludes superior vena cava, may cause new vessel formation in anterior chest wall - Pericardial effusion (severe SOB, cardiac tamponade): from pericardial/cardial involvement |
|
|
Paraneoplastic syndromes in lung cancer
|
= symptoms not resulting directly from the tumor. Occur more frequently in lung than any other tumor.
NSCLC: - squamous cell carcinoma frequently associated with hypercalcemia - NSCLC associated w/ various skeletal/connective tissue syndromes Small Cell LC (notorious for paraneoplastic syndromes) - inappropriate secretion of ADH causing low sodium - Ectopic ATCH secretion causing Cushing's syndrome - Neurologic/myopathic syndromes, most commonly Lambert-Eaton myasthenic syndrome (autoimmune reaction forming antibodies to presynaptic voltage-gated channels at neuromuscular junction) - Hypertrophic osteoarthropathy: new bone formation outside diaphyses of long bonds w/o coritcal destruction. Causes stiffness, warm/firm leg swelling - Digital clubbing |
|
|
Lung cancer diagnosis/workup
|
H&P: lymphadenopathy, hepatosplenomegaly, hemotypsis, other respiratory symptoms
Radiology: CT (or CXR), PET scan, bone scan (for bone metastases), endobronchial ultrasounds (lymph involvement), brain MRI, bone marrow aspiration (rarely done anymore) Biopsy: bronchoscopy, needle biopse, thoratoromy Prognosis depends on stage at diagnosis, performance status, smoking status |
|
|
Lung cancer staging and treatment options
|
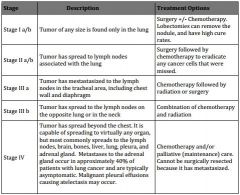
|
|
|
Treatment strategies for lung cancer
|
NSCLC: treatment depends on whether it is localized or metastatic
- surgery: mediastinoscopies (lymph >1cm), video assisted thorascopy (lymph, tumor excision), thoracotomy (pulm resections) - radiation: external beam, brachytherapy. For tumors too large to be removed, inadequate pulm function for resection - Chemo: (most present at stage IV, so strategy is to maximize time) cisplatin, carboplatin, new agents, targeted agents (EFGR inhibitors, Alc, Ras, angiogenesis inhibitors) SCLC: more aggressive but more responsive, by definition systemic - limited stage (one lung, mediastinum and ipsa/contralateral supraclavicular lymph nodes or disease encompased by 1 radiotherapy port). Limited to the chest, treat w/ chemo (cisplatin or carboplatin), concomitant radiation, prophylactic cranial irradiation - Extensive stage: spread beyond thoracic cavity (often to adrenals, bone, liver, bone marrow, brain, contralateral lung and nodes). Not curable, extend life with chemo, palliative radiation |
|
|
Rationale for lung cancer screening
|
- early detection helps increase survival rates, helps stage disease
- 4 Stages: 1) Stage I i. Confined to lungs ii. 5 year survival = 50% 2) Stage II i. Spread to nearby lymph nodes ii. 5 year survival = 30% 3) Stage III i. Spread to distant nodes or nearby organs ii. 5 year survival = 5-15% 4) Stage IV i. Spread to distant sites ii. 5 year survival < 2% |
|
|
10 criteria for effective screenign
|
1. serious disease
2. high prevalence of detectable preclinical disease 3. screen detects little pseudodisease 4. high accuracy (sensitivity/specificity) for detectable preclinical phase 5. tests detect disease before critical point (before metastasis/in-curable) 6. screening test causes little morbidity 7. test is affordable and available 8. treatment exists 9. treatment is more effective before symptoms start 10. treatment is not too risky or toxic |
|
|
lead-time bias
|
bias intrinsic to using metric of "survival years after diagnosis." Early detection will improve this metric but not necessarily change natural course of disese
|
|
|
length-time bias
|
- bias of rate of disease progression vs. screening intervals
- screening is more likely to detect indolent (slow-progressing) disease and missing rapidly-progressing/severe disease - so not-necessarily catching the most severe disease |
|
|
over-diagnosis bias
|
- bias due to the presence of pseudodisease
- screening labels more disease than is actually active, resulting in an increased survival rate in the screened population |
|
|
stage migration bias
|
- more accurate staging results in better classification of patients so that the relative standards for their class of disease are different:
- i.e. healthier patients w/ stage IV disease are correctly stage and so bring up stage IV stats (worse expectations) and no longer bring down stage III stats |
|
|
International Early Lung Cancer Action Project
|
i. Actually funded by a tobacco company…
ii. Not randomized – all patients were screened w/ CT iii. Outcome measure is cancer diagnosis/stage iv. Diagnosed 484 patients with lung cancer, and 412 patients as Stage I this study 1. Stage I patients – 88% 10-year survival rate Biases: lead-time, length-time, over-diagnosis, stage-migration, conflict of interest |
|
|
National Lung Screening Trial
|
i. Randomized – half were screened w/ CT, another half w/ CXR for 3 years
ii. Outcome measure is lung cancer mortality iii. 247 deaths/100,000 person-years w/ CT screening vs. 309 deaths/100,000 person-years w/ CXR screening 1. CT screening decreased mortality by 20% as compared w/ CXR screening. Still not clear if it is cost effective though |
|
|
Cost effectiveness
|
= comparison of 2 or more healthcare conventions, usually cost and effect (quality of life * years)
dominance refers to how cost effective an outcome is Dominance: maximizes both cost and effect non-dominance: maximizes only cost or effect |
|
|
Squamous cell carcnioma
|
predisposing: smoking
incidence: 2 most common type gross: centrally located, polypoid, involve larger bronchi (often grow along it causing obstruction/atalectasis and severe bleeds), can cause cavitation, gritty white appearance micro: keratin pearls, intracellular bridges (desmosomes), fibrous stroma (light purple) paraneoplastic: parathyroid like activity |
|
|
Adenocarcinoma
|
predisposing: often in non-smokers/women, also smokers, bronchioalveolar subtype is not linked to smoking (better survival, insitu, EGFR linked)
incidence: most common type gross: peripheral and subpleural (not associated w/ bronchi except for broncioloalveolar), solitary or multiple masses, mucoid (shiny), hard, invade pleura micro: glands, mucin containing cells (PAS stain) paraneoplastic: hypercalcemia, BAC can cause hypertrophic osteoarthropathy |
|
|
large cell carcinoma
|
predisposing: smoking, environmental exposures
incidence: usually poor prognosis gross: peripheral, large, necrotic/cavitating micro: large pleomorphic cells with leukocyte fragments in the cytoplasm, lack characteristics of other NSCLC |
|
|
small cell carcinoma
|
predisposing: smoking (strong), poorest survival (usually present with extensive, inoperable--metastatic by definition)
gross: central, mucoid micro: smal blue cells w/ salt& pepper chromatin, crush artifact paraneoplastic: ectopic ACTH/ADH (Cushing,s SIADH), superior vena cava syndrome, Pancoast syndrome, Horner's syndrome, Lambert-Eaton syndrome |
|
|
carcinoid tumor
|
= neuroendocrine tumor that is frequently benign
- central or peripheral - high 5 year survival rate - gross: polypoid lesion that may invade the airway, filling the lumen - micro: medium cells w/ lots of cytoplasm, no mitotic figures or necrosis - highly vascular--should NOT be biopsied, risk of bleeding - paraneoplastic: secrete serotonin (flushing, diarrhea, salivation, wheezing) |
|
|
mesothelioma
|
= pleura tumor of mesothelial cells, associated with asbestos exposure, very malignant (<10% @ 5yrs)
- results in hemorrhagic pleural effusions and pleural thickening (pleural plaques are benign but indicative of asbestos exposure) - cancer appears as a thick rind on gross examination, invades and restricts the lung. Visible on CXR |

