![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
What is the monomer for proteins? How does the structure of the monomer look like? |
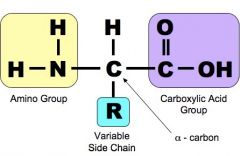
Ans : Amino acid |
|
|
What are the 4 levels of structure of a globular protein? |
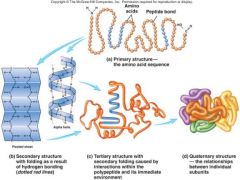
Ans : Primary, Secondary, Tertiary and Quaternary Structure |
|
|
Describe the primary Structure of Proteins. |
- Primary structure of proteins refers to the number, sequence and variety of amino acids in a protein. - Structure is linear - Type of bonds found within this structure is peptide bond |
|
|
Briefly Describe the Secondary Structure of Proteins.
|
-Repeatedcoiling or foldingof polypeptide chains into regularpatterns that stabilize protein structure.
- Stabilized by hydrogen bondingbetween amino acid residues. - Two types of secondary structure -->α-helix and β-Pleated Sheet
|
|
|
Describe the Tertiary Structure of Proteins.
|
> Complex and precise threedimensional structure formed from the extensivebending and further folding of the secondary structure/polypeptide
> Stabilized by interactions between the R groupsof the amino acid residues. > Hydrogenbonds, Hydrophobicinteractions, Ionicbonds, and Disulfide linkage (H2ID) are form between the R groups to stabilize the structure. |
|
|
Describe the Quaternary Structure of Proteins.
|
>Refers to the interaction between two or more polypeptide chainstoform an intact functional protein.
> Hydrogen bonds, Hydrophobic interactions, Ionic bonds, and Disulfide linkage (H2ID) |
|
|
Explain the following terms
(i) Zwitterion (ii) Isoelectric point |
(i) Zwitteriondipolar ions (i.e. ions having botha positive and a negative charge).
(ii) Isoelectric pointpH at which a particular amino acidis neutral and mainly found in the zwitterionic state. |
|
|
Give one example of Globular Protein and Fibrous Protein with Quaternary structure.
|
Ans :
>Haemoglobin (example of a globular protein) > Collagen (example of a fibrous protein) |
|
|
Relate the structure of Haemoglobin to its function. |
1. Globular & compact shape --> Allows many molecules to be packed in a red blood cell.
2. Made of 4 subunits. Each subunit binds one molecule of oxygen--> Increases capacity for transport of oxygen. 3. Each subunit is globular, with a hydrophobic core and a hydrophilic exterior --> Soluble in an aqueous medium such as cytoplasm of Red Blood Cell. 4. Each subunit possesses a deep hydrophobic cleft -->Provides a binding site for haem group. 5. Haem group contains a porphyrin ring bound to an iron ion --> Allows reversible binding of oxygen, enhancing release in metabolically active tissues. |
|
|
Relate the structure of Collagen to its function.
|
1. Large size, large number of neutral, non-polar amino acids (glycine, proline, hydroxyproline & Hydroxyllysine ) -> Insoluble in water ->perform a supportive role in plants.
2. Extensive hydrogen bonding between polypeptide chains to form tropocollagen and further cross-linkage ( aldo linkages) to form collagen fibers ->Provides great tensile strength |
|
|
Whatare the structural features of an α-helix?
|
α-helix
> Right-handed helix. > Stabilized by intramolecular hydrogen bonding between C=O and N-H group in peptide bonds. >α-helix makes a complete turn every 3.6 amino acid residues. |
|
|
What are the structural features of aβ-pleated sheet?
|
β-Pleated Sheet
> Polypeptide chain folds back and forth or lies parallel to form sheets. > Stabilized by hydrogen bonding between C=O and N-H groups from peptide bonds in the adjacent chains. >“Pleated” because α-carbon in the amino acid residues lies alternately above and below the plane of the sheet. |

