![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
93 Cards in this Set
- Front
- Back
|
What are the essential amino acids that must be acquired through diet?
|
Histidine
Isoleucine Leucine Lysine Methionine Phenylalanine Threonine Tryphtophan Valine |
|
|
What is the primary proteolytic enzyme of the stomach?
|
pepsin
maximally active at pH 2 denatures proteins to random coils easier for proteolysis |
|
|
Once in the intestinal lumen, where do the other enzymes come from?
|
pancrease.
pancrease sends them as inactive zymogens that become active and very specific |
|
|
what is aminopeptidase N?
|
resides in plasma membrane of intestinal cells
digests proteins from amino end and makes di and tri peptides which are released into the blood to go to other tissues. |
|
|
The identity of what feature determines the half lives of proteins?
|
amino-terminal residues
There are highly stabilizing ends (ala, cys, gly, met, pro, ser, thr, val) destabilizing (arg, his, Ile, leu, lys, phe, trp, tyr) |
|
|
What is Ubiquitin?
|
a small protein present in all eukaryotic cells that signals proteins for destruction.
|
|
|
What are two highly stabilizing amino terminyl residues?
|
Ala
Pro |
|
|
What are two destabilizing amino terminal residues?
|
Arg
Lys |
|
|
Where is the Ub attached to proteins?
|
has an isopeptide bond between the carboxy terminus of Gly of Ub and a Lys E-amino group on the target protein
|
|
|
What is E1?
|
Ub-activating enzyme
adenylates Ub and is transferred to sulfahydryl thioester bond on E-1 |
|
|
What is E2?
|
E2 is Ub conjugating enzyme
gets Ub from E1 and forms thioester bond |
|
|
What is E3?
|
Ub protein ligase
catalyzes the transfer of Ub to E-amino group on the target protein Only E3 recognizes the target protein |
|
|
What Lys residue does Ub use as catalytic site?
|
Lys 48
|
|
|
How many poly Ub make for a strong signal?
|
4 or more
|
|
|
What determines whether a protein will be Ub'd?
|
N-terminal residue (destabilizing Arg, Lys, Leu) make it much more of a target
(stabalizing like Met, Ala, Pro make it last longer and avoid being Ub'd) |
|
|
What is the proteasome (26A proteasome)?
|
the executioner
has 2 components: --20S catalytic unit --19S regulatory unit |
|
|
Describe the 20S catalytic subunit of proteasome.
|
barrel structure--sealed
(Think of the 2 19S subunits as bouncers to Tao) contains the catalytic sites (3) |
|
|
What do the 19 S regulatory subunits of 26S proteosome bind to specifically?
|
poly Ub'd proteins only
|
|
|
Describe how the 3 catalytic sites in the 20S subunit work.
|
hydroxyl group of a Thr residue becomes a Nu and attacks the carbonyl groups of peptide bonds
|
|
|
Describe how the 19S 'bouncers' regulate protease activity
|
bind only to Ub'd proteins
AAA class ATPases unfold the substrate Ub'd protein and cause conformational changes in 20S subunit. and then an isopeptidase cleaves off the Ub conserving it for recycling. |
|
|
How are carbon skeletons that will be used for TCA cycle derived from free amino acids?
|
NH2 group is removed for urea production
|
|
|
Describe how the inflammatory response is an example of how protein degradation regulates biological functions.
|
Some proteins are regulatory proteins. When those cells get degraded, the targets of those proteins are effected.
So picture NFkB:1-kB. In the cytoplasm it is inactive because it has an inhibitory protein bound to it. When that protein (1-kB) gets degraded, suddenly the leftover NF-kB is free to initiate pro-inflammatory gene expression in the nucleus |
|
|
How does Bortezomib (Velcade) work?
|
proteasomal inhibitor.
inhibits the degradation of tumor suppressors. |
|
|
Where is the major site of amino acid degradation?
|
liver
|
|
|
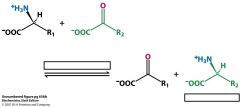
Describe what happens in a.a. degradation in the liver.
|
a-amino group transferred to an a-ketoglutarate (aminotransferase) to make glutamate and then it is oxidatively deaminated (glutamate dehydrogenase) to free NH4+
|
|
|
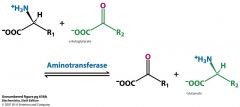
What are two important aminotransferases?
|
Asp aminotransferase
Asp + aKG<-->OAA + Glu Ala aminotransferase Ala + aKG<--> pyruvate + Glu |
|
|
What does glutamate dehydrogenase do?
|
oxidative deamination
dehydrogenation of C-N bond and hydrolysis of Schiff base Can use NAD+ or NADP+ |
|
|
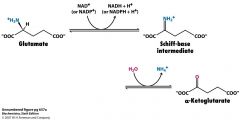
What accessory do aminotransferases contain that facilitate deamination?
|
PLP
forms the covalent Schiff base linkage with the Lys residue on amino acids |
|
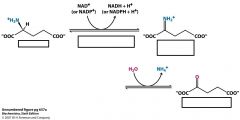
Name the substrates and the enzymes
|
Enzyme is glutamate dehydrogenase
|
|
Name the substrates and the enzyme
|
|
|
|
Which two amino acids permit direct deamination?
What are the enzymes called? |
Ser and Thr
Ser and Thr dehydratases (because they are dehydrated) |
|
|
Why are Ser and Thr allowed to be directly deaminated without going through the a-ketoglutarate route?
|
B-carbon hydroxyl in both of these amino acids allows for the dehydration and subsequent deamination.
|
|
|
Can muscle tissue use amino acids as a fuel source?
|
Yes, branched amino acids can be used during prolonged exercise and fasting
|
|
|
What happens to the Nitrogen released when muscles use branched amino acids for fuel?
|
Transported to the liver as pyruvate (Ala cycle puts nitrogen on pyruvate to get Ala which goes in blood to liver to be made back into pyruvate again when liver deaminates it)
Glutamine synthetase makes glutamine from glutamate then it's sent to liver to be deaminated there into urea. |
|
|
Where does the 2 nitrogen atoms come from in the Urea Cycle?
Where does the carbon come from? |
One N is from Asp and the other is from free NH4+
Carbon is from HCO3- |
|
|
Where does the Urea cycle take place?
|
mitochondrial matrix
|
|
|
What is the enzyme associated with the formation of carbamoyl phosphate in Urea cycle?
|
carbamoyl phosphate synthetase (CPS)
|
|
|
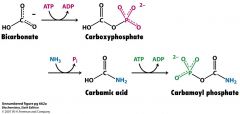
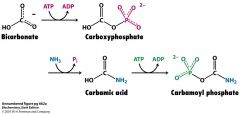
What are the 3 steps in making carbomyl phosphate?
|
HCO3- + ATP--> carboxyPi
carboxyPi + NH3--> carbamic acid carbamic acid + ATP --> carbamoyl Pi |
|
|
How many ATP are used to couple NH3 to HCO3-?
What else is needed for this reaction to go forward? |
2 ATP
N-acetylglutamate |
|
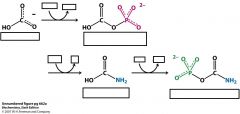
What is the enzyme that catalyzes these three reactions and what are the cofacters and substrates?
|
Carbamoyl Phosphate Synthetase
|
|
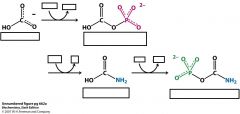
What is the enzyme that catalyzes these three reactions and what are the cofacters and substrates?
|
Carbamoyl Phosphate Synthetase
|
|
|
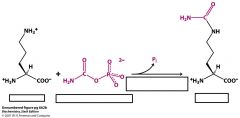
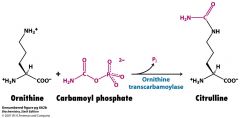
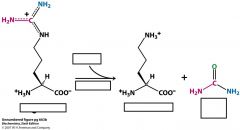
Where does the change from ornithine to citrulline take place?
Where does citrulline condense with aspartate to make argininosuccinate? |
ornithine uses ornithine transcarbamolyase to turn into citrulline in the mitochondrial matrix.
Citrulline condenses with aspartate in the cytoplasm |
|
|
what type of amino acids are ornithine and citrulline?
|
non-protein making amino acids
|
|
|
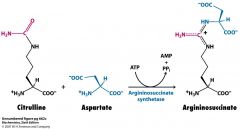
What is argininosuccinate syntsynthetase?
|
argininosuccinate synthetase is the enzyme that catalyzes the reaction from citrulline to argininosuccinate in the cytoplasm.
Needs ATP |
|
Name the substrates and the enzyme
Where does this reaction take place? |
|
|
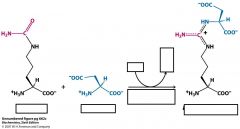
Name the substrates, cofactors and enzyme
|
|
|
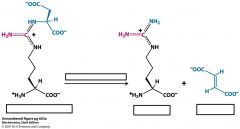
Name substrates and enzyme
|
|
|
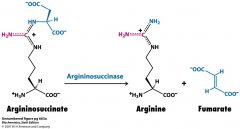
name the substrates, cofactors and enzyme
|
|
|
|
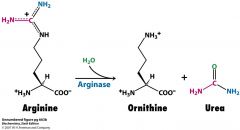
How many ATP are consumed to make one molecule of urea?
|
4 ATP
|
|
|
What is a product of the urea cycle that is important for gluconeogenesis?
|
fumarate
fumarate-->malate-->OAA |
|
|
What are the fates of OAA?
|
transaminated to Asp
gluconeogenesis to glucose condense with acetyl CoA to make citrate |
|
|
What are the 7 fates of deanimated carbon skeletons?
|
pyruvate, acetyl CoA, acetoacetyl CoA, OAA, fumarate, a-ketoglutarate, succinyl CoA
|
|
|
What are the two solely a-ketogenic amino acids?
|
Leu, Lys
|
|
|
What are the 4 a.a.'s that are both keto and gluco-genic
|
Ile, Phe, Trp, Tyr
|
|
|
What reaction does Alanine aminotransferase catalyze?
|
Ala + aKG ----> pyruvate + Glu
|
|
|
What reaction does Ser dehydratase catalyze?
|
Ser---> pyruvate + NH4+
|
|
|
What are the three ways Cys can be turned into pyruvate?
|
H2S, SCN- or SO32-
|
|
|
How does Gly become pyruvate?
|
Add hydroxymethyl group to make Cys
|
|
|
How does Threonine become pyruvate?
|
2-amino-3-ketobutyrate '
|
|
|
How does Trp become pyruvate?
|
3 C's become Ala then pyruvate
|
|
|
How does Asp become OAA?
|
Asp aminotransferase
Asp + aKG---> OAA+ Glu |
|
|
How and what does Asp become to enter metabolic pathway?
|
Asn ----> NH4+ + Asp
via asparaginase In two ways, it either becomes transaminated to OAA or becomes fumarate in Urea Cycle |
|
|
How many carbons funnel into glutamate and a-ketoglutarate?
|
5 carbon amino acids
|
|
|
How is a-ketoglutarate made?
|
glutamate is oxidatively deanimated to a-ketoglutarate by the enzyme glutamate dehydrogenase
|
|
|
How does His become glutamate?
|
His --> 4-imidazolone 5-propionate --> N-formiminoGlu -->Glu
|
|
|
What reaction does glutaminase catalyze?
|
Gln (glutamine) hydrolyzed to glutamine and NH4+
|
|
|
How do Pro and Arg become glutamate?
|
Each are converted to Glu-y-semialdehyde and then get oxidized to glutamate
|
|
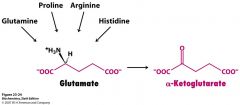
Describe how each one becomes glutamate.
|
Gln-->glutaminase
Pro & Arg---> Glu-y-semialdehyde-->glutamate His---> 4 imid-->N-formimi-->glutamate |
|
|
Name 3 non-polar amino acids and how they enter the metabolic pathway.
|
Met
Ile Val Propionyl CoA--->methylmalonyl CoA--->succinyl CoA |
|
|
Name the 7 step program Met users have to do to get to Succ CoA.
|
adenylation
S-adenosylmethionine (SAM) homocys aKB enzyme complex a-ketoacid dehydrogenase complex propionyl CoA Succinyl CoA |
|
|
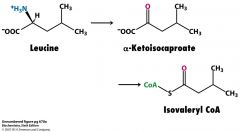
What is the branched chain a-ketoacid dehydrogenase complex a homolog of ?
What reaction does it catalyze? |
E3 from pyruvate dehydrogenase
oxidative decarboxylation of Leu, Val, Ile |
|
|
What is isovaleryl CoA derived from?
what is the enzyme? Who gets the electrons? |
Leu
isovaleryl CoA dehydrogenase FAD--->FADH2 |
|
|
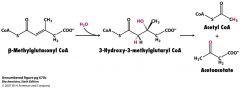
Once Isovaleryl CoA gives up its electrons what does it become?
|
B-methylcrotonyl CoA and then B-methylglutaconyl CoA
|
|
|
How do you get from B-methylglutaconyl CoA to acetyl CoA and acetoacetate?
|
remember ketone bodies? Like that
B-methylglutaconyl CoA + H20--->3-hydroxy-3-methylglutaryl CoA (HMG-CoA--->cleaved into acetyl CoA + acetoacetate |
|
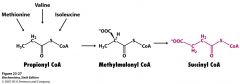
Explain what is happening here.
|
memorize the pieces and parts
|
|
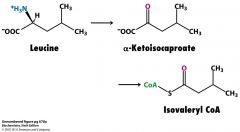
|
Oh god.
|
|
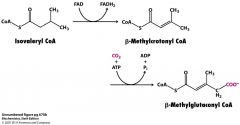
Why do I have to know this???
|
Penance
|
|
shoot me now
|
please
|
|
painful,
|
isn't it?
|
|
|
What is used to break up aromatic rings to degrade the amino acids?
|
O2 and oxygenases
|
|
|
What is the enzyme that degrades Phe?
|
Phe hydroxylase
adds O2 to make Tyr One O goes on Tyr and other is in H20 |
|
|
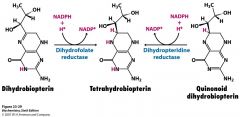
What is tetrahydrobiopterin?
|
electron carrier (deriviative of cofactor biopterin)
|
|
|
What is dihydrofolate reductase?
|
reduces dihydrobiopterin to for tetrahydrobiopterin
|
|
|
Once you have Tyr, what is the enzyme that transaminates it?
|
p-hydroxyphenylphyruvate
|
|
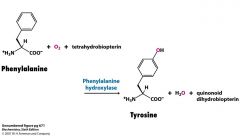
Show where the O's are going
|
One on Tyr and one in water
|
|
Have I mentioned...
|
...how little I like biochem right now?
|
|
|
What does p-hydroxyphenylpyruvate hydroxylase catalyze?
where are the O2's? |
takes you from p-hydroxyphenylpyruvate to homogentisate
Both O's are in the product (ring + carboxyl) |
|
|
What happens when homogentisate oxidase reacts with homogentisate?
|
cleaving of the aromatic ring that has the 2 O's
yields 4 maleylacetoacetate |
|
|
How does one get from 4 maleylacetacetate to get to fumarate and acetoacetate?
|
isomerization of 4-maleylacetoacetate to 4 fumarylacetoacetate and then hydrolysis to fumarate and acetoacetate.
|
|
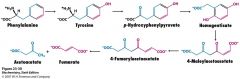
Why do I need to know this???
|
Because. That is the only reason.
|
|
|
What's Trp's story?
|
needs a few oxygenases.
uses Trp 2,3-dioxygenase to cleave the pyrrole ring so you get to N-formlykynurenine needs a dioxygenase |
|
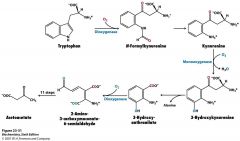
ugh...
|
...squared.
|
|
|
What is phenylketonuria?
|
lack of Phe hydroxylase
20% increase in Phe in blood and body fluids causes severe mental retardation if left untreated decreased brain weight and defective myelination causes unknown. low Phe diet is only treatment beginning before age 1 |

