![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
The Particle Theory of Matter |
1.All matter is made up of tiny particles that have empty spaces between them 2. Different substances are made up of different kinds of particles 3.Particles are in constant random motion 4.The particles of a substance move faster as its temperature rises 5.Particles attract each other |
|
|
Solids |
-are formed when the forces of attraction between particles hod the particles in close contact with each other -rigid,fixed shape,fixed volume |
|
|
Liquids |
-are formed when solids are heated -this will cause the particles to gain energy and begin to move faster -particles start sliding past each other -not rigid, no fixed shape, fixed volume |
|
|
Gases |
-are formed when liquids are heated -the particles gain so much energy that they begin to fly apart -at this point the particles are so far apart that attraction has little impact -not rigid, no fixed shape, no fixed volume, can be quashed |
|
|
Pure Substances |
-substances that are only made up of one type of particle (ex.distilled water) -2 types (Element and Compound) |
|
|
Element |
-any substance that cannot be broken down into any simpler substance (ex. Oxygen) |
|
|
Compund |
-a pure substance that is made from two or more elements that are chemically combined together (ex.Salt) |
|
|
Mixture |
-a substance that is made up of two or more different types of particles -can be solid,liquid,gas ex. table, salt and distilled water, air -2 types (Heterogeneous and Homogeneous) |
|
|
Heterogeneous |
-a mixture with different visible parts ex.computer,chicken noodle soup - 2 types (Mechanical and Suspension) |
|
|
Mechanical |
-mixtures in which the substances are distinguishable from each other ex.milk and cereal in a bowl |
|
|
Suspension |
-cloudy mixtures in which one substance is held within another ex.tomato juice |
|
|
Homogeneoous |
-a mixture that looks as though it is a pure substance but is not ex.salt water compared to distilled water -1 type (Solution) |
|
|
Solution |
-mixtures that look like a pure substance but contain more than one type of particle (you cannot distinguish) |
|
|
Alloy |
- a solid solution of two or more metals ex.bronze is a 93% and 7% tin |
|
|
Order |
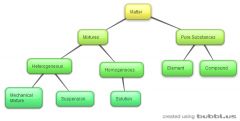
|
|
|
physical change |
-a change in which the appearance changes but the substance doesn't *melting,boiling,breaking, |
|
|
chemical change |
-a change in the starting substance or substances and the production of one or more new substances *the original substances don't disappear but are rearranged to form a new substance or substances *color change,bubbles(not from heating),odor change, formation of a solid (usually powdery:precipitates), change in temperature or light |
|
|
density |
D=M/V M=DxV V=M/D *kg (density is in grams) : x 1000 |

