![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
124 Cards in this Set
- Front
- Back
|
What are the properties of LTP?
|
1. Cooperativity: A crucial number of presynaptic fibers must be simultaneously activated that is they must cooperate to elicit LTP (threshold stimulation strength must be used)
2. Input (synapse) Specificity : When LTP is induced by stimulation at one set synapses, it does not occur in other inactive synapses that contact the same cell. 3. Associativity :Week stimulation will not trigger LTP by itself But if one pathway weekly activated simultaneously when a neighboring pathway onto the same cell is strongly activated, both synaptic pathways undergo LTP. LTP is associative requiring both pre and postsynaptic activity. 4. Persistence:LTP can last hours in an in vitro and months in vivo LTP is often divide into two phases, an early phase that is independent of protein synthesis, and last for one to five hours. E-LTP produces a potentiation by making the postsynaptic side of the synapse more sensitive to glutamate by adding additional AMPARs into the postsynaptic membrane. Conversely. L-LTP, which lasts days to months requires new protein synthesis. These proteins include, glutamate receptors (AMPARs), transcription factors (CREB), immediate early genes like c-fos, and c-jun, and other structural proteins that enhance existing synapses and form new connections. -on the same synapse, can stimulate diff. kinds of LTP |
|
|
What is synaptic plasticity?
|
Synaptic plasticity is the ability of the of synapse to change in strength
The strength of a synapse is defined by the change in transmembrane potential resulting from activation of the postsynaptic receptors (iontropic and mettabotropic). Synaptic plasticity is activity-dependent change in synaptic transmission |
|
|
What r the types and phases of LTP?
|
Types of LTP
NMDA receptor dependent NMDA receptor independent Phases of LTP Initial LTP (STP), ~1hr, NMDAR-dependent and protein synthesis-independent phase. E-LTP, 2-3 hrs, protein synthesis-independent phase, insertion of AMPA receptors into postsynaptic membrane. L-LTP, 2-3 hrs, days to months, synthesis of new proteins, transcription factors, and structural proteins that enhance existing synapses and form new connections. |
|
|
What generates the resting membrane potential?
|
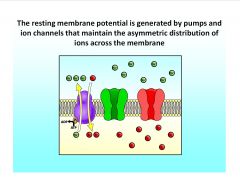
|
|
|
Briefly describe equilibrium potential.
|
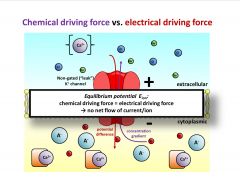
Equilibrium potential Eion:
chemical driving force = electrical driving force → no net flow of current/ion |
|
|
Describe the distribution of major ions across the neuronal membrane.
|
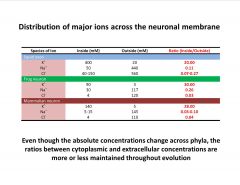
|
|
|
What is the equilibrium potential of K, Na, and Cl?
|
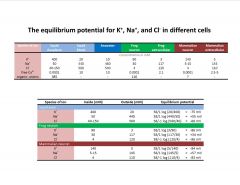
|
|
|
What is the relationship between
the equilibrium potential for a species of ion (Eion) and the resting membrane potential (Vm)? |

Glia cells have very hyperpolarized membrane potentials (-80 to -90 mV in mammals), close to EK (b/c they have very few Na channels).
However, neurons have membrane potential more typical between -60 and -70 mV b/c the contribution of the Na channels pulls the Vm away from Ek. |
|
|
Describe the input resistance.
|
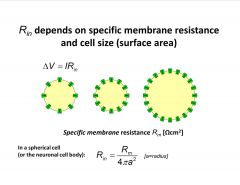
|
|
|
Design a simple experiment to figure out the input resistance of a membrane.
|
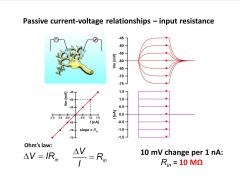
-inject current and see what happens to voltage
-if no voltage-gated ion channels activated, response behaves linearly (described by ohm’s law) -voltage change depends on current and input resistance of cell (change in voltage/current=input resistance slope of fn on bottom left graph) -input resistance=10 mega ohms (typical value for large invert. axon) |
|
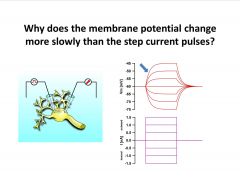
|
|
|
|
Briefly describe axial resistance and some of its determinants.
|
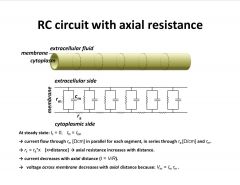
-model of sys. using simple ex.
-every segment has isopotential -current flows through mem resist. and capacitance -axial resistance is the resistance of the cytosol filling the circuit resistance as current flows down the tube (vs. across mem.) -current decreases with dist. so less current flows across mem at these points |
|
|
What is the length constant?
|
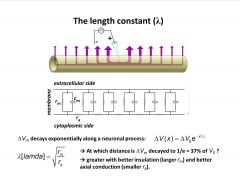
-can we quantitatively describe how voltage decays with distance
-exponential function: change in voltage= maximal voltage time an exponential constant -lambda= length constant square of mem. resis. / axial resist. -bigger length constant from better insulation (less ion channels would yield a further spreading potential, or smaller axial resistance better conducting cytosol) --better axial conduction is like drinking water vs. a viscous fluid -better insulation on left, holes on right cause loss of suction power |
|
|
What is lambda dependent on?
|
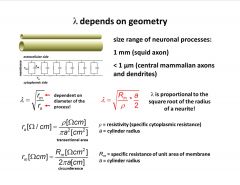
-lambda depends on geometry size range of neuronal processes is quite varied
-resistance depends on radius (both mem. and axial) -lambda is larger if diameter of processes are larger -larger diameter will conduct voltage over long distances as compare to smaller processes |
|
|
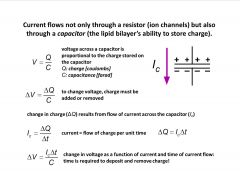
How does capacitance influence current flow in a cable?
|
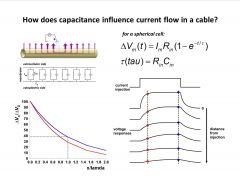
-capacitance slows down response ot ohm’s law
-electrodes at diff. dist. -blue=meas. at steady state, red=voltage response before steady state (sharper decay of signal faster signals are attenuated more) |
|
|
In relation to the length constant, if you want to make a fast axon, what should you do to it?
|
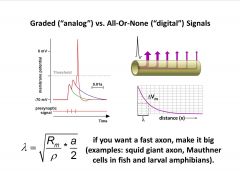
-important consequence of length constant: potential travels down axon at finite speed, and speed depends on length constant (how far down the cable is the passive spread able to conduct the voltage above threshold)
-big axons in escape systems (need fast responses) -if you want a fast axon, make it big…or increase Rm ! -insulate and remove most of the ion channels via myelination to increase the membrane resistance -Na channels only at nodes, and K channels only around nodes |
|
|
Which ion carries the action potential?
|
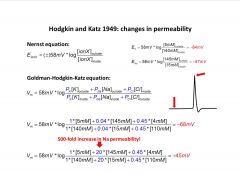
-action potential is mostly carried by Na reduced Na slows the AP
-Na carries the current -know that Na is what changes during an AP, and know the Voltage of an AP, so you can back calculate the permeability (from .04 at rest to 20 during AP) |
|
|
When analyzing voltage-clamp data, how do you differentiate between inward and outward currents?
|
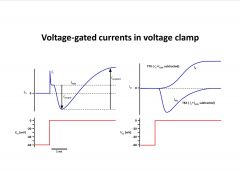
-TTX blocks Na channels
-STX blocks Na Channels too -TEA blocks K channels -the inward current (Na) activates rapidly, peaks, and then diminishes even though the voltage is held constant |
|
|
Describe The “ball and chain” model of channel inactivation.
|
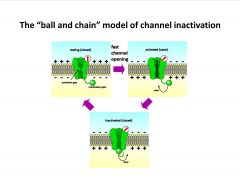
-2 gates: activation gate opens rapidly following depolarization, and then the inactivation gate closes slowly (reopens at resting potential)
|
|
|
Describe the reversal potential?
|
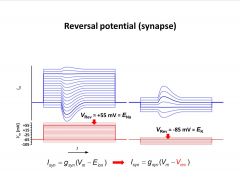
-if the driving force is 0 (equil. potential of the ion), the current becomes 0 and then reverses
-excitatory synaptic potential in this ex. is likely carried by Na on left and K on right |
|
|
What are the gating factors?
|
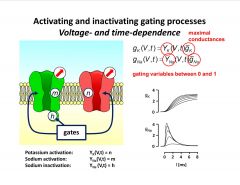
Y=gating variable that scales to a maximal conductance (g bar, which equals all channels open)
-voltage and time-dependent scaling factor; all open=1, none open=0 |
|
|
What effect does conformational changes in gating process have on the mathematical representation?
|
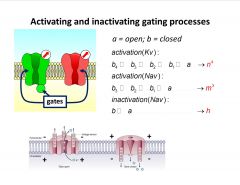
-gating processes aren’t isolated events
-open to close involves about 4 conformational changes -inactivation process of Na channel is the only one that behaves as a simple exponential function |
|
|
What is the action of the delayed rectifier?
|
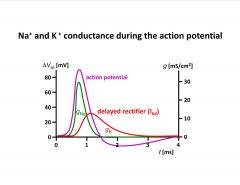
-called delayed rectifier b/c it brings the action potential back down to resting after a brief delay
|
|
|
Describe the absolute and relative refractory periods.
|
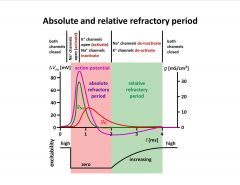
-harder to elicit AP during relative refractory period
-limits max frequency of sustained firing -sustained spike frequency is limited by refractory period (1/0.004s = 250 Hz) |
|
|
How is the action potential threshold determined?
|
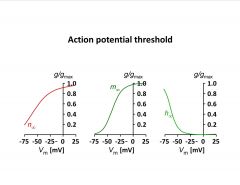
-gating variables have to be multiplied (activation and inactivation gate at -60V, 10% of 10% of Na channels are truly opened
-K only has an activation gate -properties of all the gates on the 2 channels add up to form a discrete threshhold |
|
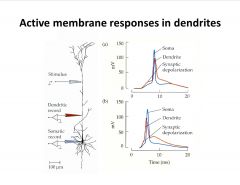
Where were each of these membrane responses generated?
|
-generated in soma and travel to dendrite up top, generated in dendrite and travel to soma at bottom
|
|
|
What is the general appearance of voltage-gated ion channels?
|
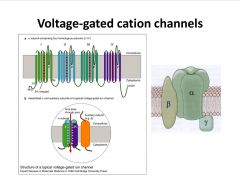
-have alpha, pore-forming subunits that consist of 4 parts; each subunit has 6 TM regions, with the 4th being charged (Voltage sensor); pore-forming loop between 5 and 6 TM regions
|
|
|
What are the patch clamp variations and what are they used for?
|
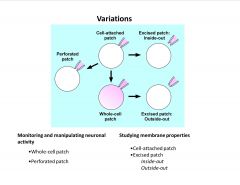
-whole cell patch allows dialysis (to wash in drugs or other variables)
|
|
|
Say a few words about the contribution of voltage-gated channels to the
resting membrane potential. |
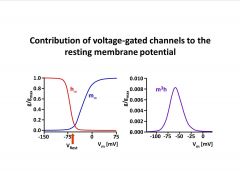
-multiply m and h
|
|
|
What is the relationship between unitary currents and whole cell currents?
|
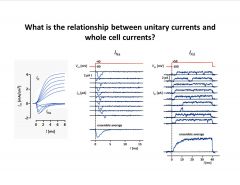
The macroscopic currents we measure in a whole-cell voltage-clamp experiment reflect the opening statistics of a population of unitary (single-channel) currents!
-whole cell Na current at diff. steps on left -patch clamp recording on 2 right panels; rapid after step but doesn’t stay open even with prolonged stimulus; avg. of all traces on bottom |
|
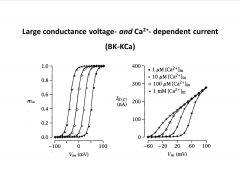
Interpret this trace.
|
-BK= big postassium
-where they sit on the Vm depends on Ca (the more Ca present, the easier it is to activate this current) |
|
|
What are the types of synapses? Some properties of each?
|
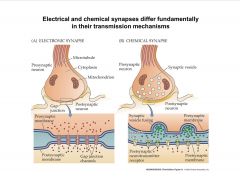
-electrical synapses:
-have direct cytoplasmic connections;cytoplasmic connection est. via gap junctions -not polarized (info flows both ways) -really fast rely on passive conduction (effectively, no delay in synaptic transmission) -channels (gap junctions) are much larger than many ionic pores (can allow passage of larger molecules like second messengers, and ATP) Synchronized neural activity (Crayfish escape reflex, mammalian brainstem respiratory networks, hypothalamic secretory cell groups) |
|
|
What are some Presynaptic proteins implicated in neurotransmitter release (on the synaptic vesicle)?
|
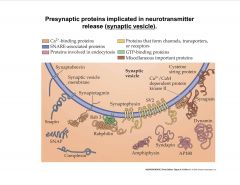
|
|
|
What are some Presynaptic proteins implicated in neurotransmitter release (at presynaptic membrane)?
|
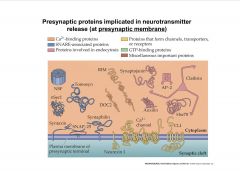
|
|
|
Describe a model for Ca-triggered vesicle fusion.
|
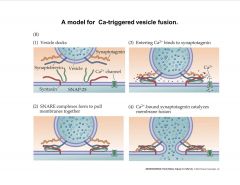
|
|
|
What is the role of DSCAM's in the nervous system?
|
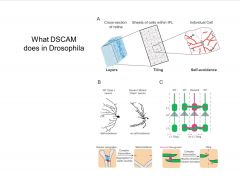
-2 processes with same variant of DSCAM-1 will repel one another (self-avoidance)
-DSCAM-2 is responsible for tiling (not as many variants as -1) -DSCAM: 12 potential exons in green, 48 in blue, 33 in yellow, 2 in red; only 1 from each group is selected; each is an IgG like domain (lots of splice variant combo's yielding huge amt of diversity much like Ig's) |
|
|
Describe a modern experimental paradigm to characterize axonal transport.
|
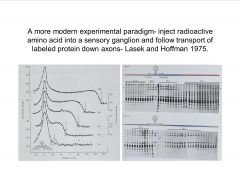
-right, graphs of gels done on segments of the axons after radio-labelling to id proteins being transported and relative times of transit
|
|
|
What are the Major Rate Components of Axonal Transport?
|
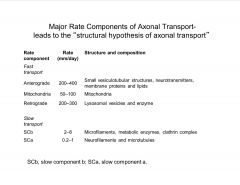
-distinct rates of transport (diff cargo goes at diff. rates and by diff. mechanisms)
-kinesins=-anterograde transport motor |
|
|
What are the Major Rate Components of Axonal Transport?
|
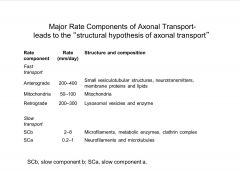
-distinct rates of transport (diff cargo goes at diff. rates and by diff. mechanisms)
-kinesins=-anterograde transport motor -dyneins: for retrograde transport of material (a.k.a. MAP1c) |
|
|
What are the Types of peripheral axon?
|
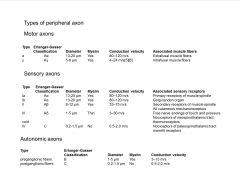
-classified by diameter and myelination state
-smaller diameter fibers are slower |
|
|
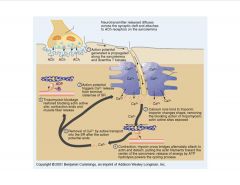
Describe the general mechanism of muscle contraction.
|
|
|
|
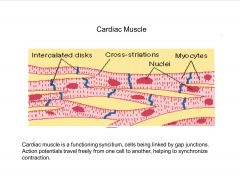
Describe cardiac muscle.
|
|
|
|
What fx does Ca have on cardiomyocytes?
|
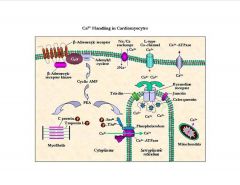
phospholaman, when phospho., inhibits SERCA, the sarcoplasmic reticuluum calcium ATPase, which results in more Ca in cytoplasm and more contraction
|
|
|
Most blood vessel smooth muscles contract
when given NE or E, but muscle and cardiac blood vessels relax- why? |
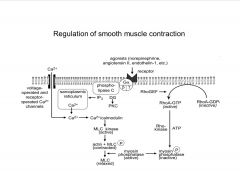
Alpha1-adrenergic receptors in most blood vessels, beta-adrenergic in heart and muscle
|
|
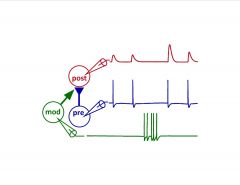
What does this image depict?
|
Priming of postsynaptic responses by neuromodulation
-pre-syn firing leads to EPSP…fairly fixed at low freq -just b/c modulatory neuron fires doesn’t immediately elicit a response, but the next EPSP of the post-syn. cell has increased amplitude |
|
|
What are some targets of metabotropic receptors?
|
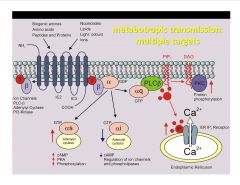
alpha-s=stimulating, alpha-i=inhibitory, alpha-q=leads to Ca release and other downstream activity (like PKC activation)
|
|
|
What are the different sites of neuromodulation?
|
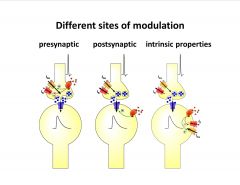
|
|
|
What causes Fast and slow responses to ACh in autonomic ganglion neurons?
|
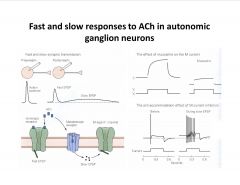
-dual post-syn response (fast and slow) due to the fact that the same transmitter (Ach) opens an ionotropic receptor (nicotinic) and a metabotropic receptor (muscarinic) leading to a second messenger cascade that closes a K channel
|
|
|
What's one mechanism by which serotonin exerts a modulatory function?
|
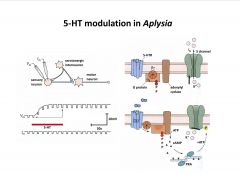
-close K channel via phosphorylation
|
|
|
What are some ways to Modulate voltage-gated ion channels?
|
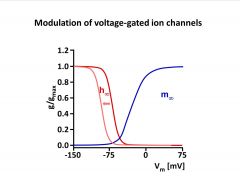
-modulator could cause a voltage-gated channel to be more or less open (changes m)
-could change voltage-dependence (shifts curve to more polarized or less polarized state) -can shift range over which channel is activated (changes slope) -can effect inactivation (shits h) |
|
|
What are the Cellular targets of neuromodulators?
|
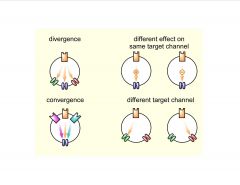
|
|
|
What is cotransmission?
|
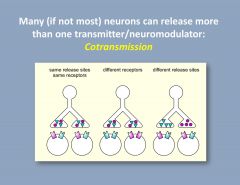
|
|
|
What is one way to modulate cotransmission?
|
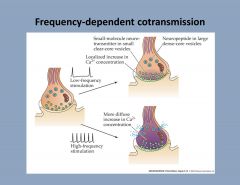
|
|
|
How are presynaptic receptors activated?
|
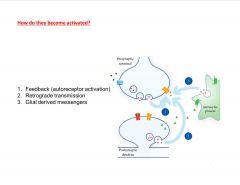
AND:
Spillover Volume transmission Axo-axonic synapses |
|
|
What are some Presynaptic Ionotropic mechanisms for ihibition of release?
|
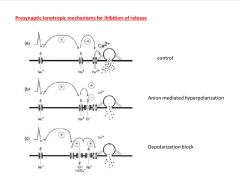
-depolarization block due to Na inactivation gates being activated
|
|
|
What are some Presynaptic Ionotropic mechanisms for ihibition of release (part B)?
|
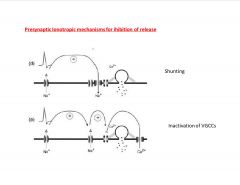
|
|
|
What are the sensory receptor types?
|
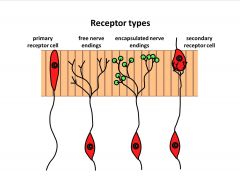
|
|
|
What's a common theme of sensory receptors?
|
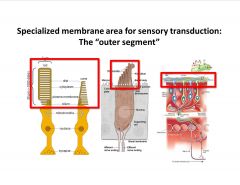
-elaborate, specialized area responsible for signal transduction
-diversity of diff cells within one sense -many receptor cells are ciliated epi’s -largest ear hair cell is a cilia -entire outer seg of retinal receptor is at the end of a cilia |
|
|
What mediates sensory signal transduction?
|
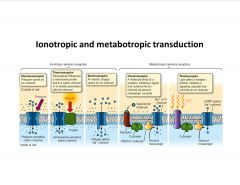
-pressure opens cation channels in mechanoreceptors, etc.
|
|
|
What is the Weber-Fechner law?
|
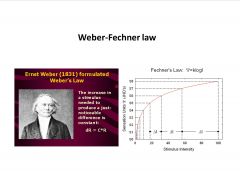
log relationship between detection threshold and stimulus intensity
-we detect the % diff. between a broad range of stimuli intensity (wouldn’t notice a 50 gram diff. in a 20kg suitcase but would for a light tennis ball) |
|
|
What are some response properties of different mechanoreceptors?
|
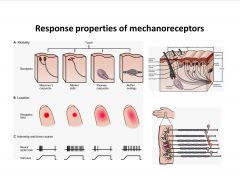
-diff. responses dependent on diff aspects of mechano-sense (velocity, acceleration, or direction)
|
|
|
What's one way that visual receptors enhance contrast?
|
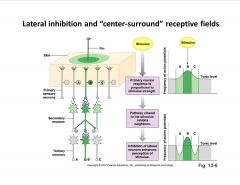
-contrast enhancement--> sharp signal about position of stimulus; lower detection threshold level by laterally inhibiting surrounding area
|
|
|
What are the two general models for mechanotransduction involving plasma membrane ion channels?
|
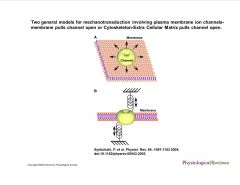
|
|
|
What is the major class of proteins involvec in C. elegans mechanoreception?
|
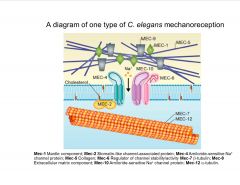
|
|
|
What is a Proposed model of a mechanosensor in Vascular Smooth Muscle Cells?
|
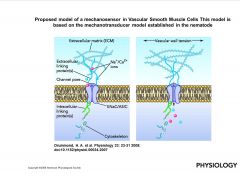
|
|
|
What is involved in signal transduction of sweet and bitter tastes?
|
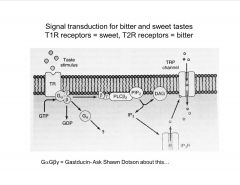
|
|
|
How are Trp channels involved in photo-reception?
|
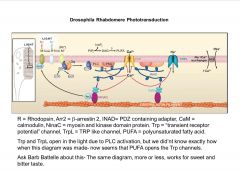
|
|
|
In general, how do hair cells work?
|
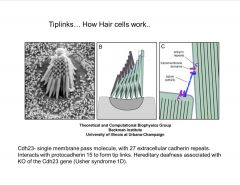
|
|
|
Where are rhodopsin containing discs in rods?
|
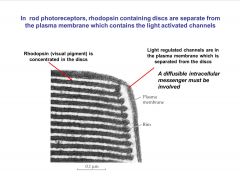
|
|
|
How are the opsins grouped, and what differentiates them?
|
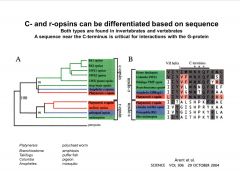
|
|
|
What is the Current model of activation in vertebrate rods?
|
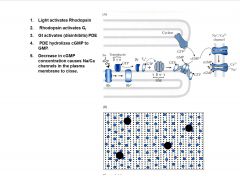
|
|
|
What are the Relative proportions of transduction proteins?
|
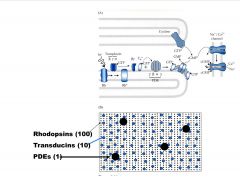
|
|
|
What happens to photoreceptor transmitter release in the light?
|
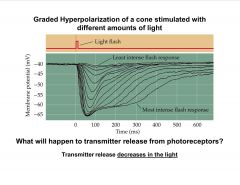
|
|
|
Describe The photoresponse in vertebrate rods.
|
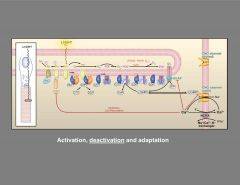
|
|
|
How is Gt inactivated?
|
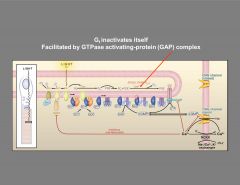
G proteins are inactivated by hydrolysis of GTP to GDP.
Catalyzed by GAP (GTPase activating complex) PDE is inactivated when the GTP on the G protein is hydrolyzed to GDP. |
|
|
How are cGMP levels in the photoreceptor restored?
|
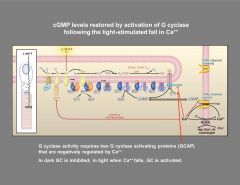
|
|
|
How is Ca++ feed-back useful for light adaptation?
|

|
|
|
How do scallop eyes work?
|
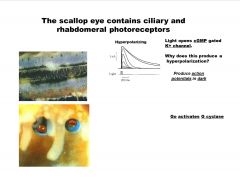
|
|
|
Describe Phototransduction in Drosophila?
|

|
|
|
What role does Ca play in phototransduction of Drosophilia?
|
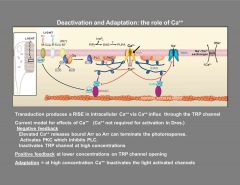
|
|
|
What is the max EPSP equal to? And what is N exactly?
|
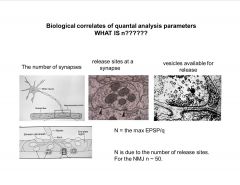
|
|
|
What influences p?
|
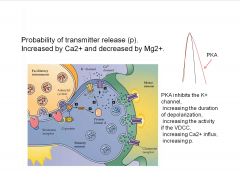
-changing transmitter release changes synaptic strength
-PKA delays K channel closing making a wider AP curve and allowing more Ca to enter the cell |
|
|
Describe paired pulse facilitation.
|
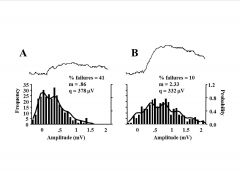
-bigger synaptic response from second pulse
-2nd pulse: more packets released due to Ca |
|
|
What are silent synapses?
|

|
|
|
Describe the taste bud cell types.
|
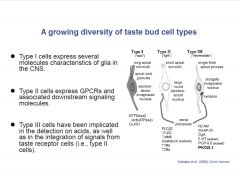
|
|
|
What are the types of taste receptors?
|
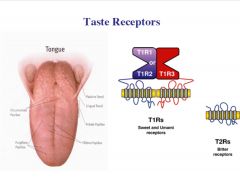
|
|
|
What comprises the sweet taste receptor?
|
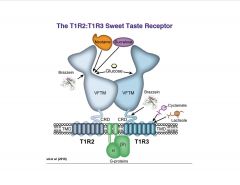
|
|
|
What are the mammalian taste receptors, cells, and ligands?
|
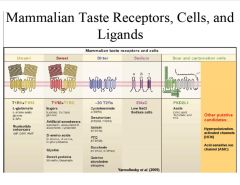
|
|
|
What is the typical sequence of events in taste transduction?
|
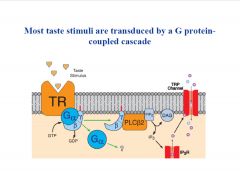
|
|
|
Describe the intracellular cascades of receptor and downstream effectors initiated by stimulation of gpcr's in taste receptor cells.
|
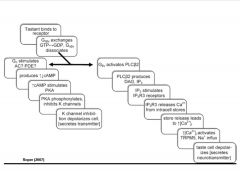
|
|
|
Link the different taste receptors to their corresponding G-protein and effector(s).
|
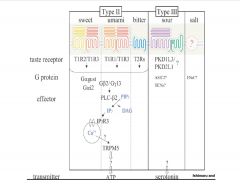
|
|
|
Describe the taste receptor signaling cascade.
|
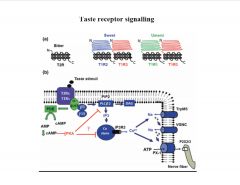
|
|
|
What are the 2 possible types of coding in chemosensory systems?
|
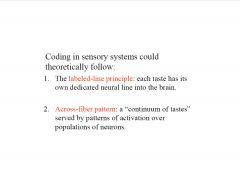
|
|
|
Briefly describe the olfactory system.
|
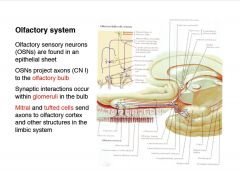
|
|
|
Describe the olfactory epithelium.
|
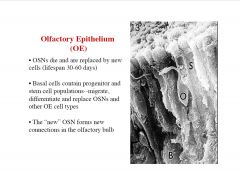
|
|
|
Briefly describe the olfactory sense neuron's.
|
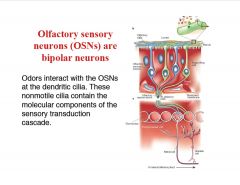
|
|
|
Describe the connections in the main olfactory system.
|
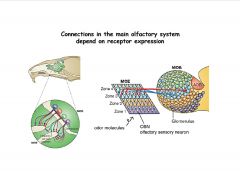
|
|
|
Describe the coding in the olfactory system.
|
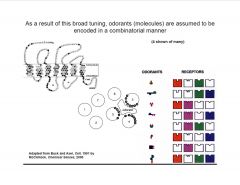
|
|
|
What are the major molecular components of the olfactory transduction cascade?
|
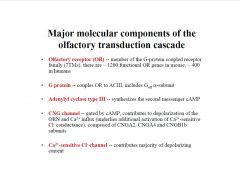
|
|
|
What is the signal transduction mechanism in V1R VSN's?
|
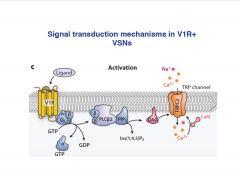
|
|
|
What role does Ca play in the action potential?
|
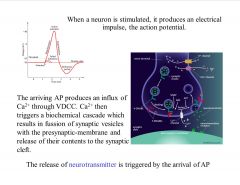
What happens when a neuron is sufficiently stimulated, it produces an electrical impulse (primary function of a neuron), the action potential. As you can see in this figure, when a neuron is sufficiently stimulated, it produces an AP. If the stimulus is not sufficient it will not produce an AP.
The arriving AP produces an influx of Ca2+ through VDCC. Ca2+ then triggers a biochemical cascade (as shown in this figure) which results in fussion of synaptic vesicles with the presynaptic-membrane and release of their contents to the synaptic cleft (gap between the pre and post synaptic membrane). |
|
|
What is the Sequence of Events in Presynaptic Terminal for the Release of Neurotransmitter?
|
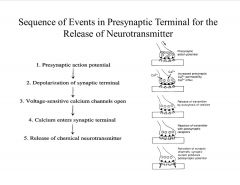
|
|
|
What is the mechanism of LTP induction?
|
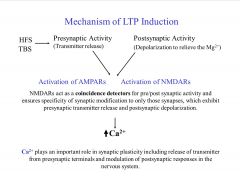
Depolarization is necessary to remove the Mg block.
|
|
|
What is the mechanism of LTP induction?
|
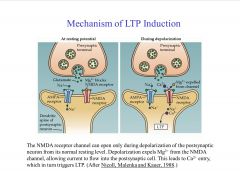
Induction of LTP depends on a substantial rise in postsynaptic Ca2+. During LFS glutamate released by presynaptic terminal binds with NMDA, Kainate glutamate receptors.
-depolarization releases the Mg block on the NMDA receptor allowing Ca to come inside (enhances LTP) |
|
|
What is a typical time course of LTP?
|
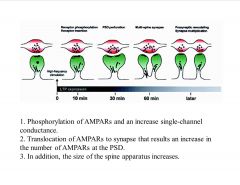
Within ten minutes of LTP induction, activation of Ca2+-dependent signal-transduction pathways results in phosphorylation of AMPA receptors and an increase in their single-channel conductance. In addition, the size of the spine apparatus increases, and AMPA receptors are delivered to the postsynaptic membrane by exocytosis of coated vesicles. This membrane insertion also leads to an increase in the size of the postsynaptic density (PSD) and, eventually, to the production of perforated synapses within the first 30 minutes. At one hour, through an unknown mechanism, some synapses use the expanded membrane area to generate multi-spine synapses (where two or more spines contact the same presynaptic bouton). Concomitant retrograde communication, possibly through cell-adhesion molecules, would trigger appropriate presynaptic structural changes, eventually increasing the total number of synapses.
-bifurcation of existing synapse…enhances potentiation |
|
|
What is the Role of CaMKII in AMPARs-mediated transmission during early LTP?
|
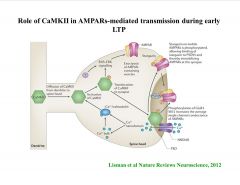
-CamKII can increase NMDAr function, phosphorylate AMPA receptors to increase conductance, or phos. stargazin to bring the AMPAr to the post-syn. density
|
|
|
What is the general scheme of LTP?
|
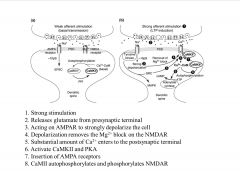
|
|
|
Discuss silent and whispering synapses.
|
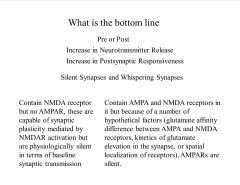
Two other alternate hypothesis, which received some attention, possibility of decreased re-uptake of glutamate, leading to increased synaptic glutamate levels, other, altered kinetics of glutamate release, where same number of glutamate molecules are released but at faster rate, such that the peak glutamate concentration is higher.
Silent synapses are synapses that contain NMDA receptor but no AMPAR, these are capable of synaptic plasticity mediated by NMDAR activation but are physiologically silent in terms of baseline synaptic transmission Whispering synapse has both AMPA and NMDA receptor in it but because of a number of hypothetical factors (glutamate affinity difference between AMPA and NMDA receptors, kinetics of glutamate elevation in the synapse, or spatial localization of receptors), AMPA receptor are silent. |
|
|
What is afterhyperpolarization? How is it implicated in LTP?
|
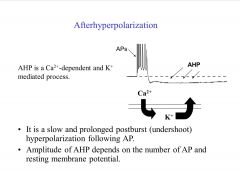
-AHP can impair the induction of LTP
|
|
|
Is LTD NMDAr dependent?
|

|
|
|
How does calcium influence LTD and LTP?
|
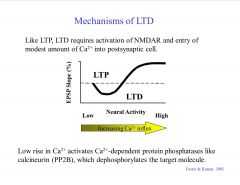
|
|
|
Compare and contrast LTP and LTD?
|
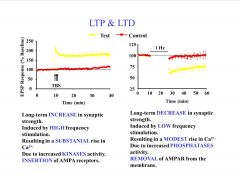
|
|
|
Give a basic etiology of Huntington's disease.
|
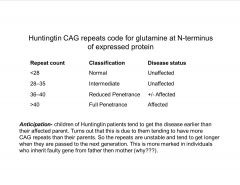
|
|
|
What are the models of cerebral ischemia?
|

|
|
|
What are some of the advantages and disadvantages of MACO models?
|

|
|
|
What role does calcium play in stroke?
|
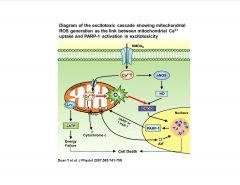
NMDA receptor activation leads to intracellular Ca2+ overload after ischemia
|
|
|
What are the acute neurochemical changes after ischemic stroke?
|
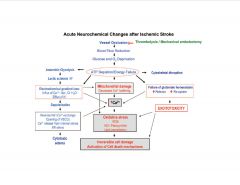
|
|
|
What is the timecourse of neutrophil infiltration after a stroke?
|
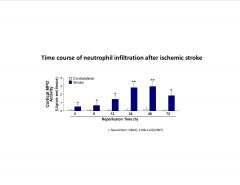
|
|
|
What are presynaptic mechanisms for facilitation of release?
|
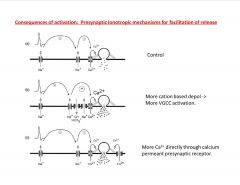
|
|
|
What are 2 classes of retrograde messengers?
|
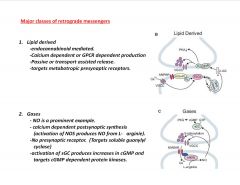
|
|
|
What are 2 of the major classes of retrograde messengers?
|
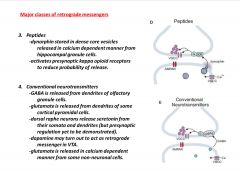
|
|
|
What is one major class of retrograde messenger?
|
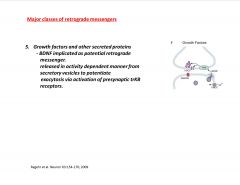
|
|
|
describe Otto Loewi's major experiment.
|
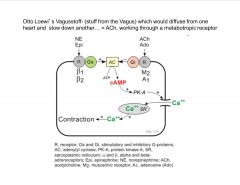
|
|
|
What are the Trp channel family relatives?
|
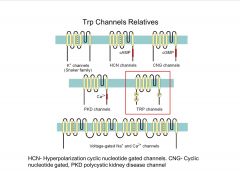
|

