![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
5 Cards in this Set
- Front
- Back
|
Nonpolar covalent bonds |
Have a electronegativity difference less than 0.5 |
|
|
Polar covalent bonds |
Have a electronegativity difference between 0.5 and 2 |
|
|
Ionic bonds |
Have an electronegativity difference over 2 |
|
|
Dipole moments |
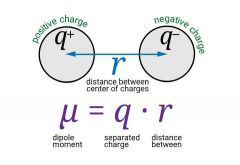
A dipole moment is defined as the magnitude of the charge times the distance between the charges. Symmetrical structures such as carbon dioxide, methane and benzene has a zero dipole moment due to the symmetrical structures. |
|
|
Formal charges |
Is defined as number of valence electrons minus number of bonding electrons divided by two minus the number on non-binding electrons |

