![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
87 Cards in this Set
- Front
- Back
|
How does glucagon increase blood glucose levels?
(what 3 processes?) |
1.Stimulation of hepatic glycogenolysis -> blood glucose levels can double within minutes
2.Stimulation of hepatic gluconeogenesis (via increased amino acid uptake) 3.Stimulation of lipolysis in fat tissue in very high concentrations |
|
|
Which type of diabetes is potentially reversible?
|
Diabetes type 2
|
|
|
Two types of hormones (in terms of where originate):
|
1. Tissue hormones - produced by individual endocrine cells; not in large glands
2. Glandular hormones - e.g. cytokines |
|
|
Which 2 organ systems produce the most hormones?
|
1. CNS (brain)
2. GI |
|
|
What are endocrines or hormones? functions?
|
Produced in a gland and secreted directly into circulation w/o ducts
chemical messangers, regulators of homeostasis and metabolic pathways, etc. |
|
|
autocrine hormones
|
affect cell of production
|
|
|
Are endocrine hormones present in high concentrations?
|
No, always in extremely low concentration (pg - ug/ ml), which why they are difficult to detect.
|
|
|
4 Types of hormones in terms of chemical structure?
(e.g. peptide hormones, and ? ) |
1. peptide hormones (like insulin)
2. steroid hormones (e.g. vit.D) 3. amines (e.g. dopamine, epinephrine) 4. eicosanoids (e.g. prostaglandins) |
|
|
which hormone type is most common?
|
peptide hormones
|
|
|
what hormones are hydrophilic?
|
peptide hormones
|
|
|
which hormones are lipophilic?
|
steroid hormones
|
|
|
What is term Dr. Zeigler uses for parts of hormone precursor which are cleaved off during protein/peptide synthesis? Are these cleaved off pieces ever active on their own?
|
pre-pro sequences; can be active compounds also!
|
|
|
What are differences between nervous and endocrine system?
|
endocrine system is slower to react, much slower, because it relies on chemical messengers
also endocrine system responds to INTERNAL stimuli; while nervous system responds to external ones. |
|
|
How long (approx.) does it take protein hormone to be produced?
|
~45-60 minutes
|
|
|
How long does it take for protein hormone to be released?
How are hormones released so quickly, when protein synthesis takes so long? |
2-5 minutes
* b/c after prepro seq. cleaved off, hormone stored in secretory granule or vesicle where can secrete immediately upon stimulation via exocytosis * also hydrophilic so dissolves easily in plasma |
|
|
What type of hormones made in adrenal cortex:
A. peptide hormones? B. steroid hormones? C. amines? D. eicosanoids? |
* hormones made in adrenal cortex are steroid h's
e.g. mineralocorticoids (aldosterone) and glucocorticoids (cortisol). * also a secondary site of androgen synthesis. |
|
|
True or false: Protein hormones generally have longer half-life then steroids.
|
False, b/c proteases are found everywhere in tissues, esp. concentrated in blood flow of liver&kidney.
|
|
|
Steroid Hormones are derived
from ? In one sentence, describe how made. |
Steroid Hormones are derived
from Cholesterol * stimulus activates enzymes -> starts a series of 3 conversions to produce steroid hormones |
|
|
How are steroid transported in plasma?
What % of bound? |
* not hydrophilic, are lipophilic, so need to attach to carrier (like albumin) in plasma
1-10% are in free form=active; rest is bound=inactive (acts as a hormone reserve in plasma) |
|
|
Why is half life of steroids extended (last longer than peptide hormones)?
|
* binding to albumin helps protect steroids from degradation and quick destruction in liver (longer half-lives -> hours to days)
* also makes "reservoir" in circulation |
|
|
How are most steroids metabolized?
|
glucuronidation in liver
|
|
|
How tissues get cholesterol?
Flows around body in what form? |
* from diet (although can be converted from Acetyl CoA)
* TriG and cholesterol all condensed into VLDL * LPL off loads cholesterol in tissues making steroids |
|
|
Receptor location for hydrophilic hormones?
Describe these receptors; are they complex? |
* Cell membrane (cannot diffuse through); so receptor on surface of membrane
* most of these receptors are very large and complex w/external binding portion and internal transducing portion |
|
|
Receptor location for lipophilic hormones:
|
* Cytoplasm or Nucleus; receptor inside target cell b/c lipophilic hormones have no problem entering cell to bind to it
|
|
|
All hormones affect their target tissues by forming first a ________ , which alters the activity of their target cells.
|
HORMONE - RECEPTOR complex
|
|
|
We know number of particular receptor can increase and decrease; which is more common: up regulation or down regulation?
|
down regulation
|
|
|
What is example of up regulation of receptor number?
|
Example: Aldosterone (steroid) initiates synthesis of enzyme ATPase in its target cells -> stimulates Na/K pumps
|
|
|
True or false: Antagonist does opposite action of hormone.
|
False, antagonist simply blocks binding of hormone to receptor.
|
|
|
Lipophilic hormones induce protein synthesis, w/newly formed proteins being mostly enzymes, which now stimulate or inhibit certain metabolic pathways = ?
|
= METABOLIC EFFECT
|
|
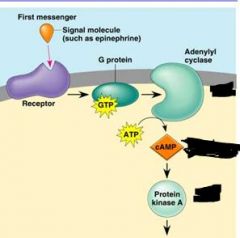
In following example: which represents the secondary messanger?
|
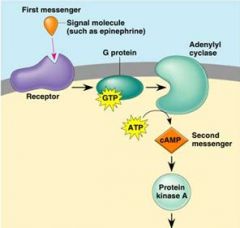
cAMP
Note: The first messenger (= hormone) remains outside of its target cell b/c cannot enter, but transmits its message via cAMP = second messenger |
|
|
phosphorylation is usually stimulatory or inhibitory event?
|
stimulatory
|
|
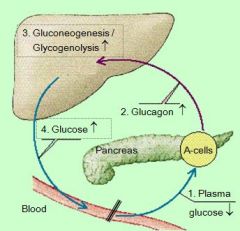
This an example of a _______feedback loop: effect of plasma glucose levels on glucagon secretion
|
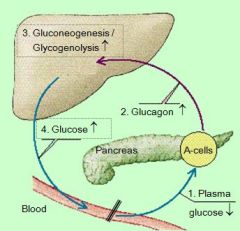
NEGATIVE FEEDBACK LOOP
|
|
|
What is stimulated (to start) if glycogen stores are full?
|
lipogenesis, creation of fat
|
|
|
What cells are not affected by insulin? (examples)
|
glucose independent cells
neurons, retina, lens, blood cells, pancreatic B cells, kidney, GI mucosa, placenta |
|
|
?% of body cells contain insulin
receptors |
80% of body cells contain insulin
receptors = insulin-sensitive tissues |
|
|
Where is insulin receptor located?
what type of mechanism? |
* its a protein, so on cell membrane
- called a tyrosine kinase * 2nd messanger mechanism (these are usually G-coupled) but not sure * insulin binds to external part alpha subunit, and stimulates beta subunits |
|
|
After insulin – receptor binding (= in
insulin-sensitive tissues): what happens? |
Beta parts of receptor become the
activated tyrosine kinase (= second messenger) -> leads phosphorylation of various enzymes |
|
|
What is stimulated by insulin binding?
|
* glucose uptake by GLUTs
* fat uptake and storage in fat tissue via lipoprotein lipase enzyme - extracts fat from VLDL or chylomicrons * inhibits hormone specific lipase * inhibits proteases / proteolytic enz. / protein brk.down * stim. protein temporary storage and synthesis (organ function, growth, clotting factors & albumin in liver) |
|
|
What happens without w/o insulin, but with circulating chylomicrons?
|
LDL not active, full of chylomicrons
|
|
|
Glucagon opposes Insulin effects on _____ in the LIVER
Glucagon ____ blood glucose level |
Glucagon opposes Insulin effects on carbohydrate metabolism (which were storage, breakdown)
Glucagon INCREASES blood glucose level |
|
|
How does glucagon increase blood glucose levels?
(by stimulating what 3 processes?) |
1.Stimulation of hepatic glycogenolysis -> blood glucose levels can double within minutes
2.Stimulation of hepatic gluconeogenesis (via increased amino acid uptake) 3.Stimulation of lipolysis in fat tissue in very high concentrations |
|
|
What might stop glucagon from secreting?
What cells are involved? |
high blood glucose, like after high carb. meal
(pancreatic alpha cells are insulin-sensitive: high plasma glc -> high insulin -> glc uptake into alpha cells -> glucagon secretion is inhibited!) |
|
|
Main signal for glucagon release
|
HYPOGLYCEMIA -> stimulates glucagon secretion
|
|
|
high plasma amino acid levels ___ glucagon secretion
|
also stimulate glucagon secretion
but only when blood glc levels are low (A.A. are then channeled into gluconeogenesis) |
|
|
= IDDM
|
Type I DM
circulating insulin levels are low |
|
|
What causes Type I DM?
|
Causes: islet cell destruction b/o pancreatitis, senile degeneration, autoimmune disease -> hyperglycemia (b/c low insulin; cells can't take up glc.)
|
|
|
Definition of Diabetes Mellitus:
|
absolute or relative lack of insulin leading to impaired
carbohydrate, lipid and protein metabolism |
|
|
Non-insulin dependent DM =
|
Type II DM
|
|
|
Causes of Type II DM?
Insulin resistance? |
* something goes wrong with insulin receptors, not binding insulin right, or not enough of them
* insulin present but not responding to it; known as insulin resistance aka "relative" lack of insulin |
|
|
%2 of ___ suffer from diabetes m.
|
%2 of burmese cats
|
|
|
Are beta cells of pancreas intact in type 2 diabetes?
|
yes at first, insulin still being made in excess as way to compensate blas
eventually the beta cells become "exhausted" and type I diabetes develop |
|
|
Secondary type diabetes or type 3 diabetes
|
high levels of other hormones like cortisol
high glucagon |
|
|
Which type of diabetes has normal insulin levels?
|
Type II DM
|
|
|
Which hormone responds to high osmoregularity in plasma? causes what?
|
ADH, causes incr. in thirst
|
|
|
two initial clincal signs of diabetes?
|
1a. rapid weight loss
(b/c of excess lipolysis, proteolysis), 1b. polyphagia (b/c body thinks it's hypoglycemic) 2. glucosuria |
|
|
What is consequence of excess lipolysis?
|
Excessive Lipolysis -> FATTY LIVER and KETOACIDOSIS -> diabetic COMA
FYI: Ketones produced when FA broken down in liver. Before this FA are enz. broken down via β-oxidation to form acetyl-CoA. However, if conditions not right for TCA cycle (intermediates not present), A/CoA become ketones instead.. In high lvl. lower pH of blood, leading to 'ketosis' |
|
|
Glycated Proteins/Acidosis/Proteolysis/Swelling further lead to?
|
* vascular damage -> microangiopathies / neuropathies
(e.g. glomerula, TIBIAL nerve/cat, retina/humans) |
|
|
What is consequence of weight loss and proteolysis?
|
proteolysis -> WEIGHT LOSS, muscle weakness and reduction of organ functions
|
|
|
At what glc. plasma level will glucose start appearing in urine?
|
if over 180mg/dL
|
|
|
what 2 clinical signs of diabetes usually appear as a result of glycosuria?
|
1. polyuria
2. polydipsia (incr. thirst) |
|
|
Does polyphagia (as hypothalamic satiety centre is insulin-dependent) continue?
|
initially only as hypothalamic satiety centre is insulin-dependent; later anorexia and vomiting following tissue damages a/o acidosis
|
|
|
What tissues get too much glucose with diabetes?
Do do these tissues with excess glucose? |
insulin independent tissues (neurons, liver, RBC, etc.)
do not have a lot of glucogen storing ability, also cannot convert to FAs ~form complexes w/proteins -> glycated proteins |
|
|
Are glycated proteins functional?
Examples? |
No
glycated hemaglobin! (A chain more often that B chain), after ~6 wks. of hyperglycemia |
|
|
What are glycated proteins in plasma called (e.g. glucosa + albumin)?
Time frame? (like when does this start to happen?) |
fructose amines
2 weeks |
|
|
What glycated protein test is most frequently done in vet medicine?
|
fructose amine test, for detecting glc. & albumin complexes
|
|
|
In the tissues excess glucose is converted into ___ in the lens?
Eventual consequence of this? |
sorbitol
H2O acc. in lens, gets cloudy -> nerve damage and blindness |
|
|
What are animals with diabetes prone to UTI?
|
tract has lots of sugar in it
|
|
|
which type of diabetes most common in cats?
|
DM type 2
|
|
|
how would u treat DM type 1?
|
give insulin
|
|
|
Why do u have to inject insulin?
|
have to inject all peptide hormones otherwise get degraded by the acid in stomach, etc.
|
|
|
why are steroid able to taken as pills?
|
lipophilic
|
|
|
what maintenance diet wud you feed animal with diabetes?
|
high in quality protein, low soluble carbs, low FA (b/c often obesity is cause, and leads to fatty liver), high in fiber
|
|
|
Which type of diabetes is potentially reversible?
|
diabetes type 2
|
|
|
how would you treat T2 diabetes to begin recovery process and why?
|
would give insulin along with exercise so BW reduced; this gives exhausted beta cells in pancreas (have churning out extra insulin) a chance to recover and make insulin again on own
|
|
|
What must be functional in animal to recover?
(unanswered questions) |
* Beta cells can't be completely shot
* Need at least some insulin receptors on insulin dep. tissue >But aren't receptors the source of problem with T2?? |
|
|
What is course of action if animal recovers from T2 diabetes? (What would you watch for?)
|
* animals blood glucose levels must monitered closely
* if beta cells making insulin again, must reduce or stop insulin supplements * otherwise can become hypoglycemic |
|
|
Could you cure/reverse type 1 diabetes?
|
Theoretically could do pancreas transplant, but normally only done in human medicine
(remember in type 1 is absolute of insulin, so beta cells not functional at all) |
|
|
How treat diabetes type 3?
(unanswered question) |
Secondary DM or Type III ( TRANSIENT DM)
* high levels of other hormones like cortisol, or glucagon * caused by DM Type II; insulin resistance Does it fix itself? What is connection between cortisol and insulin/hyperglycemia? |
|
|
If glucagon levels really HIGH, which metabolic pathway favored?
|
lipolysis (2.3.1)
|
|
|
Where is glucagon made?
|
secreted in pancreatic
α cells AND stomach <- gut glucagon |
|
|
Half life of glucagon?
|
5 minutes (it's a peptide - shorter half life than steroid, b/c proteases are everywhere)
|
|
|
How are pancreatic alpha cells and beta cells different aside from the hormones they make?
|
* Beta cells are insulin independant (they just make it)
* alpha cells are insulin dependent |
|
|
What happens when insulin binds alpha cells?
(unanswered question) |
glucagon secretion stops being released
(remember peptide hormones stored, so inhibiting synthesis..i dont think) |
|
|
Name two most common health problems affects in ferrets:
|
1. Adrenal disease - excess sex steroids
2. Insulinoma - tumor in beta cells of pancreas |
|
|
True or False: Regarding adrenal disease in ferrets, characterized by rise in cortisol & aldersterone.
|
False!
Ferrets w/adrenal disease have excess sex steroids (estrogens > androgens); but cortisol & aldosterone are normal |
|
|
Symptoms of Adrenal disease in ferrets?
|
Ferret with Adrenal Disease:
estrogens > androgens: symptoms include alopecia, enlarged vulva, prostate hypertrophy, sexual aggression, bone marrow depression (anemia) |
|
|
True or False: Insulinoma in ferrets causes rapid increase in blood glucose
|
False!!!
Insulinoma in ferrets causes rapid DROP in blood glucose because more insulin secreted |

