![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
55 Cards in this Set
- Front
- Back
|
Function of the islet of langerhans
|
secrete insulin and glucagon directly into the blood
|
|
|
delta cell
|
secretes somatostatin
|
|
|
alpha cell
|
secretes glucagon
|
|
|
b cell
|
insulin and amylin
|
|
|
Insulin allows excess carbs to be stored as what, where?
|
Glycogen in the liver and muscle tissue. After glycogen stores are full, the carbs are converted to fats and stored in adipose tissue.
|
|
|
How does insulin affect proteins
|
Inhibits the breakdown of proteins and increases the uptake and conversion of amino acids to protein
|
|
|
Insulin is first formed where? As what molecule?
|
RER
Insulin Preprohormone |
|
|
Does proinsulin have hormonal activity
|
NO
|
|
|
Is insulin carried by binding proteins through the blood?
What happens to insulin that does not bind to its target receptor? |
NO
It is degraded by insulinase, mostly in the liver |
|
|
Insulin receptor
|
Consists of 4 subunits:
2 alpha that lie entirely outside the cell 2 beta that penetrate through the membrane |
|
|
What portion of the receptor does insulin bind?
What becomes autophosphorylated? What does this activate? |
Alpha
Beta Activates Tyrosine Kinase, which in turn causes phosphorylation of multiple other intracellular enzymes including IRS |
|
|
Insulin's affects are most prominently not seen where?
|
Neurons of the brain
|
|
|
The main physiological affect of insulin is to increase glucose transport how?
|
Increases the translocation of intracellular vesicles to the membrane that contain glucose transport binding proteins.
|
|
|
How does insulin affect the transport of K and phosphate ions
|
Causes the cell membrane to become more permeable, increasing the uptake and causing a decrease in their extracellular concentration
|
|
|
During most of the day, what is the main form of energy for muscles? Why?
|
Fatty acids
Because normal resting muscle is impermeable to glucose, except when the muscle fiber is stimulated by insulin(after meals). |
|
|
Muscles during exercise |
Contraction causes translocation of GLUT-4 on the membrane which causes uptake of glucose Muscles use glucose instead of fatty acids |
|
|
Under what conditions do muscle fibers not need insulin for glucose uptake?
|
During Exercise
|
|
|
When does the muscle use insulin
|
After a meal, insulin is secreted by the pancreas and causes uptake of glucose
|
|
|
Mechanism by which insulin causes glycogen storage in the liver
|
1. Inactivates liver phosphorylase:
Causes liver to split glycogen into glucose 2. Increases the activity of glucokinase which increases the uptake of glucose into the cell. 3. Increases the activity of glycogen synthase |
|
|
How does lack of insulin cause glucose release by the liver?(between meals) |
-lack of insulin-no glycogenesis -lack of insulin activates phosphorylase: causes splitting of glycogen to glucose phosphate -activation of glucose phosphatase: takes away the phosphate from glucose, glucose can leave the liver |
|
|
Effect of insulin on the brain |
no effect
brain cells are permeable to glucose |
|
|
Hypoglycemic shock: how and why it develops |
brain uses as energy source only glucose low levels of blood glucose can cause the shock fainting, coma, seizures |
|
|
Is insulin a fat sparer? How?
|
Yes; it utilizes glucose uptake by the tissues which automatically decreases fat utilization
|
|
|
In which form is fat stored in the adipose tissue? |
In tricyglycerides, not fatty acids! fatty acids can leave the tissue and get in the blood stream |
|
|
How does insulin increase fatty acid synthesis
|
1. An increase of glucose uptake into cells, increases the amount of glycogen synthesis. Once glycogen synthesis is overloaded, the glucose is made into pyruvate, which is converted to Acetyl Co-A.
2. Excess citrate and isocitrate activate acetyl-CoA carboxylase to form malonyl-CoA. 3. Fatty acids in the liver form TG and are released in the blood. Activates lipoprotein lipase, which spilts TG's into fatty acids for transfer into adipose cells. |
|
|
Role of insulin in the storage of fat in the adipose tissues
|
-inhibition of hormone-sensitive lipase(hydrolysis of triglycerides to fatty acids)
-insulin promotes glucose transport into fat cells where it form a-glycerol(combines with TG to form fatty acids) |
|
|
How does insulin deficiency leads to atherosclerosis? |
insulin deficiency leads to excess of fatty acids in the plasma, fatty acids are converted into phospholipids and cholesterol |
|
|
How does insulin deficiency lead to ketosis?
|
1.Fatty acids in the blood without insulin causes increased activation of carnitine mechanism-> fatty acids transported to the mitochondria 2.In mitochondria beta oxidation-> fatty acids to acetyl co-A->acetoacetic acid 3.acetoacetic acid not utilized because it's in excess, converted into b hydroxybutyric acid and acetone(ketosis) |
|
|
How does insulin decrease gluconeogenesis?
|
Decreases the activity of the enzymes that promote it, so conserves substrates of gluconeogenesis(amino acids).
|
|
|
How does insulin lack affect protein storage?
|
Leads to increased protein catabolism and increased amino acid concentration.This in turn leads to amino acid breakdown and the formation of excess urea, which is excreted in the urine.
Protein wasting leads to increased muscle weakness |
|
|
How does insulin and growth hormone affect growth?
|
When given together they act synergistically to increase growth by promoting uptake of different amino acids by the cells
|
|
|
Glucose stimulation of insulin secretion |
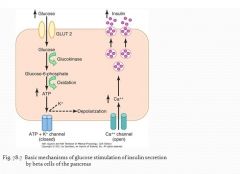
1. Glucose enters beta cells through GLUT-2 (proportional to the blood glucose) 2. Glucose converts to glucose 6-phosphate by glucokinase 3.Glucose 6-phosphate is oxidized and releases ATP 4.ATP blockes the ATP K+ channel 5.Depolarization of the membrane and activation of Ca+ voltage channels 6.Influx of Ca+ inside the cells 7.Ca+ cause fusion of insulin containing vesicles with the membrane and release through exocytosis |
|
|
glucagon,acetylcholine on insulin secretion
|
increase intracellular Ca+ levels and enhance effect of glucose |
|
|
-somatostatin+norepinephrine+leptin on insulin secretion
-sulfonylurea drugs |
-inhibit exocytosis of insulin
-stimulate insulin secretion by binding to atp k channel and blocking their activity |
|
|
How does amino acid concentration affect insulin secretion? What happens when glucose is also present?
|
Increased amino acid concentration increases insulin secretion only SLIGHTLY.
Glucose-induced secretion of insulin may be doubled in the presence of amino acids. Thus, amino acids strongly potentiate the glucose stimulus for insulin secretion. This allows for protein synthesis. |
|
|
Most potent amino acids for insulin secretion
|
Arginine and Lysine
|
|
|
Most potent astrointestinal hormones on insulin secretion |
incretins(GLP-1, GIP) released in GI after meal, enhance insulin release by b cells and inhibit glucagon production by a cells |
|
|
What is the stimulus for fat utilization in insulin lack
|
Decreased blood glucose activates gluconeogenesis
|
|
|
Major hormones secreted in response to hypoglycemia and how do they act
|
Glucagon(a cells)
inhibit utilization of glucose and promoting fat utilization very slow -Epinephrine(adrenal medulla): increasing glucose concentration during period of stress. It also increases fatty acids concentration, fat utilization a. causes glycogenolysis b. activates hormone sensitive lipase important in circulatory shock, exercise and anxiety |
|
|
Hyperglycemic hormone
|
glucagon secreted by a cells of islets of Langerhans increases glucose blood levels |
|
|
effects of glucagon
|
-glycogenolysis in the liver
-gluconeogenesis in the liver |
|
|
Glycogenolysis mechanism
|
Glucagon activates adenylyl cyclase, which causes the formation of cAMP. This in turn activates protein kinase, which activates phosphorylase B kinase. This then works to convert phosphorylase B into phosphorylase A. This promotes the degradation of glycogen into glucose-1-phosphate, which is dephosphorylated into glucose.
|
|
|
How does Glucagon affect gluconeogenesis?
|
Increases the uptake of amino acids into the liver and the conversion of them to glucose via gluconeogenesis.
|
|
|
When glucagon is maximally elevated, what is its effect on hormone sensitive adipose cell lipase?
|
Increases it, causing an increase in fatty acid concentration. Glucagon also inhibits the storage of TG's in the liver.
|
|
|
In what instance is both insulin and glucagon stimulated at the same time?
|
Increased blood amino acids
|
|
|
Effect of exercise on glucagon production |
It increases it |
|
|
What factors increase somatostatin secretion?
|
Increase Blood glucose
Increased AA's Increased FA's Increased GI hormones |
|
|
Inhibitory effects of somatostatin
|
1. acts locally on islets of langerhans and deplete synthesis of glucagon and insulin
2. decrease absorption and secretion by GI 3. decrease motility of stomach, gall bladder, duodenum |
|
|
Principal Function of somatostatin
|
Extends the time over which food nutrients are digested and assimilated into the blood. Prevents rapid exhaustion of food and therefore making it available over a longer period of time
|
|
|
Normal blood glucose levels: -in the morning -after a meal(1 hour) -feedback for control(?hours) |
-80-90 mg -120-140 mg -2 hours after last absorption |
|
|
Why is it important to maintain a constant blood glucose concentration?
|
Glucose is the only nutrient that normally can be used by the brain, retina, and germinal epithelium of the gonads.
|
|
|
In pts with sever liver disease, how is blood glucose affected?
|
It is impossible to correctly store glucose as glycogen during excess energy states, and thus, impossible for the glycogen to be broken down into glucose during hypoglycemic states.
The blood sugar is highly variable |
|
|
Syndrome associated with marked increases in ovarian androgen production and insulin resistance
|
Polycystic ovary syndrome
|
|
|
Diabetes: types |
type 1 diabetes; insulin-dependent diabetes(lack of insulin) type 2 diabetes; non insulin-dependent diabetes(decreased sensitivity of target tissues to the effect of insulin) insulin resistant |
|
|
Cause of diabetes type 1 |
injury to beta cells(viral infection, autoimmune disease) |

