![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
254 Cards in this Set
- Front
- Back
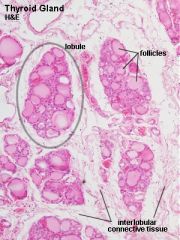
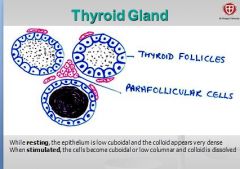
What is contained in the lobule of the thyroid gland?
|
What is contained in the lobule of the thyroid gland?
- thyroid follicles, parafollicular cells, dense networks of sinusoidal capillaries. |
|
|
ADD ADDITIONAL QUESTIONS for EXERCISES
|
ADD ADDITIONAL QUESTIONS 4 EXERCISES
|
|
|
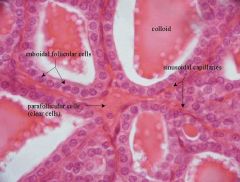
What is the structure of normal thyroid follicles?
|
Filled with gel-like substance (colloid) and lined by cuboidal follicular cells.
- colloid is the secretory product of the follicular cell (extracellular storage!). |
|
|
Two types of hormones (in terms of where originate):
|
1. Tissue hormones - produced by individual endocrine cells; not in large glands
2. Glandular hormones - e.g. cytokines |
|
|
How do thyroid cells appear at rest?
|
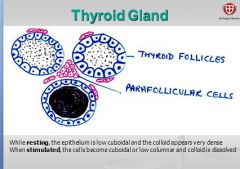
How do thyroid cells appear at rest?
epithelium appears low cuboidal very dense colloid. - colloid is the secretory product of the follicular cell (extracellular storage!). |
|
|
Which organs/tissues produce the most hormones?
|
1. CNS (brain)
2. GI |
|
|
How do thyroid cells appear when stimulated?
What is released? |
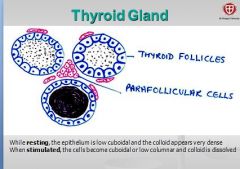
Epithelium becomes cuboidal or low columnar and colloid is dissolved. Secretes thyroid hormones thyroxin
|
|
|
What are endocrines or hormones? functions?
|
Produced in a gland and secreted directly into circulation w/o ducts
chemical messangers, regulators of homeostasis and metabolic pathways, etc. |
|
|
Parafollicular cells of the thyroid gland secrete what? function?
|
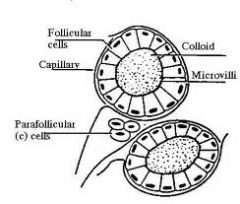
occur as single cells in the basal lamina of the follicle. Cells secrete Calcitonin (lowers blood calcium levels by suppressing bone resorption)
FYI: bone resorption = bone broken down and Ca+ released |
|
|
autocrine hormones
|
affect cell of production
|
|
|
what is function of calcitonin?
where secreted from? |
Parafollicular cells of the THYROID secrete calcitonin which LOWERS blood calcium level by suppressing bone resorption
-so counteracts break down of bone by PTH (remember PTH = incr blood calcium, incr. bone resorption/remodeling) ~when think of calcitonin, think of making bone |
|
|
Are endocrine hormones present in high concentrations?
|
No, always in extremely low concentration (pg - ug/ ml), which why they are difficult to detect.
|
|
|
major integrating link between the
nervous and the endocrine systems. |
HYPOTHALAMUS
|
|
|
Types of hormones in terms of chemical structure?
|
1. peptide hormones (like insulin)
2. steroid hormones (e.g. vit.D) 3. amines (e.g. dopamine, epinephrine) 4. eicosanoids (e.g. prostaglandins) |
|
|
receives input from various other CNS regions such as ? (6)
|
input from -> e.g. thalamus, limbic system, cortex, RAS, and sensory signals from viscera and the eye.
|
|
|
which hormone type is most common?
|
peptide hormones
|
|
|
is involved in emotions, stress, fear, control of hunger, thirst, sexual behaviour
|
HYPOTHALAMUS
|
|
|
what hormones are hydrophilic?
|
peptide hormones
|
|
|
largely controls homeostasis, e.g. osmolality, body temperature,
and controls the autonomic nervous system. |
HYPOTHALAMUS
|
|
|
which hormones are lipophilic?
|
steroid hormones
|
|
|
is a “master” endocrine gland.
|
The Hypothalamus is a “master” endocrine gland. Its many neuroendocrines influence other CNS areas as well as various peripheral endocrine glands via the Pituitary Gland.
(in past learned pituitary gland is master but this prof says otherwise) |
|
|
What is term Dr. Zeigler uses for parts of hormone precursor which are cleaved off during protein/peptide synthesis? Are these cleaved off pieces ever active on their own?
|
pre-pro sequences; can be active compounds also!
|
|
|
The Hypothalamus is connected with the PITUITARY GLAND via the
|
hypophyseal stalk.
|
|
|
What are differences between nervous and endocrine system?
|
endocrine system is slower to react, much slower, because it relies on chemical messengers
also endocrine system responds to INTERNAL stimuli; while nervous system responds to external ones. |
|
|
PITUITARY GLAND consists of what 2 parts?
|
1. ANTERIOR pituitary
2. POSTERIOR pituitary |
|
|
How long (approx.) does it take protein hormone to be produced?
|
~45-60 minutes
|
|
|
How long does it take for protein hormone to be released?
How are hormones released so quickly, when protein synthesis takes so long? |
2-5 minutes
* b/c after prepro seq. cleaved off, hormone stored in secretory granule or vesicle where can secrete immediately upon stimulation via exocytosis * also hydrophilic so dissolves easily in plasma |
|
|
neurohypophysis extension of the hypothalamus is which part of pit.gland?
What tissues/structures does it have? |
POSTERIOR pituitary
consists of nervous tissue stores and releases the 2 hypothalamic hormones produced in the Nucleus supraopticus and Nucleus paraventricularis: |
|
|
What type of hormones made in adrenal cortex:
A. peptide hormones? B. steroid hormones? C. amines? D. eicosanoids? |
* hormones made in adrenal cortex are steroid h's e.g. mineralocorticoids (aldosterone) and glucocorticoids (cortisol).
* also a secondary site of androgen synthesis. |
|
|
Nucleus supraopticus and Nucleus paraventricularis release what 2 hypothalamic hormones ??
|
the 2 hypothalamic hormones produced in the
Nucleus supraopticus and Nucleus paraventricularis: ADH and Oxytocin |
|
|
True or false: Protein hormones generally have longer half-life then steroids.
|
False, b/c proteases are found everywhere in tissues, esp. concentrated in blood flow of liver&kidney.
|
|
|
formed from Rathke’s pouch = glandular epithelium
produces and secretes hormones under hypothalamic control |
ANTERIOR pituitary = adenohypophysis
|
|
|
Steroid Hormones are derived
from ? In one sentence, describe how made. |
Steroid Hormones are derived
from Cholesterol * stimulus activates enzymes -> starts a series of 3 conversions to produce steroid hormones |
|
|
also called vasopressin
|
ADH
|
|
|
How are steroid transported in plasma?
What % of bound? |
* not hydrophilic, are lipophilic, so need to attach to carrier (like albumin) in plasma
1-10% are in free form=active; rest is bound=inactive (acts as a hormone reserve in plasma) |
|
|
Glucocorticoid
|
any hormone of adrenal cortex that affects carb,fat, and/or protein metabolism; chiefly cortisol and corticosterone
|
|
|
Why is half life of steroids extended (last longer than peptide hormones)?
|
* binding to albumin helps protect steroids from degradation and quick destruction in liver (longer half-lives -> hours to days)
* also makes "reservoir" in circulation |
|
|
ADH and Oxytocin synthesized where?
|
hypothalmus
|
|
|
How are most steroids metabolized?
|
glucuronidation in liver
|
|
|
ADH and Oxytocin stored and RELEASED from where?
|
Most of it is stored in the posterior pituitary (neurohypophysis) to be released into the bloodstream
|
|
|
How tissues get cholesterol?
Flows around body in what form? |
* from diet (although can be converted from Acetyl CoA)
* TriG and cholesterol all condensed into VLDL * LPL off loads cholesterol in tissues making steroids |
|
|
Does hypothalmus also regulate ANTERIOR pituitary = adenohypophysis?
|
Yes
|
|
|
Receptor location for hydrophilic hormones?
Describe these receptors; are they complex? |
* Cell membrane (cannot diffuse through); so receptor on surface of membrane
* most of these receptors are very large and complex w/external binding portion and internal transducing portion |
|
|
Hormones produced in hypoth. go to AP?
|
Releasing Hormones (RH) and/or Inhibiting Hormones (IH)
RHs / IHs reach the Anterior Pituitary gland (via – hypophyseal portal system) |
|
|
Receptor location for lipophilic hormones:
|
* Cytoplasm or Nucleus; receptor inside target cell b/c lipophilic hormones have no problem entering cell to bind to it
|
|
|
RHs /IHs stimulate/inhibit the production and release of hormones from the ?
What are these type of hormones known as? |
from the anterior
pituitary gland into circulation = Tropic Hormones (mostly) Tropic Hormones |
|
|
All hormones affect their target tissues by forming first a ________ , which alters the activity of their target cells.
|
HORMONE - RECEPTOR complex
|
|
|
These tropic hormones in turn stimulate ?
|
These tropic hormones in turn stimulate a peripheral endocrine gland to produce /
release its own hormones. |
|
|
We know number of particular receptor can increase and decrease; which is more common: up regulation or down regulation?
|
down regulation
|
|
|
Where are some stimulating/releasing hormones that the hypoth. makes?
Hint: gd cn G Tt P |
Gnrh
Crh GHrh Trh Prh |
|
|
What is example of up regulation of receptor number?
|
Example: Aldosterone (steroid) initiates synthesis of enzyme ATPase in its target cells -> stimulates Na/K pumps
|
|
|
GnRH = ?
GHRH = ? |
GnRH = Gonadotropin R.H.
GHRH = Growth Hormone Rel. Hormone |
|
|
True or false: Antagonist does opposite action of hormone.
|
False, antagonist simply blocks binding of hormone to receptor.
|
|
|
Gonadotropin Stimulates?
Which in term stumulates? |
Gonadotropin RH. stimulates A.P. to produce FSH and LH!!!
..gonads to make sex hormones |
|
|
Lipophilic hormones induce protein synthesis, w/newly formed proteins being mostly enzymes, which now stimulate or inhibit certain metabolic pathways = ?
|
= METABOLIC EFFECT
|
|
|
Another name for growth hormone
|
Growth h. = Somatotropin
= Somatotropic Hormone = ST(H) |
|
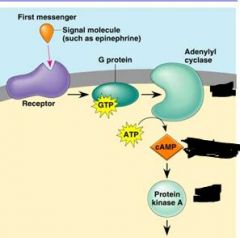
In following example: which represents the secondary messanger?
|
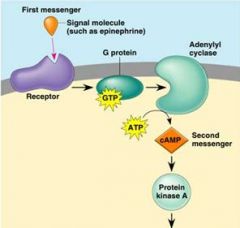
cAMP
Note: The first messenger (= hormone) remains outside of its target cell b/c cannot enter, but transmits its message via cAMP = second messenger |
|
|
Obviously GHRH stimulates AP to GH..but what peripheral gland does growth hormone stimulate?
|
Liver (and direct effects) to make Somatomedins,
which works on multiple tissues |
|
|
phosphorylation is usually stimulatory or inhibitory event?
|
stimulatory
|
|
|
Corticotropin = Adreno-corticotropic hormone = ACTH
stimulates what organ? to make what? |
adrenal cortex to synthesize Glucocorticoids (aka cortisol)
|
|
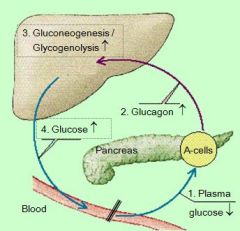
this an example of _______: effect of plasma glucose levels on
glucagon secretion |
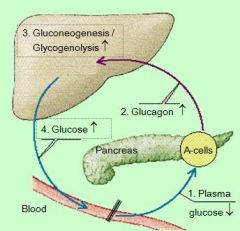
NEGATIVE FEEDBACK LOOP
|
|
|
Targets of ACTH?
|
many targets
|
|
|
What is stimulated (to start) if glycogen stores are full?
|
lipogenesis, creation of fat
|
|
|
What is TRH?
|
TRH = Thyrotropin Releasing Hormone
|
|
|
What cells are not affected by insulin? (examples)
|
glucose independent cells
neurons, retina, lens, blood cells, pancreatic B cells, kidney, GI mucosa, placenta |
|
|
Where does Thyrotropin come from?
|
ant. pituitary
|
|
|
?% of body cells contain insulin
receptors |
80% of body cells contain insulin
receptors = insulin-sensitive tissues |
|
|
Where does TSH come from?
|
Ant.Pit.Gland
Thyroid stimulating hormone and thyrotropin are same thing |
|
|
Where is insulin receptor located?
what type of mechanism? |
* its a protein, so on cell membrane
- called a tyrosine kinase * 2nd messanger mechanism (these are usually G-coupled) but not sure * insulin binds to external part alpha subunit, and stimulates beta subunits |
|
|
TSH stimulates thyroid to stimulate what?
|
T3
T4 |
|
|
After insulin – receptor binding (= in
insulin-sensitive tissues): what happens? |
Beta parts of receptor become the
activated tyrosine kinase (= second messenger) -> leads phosphorylation of various enzymes |
|
|
Where are some INHIBITING hormones that the HYPOTHALMUS. makes?
|
GHih (counteracts GHrh)
Pih (counteracts Prh) |
|
|
What is stimulated by insulin binding?
|
* glucose uptake by GLUTs
* fat uptake and storage in fat tissue via lipoprotein lipase enzyme - extracts fat from VLDL or chylomicrons * inhibits hormone specific lipase * inhibits proteases / proteolytic enz. / protein brk.down * stim. protein temporary storage and synthesis (organ function, growth, clotting factors & albumin in liver) |
|
|
Prostaglandins made in the ?
What is target organ? |
Ant.Pituitary gland
direct effects (e.g. mammary gland) |
|
|
What happens without w/o insulin, but with circulating chylomicrons?
|
LDL not active, full of chylomicrons
|
|
|
Which part (inner OR outer layer) makes the mineralcorticoids?
Which part (inner OR outer layer) makes the glucocorticoids? |
Zona glomerulosa (outer portion of AC) makes the mineralcorticoids!
Zona fasciculata (middle layer) makes the glucocorticoids! |
|
|
Glucagon opposes Insulin effects on _____ in the LIVER
Glucagon ____ blood glucose level |
Glucagon opposes Insulin effects on carbohydrate metabolism (which were storage, breakdown)
Glucagon INCREASES blood glucose level |
|
|
Low levels of calcium stimulates ?
|
Low levels of calcium stimulates release of PTH from chief cells of parathyroid gland. In addition to its effects on kidney and intestine, PTH also increases activity of osteoclasts to release calcium from bone, by stim. bone resorption.
|
|
|
How does glucagon increase blood glucose levels?
(what 3 processes?) |
Stimulation of hepatic glycogenolysis -> blood glucose levels can double within minutes
Stimulation of hepatic gluconeogenesis (via increased amino acid uptake) Stimulation of lipolysis in fat tissue in very high concentrations |
|
|
What is primary mineralcorticoids?
|
Aldosterone (makes up 90% of mineralcorticoids) secreted from outer layer (Z.G.) of A.C.
AMC |
|
|
What might stop glucagon from secreting?
What cells are involved? |
high blood glucose, like after high carb. meal
(pancreatic alpha cells are insulin-sensitive: high plasma glc -> high insulin -> glc uptake into alpha cells -> glucagon secretion is inhibited!) |
|
|
What is primary Glucocorticoids made by adr. cortex?
|
Cortisol (95% glucocorticoids secreted in mammals)
GC |
|
|
Main signal for glucagon release
|
HYPOGLYCEMIA -> stimulates glucagon secretion
|
|
|
What 2 components are required to make thyroid hormone?
|
- amino acid tyrosine and iodine
|
|
|
high plasma amino acid levels ___ glucagon secretion
|
also stimulate glucagon secretion
but only when blood glc levels are low (A.A. are then channeled into gluconeogenesis) |
|
|
How does Iodide (I-) gets into thyroid cell ?
Where does it go? in what form? |
Iodide uptake into thyroid cell via active pump btw. thyrocyte and blood vessel
Enters colloid space in follicle, Iodide oxid. by perixidase into iodine |
|
|
= IDDM
|
Type I DM
circulating insulin levels are low |
|
|
TGB? made where?
primary component of ____? |
thyoglobulin, made in follicular cells, is glycoprotein containing lots of tyrosine AA's
it is primary component of colloid |
|
|
What causes Type I DM?
|
Causes: islet cell destruction b/o pancreatitis, senile degeneration, autoimmune disease -> hyperglycemia (b/c low insulin; cells can't take up glc.)
|
|
|
difference between T1 and T2
where does all this happen? |
(iodine joins the TGB)
T1 contain only 1 iodine; T2 contains 2 iodines (still in colloid) |
|
|
Definition of Diabetes Mellitus:
|
absolute or relative lack of insulin leading to impaired
carbohydrate, lipid and protein metabolism |
|
|
what happens when form T3 or T4?
Which is find in higher conc.in plasma? |
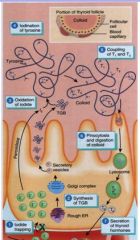
T1&T2 are mere primary peptide chains - condense, fold over and form secondary structures, couple with eachother
(still in colloid) T4 makes of 93% in thyroid hormone in plasma? |
|
|
Non-insulin dependent DM =
|
Type II DM
|
|
|
storage form of thyroid hormone
active form of thyroid hormone? what does it do? |
T4
T3 (active hormone; T4 is converted to T3 after enters target cell) |
|
|
Causes of Type II DM?
Insulin resistance? |
* something goes wrong with insulin receptors, not binding insulin right, or not enough of them
* insulin present but not responding to it; known as insulin resistance aka "relative" lack of insulin |
|
|
Thyroid hormones are controlled via the ____?
|
Hypothalamic–Pituitary–Thyroid Axis
|
|
|
%2 of ___ suffer from diabetes m.
|
%2 of burmese cats
|
|
|
Hypothalamic TRH release is
- stimulated by _? - inhibited by _? |
Hypothalamic TRH release is
- stimulated by COLD - inhibited by stress (sympathetic n.s.) |
|
|
Are beta cells of pancreas intact in type 2 diabetes?
|
yes at first, insulin still being made in excess as way to compensate blas
eventually the beta cells become "exhausted" and type I diabetes develop |
|
|
TRH stimulates anterior pituitary to
synthesize and secrete __ |
TSH
|
|
|
Secondary type diabetes or type 3 diabetes
|
high levels of other hormones like cortisol
high glucagon |
|
|
T3 or T4 is lipophilic?
transported by? |
yes
Binding of lipophylic T4 & T3 to plasma proteins (~99%), e.g. thyroxin-binding protein (& albumin); half-life of T4 in dogs: 12-14 hours half-life of T4 in man: ~6 days |
|
|
Which type of diabetes has normal insulin levels?
|
Type II DM
|
|
|
TSH stimulates thyroid gland to
synthesize and secrete thyroid hormones through: what mechanisms? |
- stimulation of iodide pump
- stimulation of iodination of tyrosine - stimulation of TGB proteolysis - stimulation of growth of thyroid cells (can lead to gland enlargement) |
|
|
Which hormone responds to high osmoregularity in plasma? causes what?
|
ADH, causes incr. in thirst
|
|
|
Increased levels of ___ inhibit further release of
TSH -> by decreasing_____? |
Increased levels of circulating thyroid hormones inhibit further release of
TSH (by decreasing TRH receptor no.) |
|
|
two initial clincal signs of diabetes?
|
1a. rapid weight loss
(b/c of excess lipolysis, proteolysis), 1b. polyphagia (b/c body thinks it's hypoglycemic) 2. glucosuria |
|
|
Thyroid hormone affect on basal metabolism?
|
INCREASES metabolic rate:
nutrient utilization up ATP formation up oxygen consumption up HEAT production up |
|
|
What is consequence of excess lipolysis?
|
Excessive Lipolysis -> FATTY LIVER and KETOACIDOSIS -> diabetic COMA
FYI: Ketones produced when FA broken down in liver. Before this FA are enz. broken down via β-oxidation to form acetyl-CoA. However, if conditions not right for TCA cycle (intermediates not present), A/CoA become ketones instead.. In high lvl. lower pH of blood, leading to 'ketosis' |
|
|
True or False: High levels of thyr.hormone lower blood cholesterol.
|
FALSE
incr. lipolysis incr. beta oxidation incr. bile secretion -> lowers blood cholesterol -> geared towards supplying fat for energy |
|
|
Glycated Proteins/Acidosis/Proteolysis/Swelling further lead to?
|
* vascular damage -> microangiopathies / neuropathies
(e.g. glomerula, TIBIAL nerve/cat, retina/humans) |
|
|
Signs of thyroid hormone deficiency on skin?
|
HD: follicle atrophy ->
bilateral ALOPECIA myxedema (edema under skin) pigmentation |
|
|
What is consequence of weight loss and proteolysis?
|
proteolysis -> WEIGHT LOSS, muscle weakness and reduction of organ functions
|
|
|
name for non-neoplastic hyperplasia of thyroid gland
cause? |
goiter
iodine deficiency, or goitergenic substances like cabbage, etc. |
|
|
At what glc. plasma level will glucose start appearing in urine?
|
if over 180mg/dL
|
|
|
How would you explain animal which has all symptoms of hypothyroidism but has normal levels in plasma?
|
T4 is converted to T3 intracellularly, so could be a problem with intracellular mechanism
|
|
|
what 2 clinical signs of diabetes usually appear as a result of glycosuria?
|
1. polyuria
2. polydipsia (incr. thirst) |
|
|
Other ways of hypothyroidism aside from testing T4 level?
|
1. inject TSH and see if T4 levels go up
2. inject thyroid hormones themselves and see if they are used |
|
|
Does polyphagia (as hypothalamic satiety centre is insulin-dependent) continue?
|
initially only as hypothalamic satiety centre is insulin-dependent; later anorexia and vomiting following tissue damages a/o acidosis
|
|
|
True or False: These are symptoms of old cat with hyperthyroidism?
* hyperactivity, nervousness (10% of cats are apathetic) * body temperature down and skin temp. decreased * tachycardia; hypertension (retinal detachment) * diarrhea; polyuria / polydipsia (thirst) (b/o medullary washout) * skin changes (matted hair or alopecia) |
all true, except body temp. would NOT go down
* would see heat intolerance and INCREASED skin temp. Remember: metabolism / functions are accelerated in all organs! |
|
|
What tissues get too much glucose with diabetes?
Do do these tissues with excess glucose? |
insulin independent tissues (neurons, liver, RBC, etc.)
do not have a lot of glucogen storing ability, also cannot convert to FAs ~form complexes w/proteins -> glycated proteins |
|
|
What causes activates trx of genes in virtually all tissues? And also stimulates following:
* synthesis of many enzymes, structural & transport proteins * general increase of metabolic activities in many tissues |
Thyroid hormone
|
|
|
Are glycated proteins functional?
Examples? |
No
glycated hemaglobin! (A chain more often that B chain), after ~6 wks. of hyperglycemia |
|
|
Causes of hypothyroidism in dogs?
Typical hormone levels? |
mostly defects of the thyroid gland (autoimmune, lymphocytic infiltration or iodiopathic atrophy)
In dogs 95% cases caused by destruction of follicular cells due to autoimmune inflammation. Typical findings are incr. conc. of TSH and low T4, may also be aB against TGB thyroperox. in blood |
|
|
What are glycated proteins in plasma called (e.g. glucosa + albumin)?
Time frame? (like when does this start to happen?) |
fructose amines
2 weeks |
|
|
True or False: These are signs of hypothyroidism?
- skin alterations are most common: (alopecia) - myxedema = accumulation of hyaluronic acid in face and tail and folded skin (sometimes in nerves) - reproductive dysfunction intolerance; heat seeking |
True
Most importantly in hypothyroidism -> reduction of metabolic rate: lethargy; weight gain; exercise Remember: metabolism / functions slow down! |
|
|
What glycated protein test is most frequently done in vet medicine?
|
fructose amine test, for detecting glc. & albumin complexes
|
|
|
Why does Cholesterol increase in hypothyroidism?
|
Cholesterol: Increase, because there is a lack of liver enzymes due to a decreased lipolysis. This means that the body is not digesting all of the fats in the diet so cholesterol levels would be elevated in blood. Fat filled feces would also be seen (according to Sjaastad p.217 constipation and passage in intestine reduced in hypoth.)
|
|
|
In the tissues excess glucose is converted into ___ in the lens?
Eventual consequence of this? |
sorbitol
H2O acc. in lens, gets cloudy -> nerve damage and blindness |
|
|
Why does Cholesterol decrease in hyperthyroidism?
|
Decreased cholest. due to a stimulation of lipolysis. Almost all fats in the body would be broken down
TH stim. production of bile juice. so micelle form. and fat abs. liver excretes of xs cholesterol w/bile as waste....so blood levels of chol. decline at high thyroid levels |
|
|
What are animals with diabetes prone to UTI?
|
tract has lots of sugar in it
|
|
|
Why wud urea (in plasma) increase in Hyperthyroid cat?
|
high; more proteolysis -> more deamination of proteins, more conversion to urea
liver not functioning well? |
|
|
which type of diabetes most common in cats?
|
DM type 2
|
|
|
TSH level in hyperthyroid cat?
|
low, b/c T4 inhibits APG?
|
|
|
how would u treat DM type 1?
|
give insulin
|
|
|
Dog got contraceptive treatment
(progesterone) for the past 2 years. Owners calls b/c heard that bitch might develop serious side effects causing her joints or bones to “grow funny”. Which condition have these owners heard about? . In this case, what causes this condition? |
Acromegaly
Progesterone-increased GH, can affect joints. Long standing progesterone levels cause GH to be released, if she is beyond sexual maturity, bone epiphysis are closed, so bones would growth in width, not length |
|
|
Why do u have to inject insulin?
|
have to inject all peptide hormones otherwise get degraded by the acid in stomach, etc.
|
|
|
List / explain the clinical signs that can be associated with Acromegaly
disorder? |
Acromegaly or hypertrophy of extremities, frontal bone and mandible (membranous bones), increased interdental space (loss of teeth), increase muscle growth. Arthrosis & arthritis due to increase in CARTILAGE growth throwing off statics of joint.
Fluid retention due to a build up of proteins circulating in blood; increases osmoregularity. Secondary diabetes could develop due to progesterone’s increase in insulin resistance. This leads to hypertrophy of heart. |
|
|
why are steroid able to taken as pills?
|
lipophilic
|
|
|
progesterone is normally released when?
|
In pregnant bitch, progesterone released during pregnancy; stimulates hypothalamus to produce GH-RH (growth hormone releasing hormone). Growth hormone makes pregn.bitch slightly insulin res. (hyperglycemic) which ensures fetus gets adequate glucose, etc. Remember fetus counts as glucose dependent tissue!
|
|
|
what maintenance diet wud you feed animal with diabetes?
|
high in quality protein, low soluble carbs, low FA (b/c often obesity is cause, and leads to fatty liver), high in fiber
|
|
|
Why are young, growing animals usually thin?
|
GH is lipolytic hormone
growing animal need protein for muscle/bone development; not fat |
|
|
Which type of diabetes is potentially reversible?
|
diabetes type 2
|
|
|
Tropic effect of GH?
|
Tropic/indirect effects (inducing release of another hormone from target tissue) on liver to produce "somatomedin" aka Insulin-like Growth factors
These are IGF effects are typical protein ANABOLIC effects, mitotic rates, A.A. uptake, |
|
|
how would you treat T2 diabetes to begin recovery process and why?
|
would give insulin along with exercise so BW reduced; this gives exhausted beta cells in pancreas (have churning out extra insulin) a chance to recover and make insulin again on own
|
|
|
Direct effect of GH?
|
* stimulates hormone sensitive lipase, to force body to use fat as energy source
* in contrast spares glucose (excess can lead to insulin resistance - DM type 2!) for unknown reason, and spare protein |
|
|
What must be functional in animal to recover?
(unanswered questions) |
* Beta cells can't be completely shot
* Need at least some insulin receptors on insulin dep. tissue >But aren't receptors the source of problem with T2?? |
|
|
Where are Somatotropins made?
|
Growth hormone (=Somatotropin) is an anterior pituitary (protein) hormone, produced by acidophils
|
|
|
What is course of action if animal recovers from T2 diabetes? (What would you watch for?)
|
* animals blood glucose levels must monitered closely
* if beta cells making insulin again, must reduce or stop insulin supplements * otherwise can become hypoglycemic |
|
|
Where are somatomedins made?
|
liver produces
somatomedins (or insulin-like growth factors = IGF) upon stim. from GH (somatotropin) |
|
|
Could you cure/reverse type 1 diabetes?
|
Theoretically could do pancreas transplant, but normally only done in human medicine
(remember in type 1 is absolute of insulin, so beta cells not functional at all) |
|
|
True or False:
Somatotropin is hyperplastic & hypertrophic |
True
Somatotropin is growth hormone |
|
|
How treat diabetes type 3?
(unanswered question) |
Secondary DM or Type III ( TRANSIENT DM)
* high levels of other hormones like cortisol, or glucagon * caused by DM Type II; insulin resistance Does it fix itself? What is connection between cortisol and insulin/hyperglycemia? |
|
|
GROWTH HORMONE DEFICIENCY in young dog?
|
Dwarfism: inherited deficiency of GH and/or somatomedins
delay in physical maturity, keep puppy coat |
|
|
If glucagon levels really HIGH, which metabolic pathway favored?
|
lipolysis (2.3.1)
|
|
|
GROWTH HORMONE DEFICIENCY if occurs later in life ?
|
Early Aging
caused by: damage to pituitary cells (tumor,hemorrhage, radiation) OR if have liver disease -> no IGF also early aging |
|
|
Where is glucagon made?
|
secreted in pancreatic
α cells AND stomach <- gut glucagon |
|
|
Panhypopituitarism
|
tumor,hemorrhage, radiation usually don't only affect cells producing GH
none of hormones produced in ant. pituitary gland are made |
|
|
Half life of glucagon?
|
5 minutes (it's a peptide - shorter half life than steroid, b/c proteases are everywhere)
|
|
|
IS there any overlap in the function of mineralcorticoids and Gluco-
corticoids? |
Yes, Cross activity exists between some members of the mineralocorticoid and
glucocorticoid group |
|
|
How are pancreatic alpha cells and beta cells different aside from the hormones they make?
|
* Beta cells are insulin independant (they just make it)
* alpha cells are insulin dependent |
|
|
True or False:
Each cortical zone is controlled by a separate mechanism ! (Zona glomerulosa, Zona fasciculata) |
True, but some cross reactivity
|
|
|
What happens when insulin binds alpha cells?
(unanswered question) |
glucagon secretion stops being released
(remember peptide hormones stored, so inhibiting synthesis..i dont think) |
|
|
Corticotropin aka ?
|
Corticotropin or ACTH
|
|
|
Name two most common health problems affects in ferrets:
|
1. Adrenal disease - excess sex steroids
2. Insulinoma - tumor in beta cells of pancreas |
|
|
Stress stimulates hypothalamus to release
|
Corticotropin Releasing Hormone
|
|
|
True or False: Regarding adrenal disease in ferrets, characterized by rise in cortisol & aldersterone.
|
False!
Ferrets w/adrenal disease have excess sex steroids (estrogens > androgens); but cortisol & aldosterone are normal |
|
|
CRH stimulates release of ___ from ___?
ACTH stimulates release of ____ |
CRH stimulates release of CORTICOTROPIN = ACTH from anterior pituitary
Cortisol (and androgens) |
|
|
Symptoms of Adrenal disease in ferrets?
|
Ferret with Adrenal Disease:
estrogens > androgens: symptoms include alopecia, enlarged vulva, prostate hypertrophy, sexual aggression, bone marrow depression (anemia) |
|
|
Main stimuli which lead to release of cortisol?
|
stress (epinephrine)
metabolic stress (hypoglycemia) pain, fever, inflammatory mediators - histamine, pyrogens |
|
|
True or False: Insulinoma in ferrets causes rapid increase in blood glucose
|
False!!!
Insulinoma in ferrets causes rapid DROP in blood glucose because more insulin secreted |
|
|
How does cortisol help bring up blood glucose?
(remember hypoglyc. is one of stimuli for cortisol release) |
These are anabolic effects of glucocorticoids!
>need to provide fuel (glucose) to muscles/brain with energy 1. mostly gluconeogenesis (from amino acids) 2. strangely glycogenesis also because gluconeogenesis generates so much glucose in liver; some goes to storage* (*Prerequisite for glucagon’s glycogenolytic effects) |
|
|
Where do anabolic effects of glucocorticoids take effect?
|
gluconeogenesis in LIVER
|
|
|
Besides stress-related release, ACTH & Cortisol secretion also follow a circadian rhythm:
In dog cortisol highest in AM or PM? In cat cortisol highest in ? |
AM - highest activity level
PM - higher cortisol for cat |
|
|
Cortisol exerts negative feedback on ___ release
|
Cortisol exerts negative feedback on CRH (hypoth.) and ACTH (apg) release
FYI: CRH is CORTICOTROPIN (ACTH) releasing hormone |
|
|
Why might artificial or synthetic corticoids be used clinically?
|
fewer side effects (cross reactivity) than natural steroids
|
|
|
Glucocorticoids/CORTISOL promote Gluconeogenesis
(from amino acids); but where do AA's come from? |
* liver supplies some
* but mostly via proteolysis from peripheral tissues/muscle (not CNS & cardiac muscle) |
|
|
Name other catabolic affects of Glucocorticoids/CORTISOL:
Where does this occur? |
Extra-hepatic tissue
=========================1. Decreased Glucose uptake (in muscle and fat tissue = insulin resistant effect); muscle must rely on fat 2. Increased Proteolysis 3. Lipolysis Redistribution of fat (abdomen) |
|
|
Animal under high-standing Glucocorticoids (for long time), what happens to liver?
|
liver enlarges, becomes full of glycogen and fat
|
|
|
What happens to GH production in older animals?
Why significant? |
decreased GH production
prof. says mitotic tissues are not maintained -> tissue aging...whatever just go along with it |
|
|
glucocorticoids’ effects on GI?
|
HCl and appetite up; but mucus down
GC's are also anti-inflammatory unfortunately so PGE2 and all prostaglandins down, leads to ulcers |
|
|
glucocorticoids’ effects on blood glucose?
|
* hyperglycemia;
* insulin resistance -> steroid diabetes aka secondary diabetes fyi: called b/c caused by GC's which are steroids |
|
|
glucocorticoids’ effects on CNS/ apg?
|
drop in synthesis of all these: ACTH,ADH,TSH,GH,
FSH/LH (leads to "Panhypopituitarism") |
|
|
2 types of neutriphils?
|
circulating pool
marginal pool |
|
|
glucocorticoids’ effects on LEUCOGRAM?
|
Lymphos & Eos numbers drop
Neutriphil (from marginal pool) increases in number in circulation platelet number increases RBC number increases |
|
|
Is the overall amount of neutriphils increasing in number?
|
No, neutrilphils from marginal pool avoid selectin-dependent capture due inhibition of binding by cortisol; consequently more neutriphils enter from marginal pool
|
|
|
Glucocorticoids prevent release of inflammatory mediators, e.g. from ?
|
from lysosomes, macrophages,
monocytes |
|
|
physiological controller of inflammatory processes
|
Glucocorticoids
|
|
|
Glucocorticoids reduce / prevent inflammations and resolve existing inflammations, probably by ?
|
deactivating inflammatory mediators
|
|
|
pre-pro-hormone of ACTH is called ?
|
Pro-Opio-MelanoCortin = POMC
|
|
|
When GC's enter cell, induce protein synthesis of ____, group of intracellular proteins which bind to trx. factors like nuclear factor kappa B to inhibit synthesis of interleukin
|
lipocortin, protein which inhibit enzymes (like phospholipase A2 and COX1/2) in cytoplasm which convert arachdonic acid to prostanglandins
|
|
|
NF kappa B is inhibited by ___?
|
cortisol receptor complex
|
|
|
True or False
Collagen synthesis increased by Glucocorticoids |
False; Collagen synthesis (part of wound healing) is inhibited by GC's
|
|
|
Which type of immune response is affected or inhibited to a greater extent: by glucocorticoids: cell mediated or humeral?
|
cell mediated immune response (T cells)
|
|
|
The cleaving of POMC gives rise to several active polypeptides:
|
1. α-MSH ( Melanocyte Stimulating Hormone)
2. β-ENDORPHIN (another stress factor to reduce incoming pain) 3. CLIP = Corticotropin-Like Intermediate Peptide 4. ACTH - controls release of Cortisol - is also crucial to maintain the viability of the entire adrenal cortex !! |
|
|
α-MSH does what?
overproduction of MSH? |
darkening of skin/mucosa
dark blotches on mucosa! |
|
|
CLIP function?
|
Not much - probably little physiological function; but pathological effects in horses similar to ACTH (aka Corticotropin)
|
|
|
ACTH another piece cleaved off from POMC is important why?
|
In addition to controls release of Cortisol,
ACTH required to maintain viability of entire adrenal cortex!!!! Disorders of ACTH production can affect all POMC compounds !! Also req. for production of mineralcorticoids? |
|
|
What might cause drop in ACTH production for long period of time?
What must been considered when treating with GC's? |
if animal treated for long period of time with GC's, tissues which make ACTH can shut down completely - entire adrenal cortex is in jeopardy
|
|
|
Hyperadrenocorticism aka?
|
Cushing’s Syndrome
excess of glucocorticoids |
|
|
Causes Cushing’s Syndrome aka Hyperadrenocorticism ?
|
1. Adenomas of adrenal glands -> excess cortisol (rare)
2. Adenomas of ant.pituitary 3. Iatrogenic = prolonged glucocorticoid therapy 4. HORSE: Pituitary Pars intermedia tumors produces P.O.M.C (and excess amounts of active metabolites) |
|
|
Which Adenomas of Cushing's is more common?
What is pathway here? What is it in excess here? |
Adenomas of ant.pituitary gland more common
Adenomas of ant.pituitary -> excess ACTH -> cortisol (common) |
|
|
Cushing’s Syndrome caused by prolonged GC treatment?
|
Latrogenic Cushing's
|
|
|
Adenomas of adrenal glands: what is pathway here?
|
Adenomas of adrenal glands -> excess cortisol (rare)
|
|
|
____ is thought to increases the ACTH efficiency 6x
|
CLIP
|
|
|
90% are ___?
|
90% are pituitary-dependent = PDH;
|
|
|
What animals is Cushings common in?
|
common in middle-aged to old dogs (boxer, poodle, terrier,dachshund), also in ponies (not horses), very rare in cats
|
|
|
PATHOPHYSIOLOGY of hyperadrenocorticism?
|
PROTEOLYSIS, LIPOLYSIS, (like in diabetes), redistribution of fat, Hepatomegaly (enlarged liver), DM3 in cats&horse (see section for symptoms!), PU/PD, ADH receptor gone, so can't reabs. H2O
|
|
|
HIRSUTISM
unusual because? |
slow hair shedding
in horses b/c in dog lose hair, color of fur gone |
|
|
These are all symptoms of final stages of what?
IMMUNODEPRESSION POLYPHAGIA PITUITARY GLAND DEPRESSION (TSH,FSH,LH, GH) -> Panhypopituitarism BEHAVIORAL CHANGES (docility in horses caused by endorphins) GASTRIC ULCERS HYPERPIGMENTATION (sometimes, if ACTH ) LAMINITIS / Horses |
Cushings - can get everything
e.g. DM3, hypothyroidism, reproductive failure (lack of LH, FSH), early aging (GH) |
|
|
TREATMENT of PDH
|
(Surgical removal of tumors; difficult so usually only in people)
> Preferred method via suppression of adrenocortex with mitotane (poison) therapy (=DDD insecticide) selectively suppress/destroy from of adrenal cortex * enz. inhibitors also available (safer) ~showed pics of dog whose fur grew back |
|
|
"Moon Face" =
|
Cushings Disease
|
|
|
How might Cushings lead to LAMINITIS / Horses ?
|
In Theory:
hypoglycemia ->Endothelins release -> vasoconstriction -> damage/hypoxia in digital joints -> LAMINITIS / Horses |
|
|
Main stimulus for ALDOSTERONE synthesis?
|
HYPOVOLEMIA & Low Blood Pressure -> Renin (released in kidney) -> converted to Angiotensin -> ALDOSTERONE
|
|
|
Other stimuli of leading to aldosterone secretion?
|
* Is also stimulated by HYPERKALEMIA
* moderately stimulated by hyponatremia (low Na+) |
|
|
WHICH tissues respond to aldersterone?
|
large intestine, salivary glands, sweat glands
|
|
|
If lack aldersterone?
Too much alderstorone? |
loss of Na+ and water, PU/PD
blood volume increases, preload incr., BP up |
|
|
Most powerful method of reducing excess BP?
|
diuresis
|
|
|
Main cause of (Hyperaldosteronism)
|
* Causes?
1. any condition which cause polyuria 2. infarction of kidney (kidney thinks it's hypervolemic but isn't) 3. Main cause -> cyst formation in kidney (polycystic renal disease = PRD) grow over time, pressure up (a.) with renal dysfunction (b.) w/o RD |
|
|
renin is vasodilator?
|
no vasoconstrictor and become angiotensin blas
|
|
|
HYPOADRENOCORTICISM =
which hormones low? |
ADDISON’S DISEASE
= deficiency in adrenal cortex hormones -> Glucocorticoids (first) and Mineralocorticoids (later) are reduced |
|
|
Why concerned about hypertension?
|
endothelial lining, brain damage, etc
|
|
|
What happens to K+, Na+, Ca+?
|
* also lost , so many + charges get metabolic alkalosis
* hypokalemic outside leads to farther diffusion of K+ out * leads to hyperpolarization of membranes, stops transmission (mild alkalosis stimulates Na/K pumps) -> weakness |
|
|
What animals is HYPOADRENOCORTICISM = ADDISON’S DISEASE more common in?
- specifically type involving immune mediated destruction of cortex |
(youngish fem. dogs)
- immune mediated destruction of cortex |
|
|
Other causes of HYPOADRENOCORTICISM = ADDISON’S DISEASE?
|
2. OVERTREATMENT for Cushings - mitotane induced adreno- cortical necrosis (mitotane therapy)
3. - long standing cortisol therapy -> depression of ACTH -> cortex atrophy > GLUCOCORTICOID Deficiency -> hypoglycemia, can't control inflammatory processes > MINERALOCORTICOID Deficiency: |
|
|
most powerful hormone controlling
plasma calcium and phosphate levels via: |
PTH is the most powerful hormone controlling
plasma calcium and phosphate levels via: |
|
|
What does aldersterone do?
|
Aldosterone stimulates: Na/K pump
- Sodium absorption - Potassium & hydrogen looser - Aldosterone stimulates renal H+ excretion (via Na+ / H+ exchanger) |
|
|
MINERALOCORTICOID Deficiency:
leads to? |
(NO ALDERSTERONE)
Na+ & WATER loss, K & hydrogen retention -> Na/K plasma ratio less than 20 (normal 30-40) (so too much K+!!!!!) |
|
|
Messed up Na/K+ ratio is a sign of? (low K+)
|
HYPOADRENOCORTICISM = ADDISON’S DISEASE
|
|
|
What stimulates release of PTH?
|
1.Hypocalcemia:
2. Hyperphosphatemia: |
|
|
Net effect of PTH
|
Plasma Calcium increases
Plasma Phosphate DECREASES |
|
|
PTH stimulates?
|
1. Stimulates bone mobilization (resorption) = increases plasma Ca and P
2. Stimulates renal calcium absorption = increases plasma Ca 3. Inhibits renal phosphate absorption = decreases plasma P 4. Stimulates activation of Vit. D = increases plasma Ca via GI absorption |
|
|
D-Hormone =
|
Calcitriol
|
|
|
Activation to D hormone occurs
in ? Requires? |
liver and kidney
PTH |
|
|
Calcitriol actions?
|
Actions:
1. increases intestinal and renal calcium and phosphate absorption 2. increases mobilization of bone (in high concentrations) |
|
|
Calcitonin stim. by?
|
- Stimulated by hypercalcemia
- Decreases activity and proliferation of osteoclasts |
|
|
Calcitonin favors __formation?
|
Favors bone formation
Decreases calcium and phosphate levels |
|
|
Calcitonin seems more important in what age group?
|
Calcitonin seems more important in young animals than adults and the
removal of thyroid gland / C cells has little effect on Ca balance |
|
|
Calcitonin secreting C-cells located where?
|
between thyroid follicle cells
|
|
|
Compensatory Hyperparathyroidism
|
1. Lack of dietary Vit D or lack of UV exposure -> reduced Ca absorption -> crooked legs
2. Dietary Ca/P imbalances 3. Renal Diseases with insufficient P excretion |
|
|
In all 3 cases:
|
body releases
PTH mobilizes Ca from bone tissue plasma Ca returns to low/normal if long-term, leads to softening of bone = DEMINERALIZATION = Compensatory Hyperparathyroidism |
|
|
Bran Disease or Big Head in Horses
|
Dietary hyperparathyroidism
- on pasture grasses high in oxalates, which binds Ca - on high bran diet = excess P |
|
|
6 months old beagle / Rubberjaw caused by?
|
Renal Hyperparathyroidism
|
|
|
milk fever
|
Parturient Paresis
Acute hypocalcemia within 72 hrs p.p. due to rapid loss of Ca via milk |
|
|
Why doesnt PTH release help?
|
PTH is released, but osteoclasts are reduced
in numbers or inactive due to dry period; takes time for them to reactivate -> Bone cannot respond fast enough -> acute hypocalcemia |
|
|
Signs of milk fever?
|
increased conductivity of Na channels -> excitability, tremors, seizures, tachycardia
- laccid paresis (may be caused / exacerbated by concomitant Mg increase) |
|
|
Prevention?
|
feed diet low in Ca+ just before animal gives birth, to force reactivation of osteoclasts!
|
|
|
where does the liver get the precursors for Gluconeogensis?
|
aa (precursors ) from muscle break down (or stored aa pool)
|
|
|
main effects of cortisol
|
provide Glucose for blood via gluconeogen. and excess Glycogen gets stored - ironic but TRUE!
|
|
|
what is the precursor of ACTH?
|
POMC - Pro-Opio-MelanoCortin
|
|
|
cleaving of POMC gives rise to several active polypeptides
|
Pro-Opio-MelanoCortin -
alpha-MSH beta endorphin CLIP ACTH |
|
|
what hormone is necessary to keep the adrenal cortex alive?
|
ACTH**
|
|
|
if an animal has been given long term exogenous cortisol (for years), can the adrenal cortex release Glucocorticoids?
|
no
|
|
|
excess of Glucorticoids eventually leads to what dz?
|
Cushings dz
|

