![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
512 Cards in this Set
- Front
- Back
|
from what are the male primordial germ cells derived and to where do they migrate?
|
* Derived from yolk sac endoderm
* Migrate to genital ridge |
|
|
from what are sertoli cells derived?
|
primitive sex cords
|
|
|
from what are leydig cells derived?
|
mesenchyme of genital ridge
|
|
|
what cells make and secrete antimullerian hormone?
|
sertoli cells
|
|
|
what cells secrete testosterone and IGF-3 in males?
|
leydig cells
|
|
|
when are testosterone levels high in males?
|
(1) In utero
(2) Shortly after birth (3) Adulthood (between puberty and senescence) |
|
|
when does spermatogenesis begin?
|
at puberty
|
|
|
what occurs in the proliferative phase of spermatogenesis?
|
Spermatogonia give rise to spermatocytes and regenerate
|
|
|
what occurs in the meiotic phase of spermatogenesis?
|
primary spermatocytes undergo maturation and division and reduce chromosome number by 1/2
|
|
|
what occurs in the spermiogenic phase of spermatogenesis?
|
metamorphosis of spermatids into mature spermatozoa
|
|
|
in what compartment does proliferative phase of spermatogenesis take place?
|
basal compartment
|
|
|
in what compartment does meiotic phase of spermatogenesis take place?
|
adluminal compartment
|
|
|
in what compartment does spermionic phase of spermatogenesis take place?
|
adluminal compartment
|
|
|
what are the three type of spermatogonia in proliferative phase (in order)
|
Type A dark (Ad): dense chromatin
Type A pale (Ap): chromatin less dense Tybe B |
|
|
what spermatogonia give rise to primary spermatocytes?
|
tybe B spermatogonia
|
|
|
what is the ploidy of primary spermatocytes?
|
diploid (4N)
|
|
|
what is the ploidy of secondary spermatocytes?
|
haploid (2N)
|
|
|
what is the ploidy of spermatids?
|
haploid (N)
|
|
|
in what phase of spermiogenesis do acrosome granules fuse and tail filaments appear?
|
golgi phase
|
|
|
in what phase of spermiogenesis does the head cap appear from acrosomal granule?
|
cap phase
|
|
|
in what phase of spermiogenesis does the nucleus and heap cap elongate, and acrosomal granule differentiate to form acrosome
|
acrosome phase
|
|
|
in what phase of spermiogenesis does cell complete differentiation?
|
maturation phase
|
|
|
what happens during the 1st cycle of seminiferous epithelium?
|
Type A gives rise to Type B spermatogonia
|
|
|
what happens during the 2nd cycle of seminiferous epithelium?
|
primary spermatocytes of the first cycle form
|
|
|
what happens in the 3rd cycle of seminiferous epithelium?
|
spermatids of first cycle appear
|
|
|
what happens in 4th cycle of seminiferous epithelium?
|
spermiogenesis is completed and spermiation begins
|
|
|
how many days for development of mature sperm?
|
64 days
|
|
|
how many days for movement of sperm to epididymis from lumen?
|
10 days
|
|
|
what are the 5 general functions of sertoli cells?
|
(1) Support and nutrition
(2) Movement and release of germ cells (3) Phagocytic function (4) Secretory function (5) Blood-testis barrier |
|
|
what cells provide nutrients to developing germ cells in males?
|
Sertoli cells
|
|
|
what cells act as a "bridge" to bring substances from capillaries to developing germ cells?
|
Sertoli cells
|
|
|
what process is defined as the release of mature sperm from the Sertoli cells?
|
Spermiation
|
|
|
what cells ingest degenerating germ cells and residual bodies in males?
|
Sertoli Cells
|
|
|
what are the names of the 4 secretory products of Sertoli cells?
|
(1) Tubule fluid
(2) Androgen Binding Protein (APB) (3) Aromatase (4) Inhibin |
|
|
what is the function of tubule fluid?
|
transports spermatozoa to epididymis and provides substrates for sperm survival
|
|
|
what is the function of androgen binding protein?
|
binds testosterone and dihydrotestosterone to increase concentration of androgens available to germ cells
|
|
|
what hormone stimulates secretion of androgen binding protein in males?
|
FSH
|
|
|
what is function of aromatase in males?
|
converts androgens to estrogens
|
|
|
what is function of inhibin secreted by Sertoli cells?
|
specifically inhibits release of FSH from pituitary
|
|
|
what cells divide the testis into basal and adluminal compartments?
|
sertoli cells (make up the blood-testis barrier)
|
|
|
which testicular compartment is accessible to blood-borne components?
|
Basal compartment is accessible to blood-borne components
|
|
|
on what hormone is maturation of spermatogonia to immature spermatids dependent?
|
FSH
|
|
|
what hormones stimulate maturation of spermatids to spermatozoa?
|
androgens (LH-dependent)
|
|
|
what hormone stimulates androgen synthesis in males?
|
LH
|
|
|
what two hormones are both required for regulation of spermatogenesis?
|
FSH
Testosterone |
|
|
what hormone binds to Sertoli cells to help initiate spermatogenesis?
|
FSH
|
|
|
with respect to testosterone production, how does FSH impact Leydig cells?
|
FSH increases the number of LH receptors on Leydig cells, increasing testosterone production (which maintains spermatogenesis)
|
|
|
which part of sperm contains mitochondria?
|
midpiece
|
|
|
what is the MT structure of axoneme?
|
9 doublets + 2 singlets in the middle (9+2 configuration)
|
|
|
what is the structure of each axoneme microtubule doublet?
|
* 2 subfibers, A and B
* Subfiber A has two dynein arms (inner and outer) that extend towards subfiber B * Central singlets are surrounded by a central sheath * Radial spokes extend from the central sheath to subfiber A |
|
|
what is the pathophysiologic cause of Kartagener's syndrome?
|
absence of dynein arms leads to immotile cilia
|
|
|
what is asthenozoospermia?
|
decreased sperm motility
|
|
|
what is necrozoospermia?
|
dead sperm
|
|
|
how long does it take sperm to migrate through epididymis?
|
10-16 days
|
|
|
With respect to sperm and sperm migration, how long for injury to testis to be seen?
|
3 months
|
|
|
where do sperm acquire capacity for motility?
|
in epididymis
|
|
|
where are sperm stored?
|
cauda epididymis and vas deferens
|
|
|
what causes release of spermatozoa just prior to ejaculation?
|
emission, caused by adrenergic contractions of cauda epididymis and vas deferens smooth muscle cells
|
|
|
what are the 4 male sex accessory glands?
|
(1) Ampulla of vas deferens
(2) Cowper and Littre glands (3) Prostate (4) Seminal vesicles |
|
|
what is the order of activity of the 4 sex accessory glands during ejaculation?
|
(1) Cowper/Littre
(2) Prostate (3) Ampulla and epididymis (containing sperm) (4) Seminal vesicles |
|
|
what is the contents and function of Cowper's gland secretion?
|
* Clear fluid rich in mucoproteins (may contain small amounts of sperm)
* Lubricates distal urethra |
|
|
what type of pituitary cells are acted on by TRH?
|
thyrotrophs
lactotrophs |
|
|
what are the 2 broad actions regulated by T3 and T4?
|
protein synthesis
thermoregulation |
|
|
how does TRH cause TSH release?
|
TRH causes TSH release via activation of phospholipid hydrolysis in thyrotropes, leading to activation of protein kinase C and release of calcium from intracellular stores
|
|
|
what type of GPCR is the TRH receptor on thyrotropes?
|
Gq type GPCR that increases intracellular calcium via PKC mechanism
|
|
|
what results from the binding of TSH to receptors on thyrocytes?
|
(1) thyroid hormone synthesis occurs, including iodide uptake
(2) release of thyroid hormone |
|
|
what amino acid is the precursor for the two thyroid hormones?
|
tyrosine
|
|
|
what mediates the uptake of iodide into the thyrocytes?
|
sodium-iodide symporter (NIS)
|
|
|
how does NIS import iodide into thyrocytes?
|
NIS actively cotransports Na+ with I-, driven by the electrochemical sodium gradient generated by the Na/K ATPase
|
|
|
where is NIS expressed?
|
thyroid
salivary glands gastric mucosa lactating mammary gland |
|
|
what results from mutations to the NIS gene?
|
hypothyroidism due to congenital iodide transport defect
|
|
|
what is the inheritance pattern of congenital iodide transport defect caused by mutation in the NIS gene?
|
autosommal recessive
|
|
|
what is pendrin?
|
anion transporter that is expressed in the inner ear, thyroid, and kidney
|
|
|
what are the symptoms of Pendred Syndrome?
|
sensorineural deafness
goiter impaired iodide organification |
|
|
where in thyroid follicular cells is Pendrin expressed?
|
the apical membrane
|
|
|
what is the function of pendrin in thyroid follicular cells?
|
transports iodide into the follicle lumen
|
|
|
what is the mechanism of action of antithyroid drugs?
|
blocks iodination of thyroglobulin
|
|
|
what is organification with respect to thyroid?
|
synthesis of T3 and T4, and storage in thyroglobulin
|
|
|
what two chemicals block active transport of iodide into thyrocytes?
|
ClO4 -
SCN- |
|
|
what is the Wolff-Chaikoff effect?
|
* Reduction of thyroid hormone synthesis following administration of large amounts of inorganic iodide
|
|
|
What is the theorized mechanism behind the Wolff-Chaikoff effect?
|
the reduction of TH synthesis is due to down-regulation of the iodide pump (NIS)
|
|
|
how long does the Wolff-Chaikoff effect last?
|
about 10 days
|
|
|
how does thyroid hormone enter target cells?
|
energy dependent transport mechanisms such as the SLC16A2 gene product
|
|
|
what gene is related to Allan-Herndon-Dudley syndrome?
|
SLC16A2 gene
|
|
|
what is Allan-Herndon-Dudley Syndrome?
|
X-linked mental retardation and hypotonia related to mutations in the SLC16A2 gene involved in uptake of thyroid hormone into target cells
|
|
|
which thyroid hormone has higher biological activity?
|
T3
|
|
|
where and how is T3 primarily generated?
|
at the target sites by mono-deiodination of T4
|
|
|
what cofactor is required by the various deiodinases?
|
selenium
|
|
|
where is Type 1 DIO found and what is its function?
|
liver and kidney cell membranes
generates most of the circulating T3 |
|
|
what is the function of Type 2 DIO?
|
converts T4 to T3
|
|
|
what is the function of Type 3 DIO?
|
inactivates T4 and T3
|
|
|
what regulates tissue responsiveness to thyroid hormone?
|
relative activities of Type 2 DIO and Type 3 DIO
|
|
|
through what type of receptors do thyroid hormones act?
|
heterodimeric nuclear receptors, typically in association with RXR
|
|
|
how does thyroid hormone effect the heart?
|
* Positive chronotropic effect: increases number and affinity of beta-adrenergic receptors
* Positive inotropic effect: enhances responses to circulating catecholamines and increases production of myosin heavy chains with higher ATPase activity |
|
|
what is Plummer syndrome?
|
* Toxic multinodular goiter
* Functioning follicular adenoma (usually benign) hypersecreting T3/T4, causing hyperthyroidism |
|
|
what is the cause of most cases of Plummer syndrome?
|
TSH-R mutations
|
|
|
is Plummer syndrome associated with hyper- or hypo- thyroidism?
|
hyperthyroidism
|
|
|
is Grave's disease associated with hyper- or hypo-thyroidism?
|
hyperthyroidism
|
|
|
how is gestational trophoblastic disease caused by TSH-R activation?
|
In molar pregnancy and choriocarcinoma, excess levels of hCG activate the TSH-R and result in gestational trophoblastic disease
|
|
|
what is the inheritance pattern of TSH resistance?
|
autosomal recessive
|
|
|
what are the relevant blood levels in regards to TSH Resistance?
|
Normal to low free-T4 levels
Elevated TSH |
|
|
what is TSH resistance?
|
Autosomal recessive condition involving loss of function mutation in TSH-R, leading to thyroid hypoplasia, normal-low free-T4, and elevated TSH levels
|
|
|
name two disorders of TSH-R gain of function mutations
|
* Congenital hyperthyroidism (autosomal dominant)
* Thyroid adenoma (somatic) |
|
|
what happens in Acute Thyroiditits?
|
Inflammation of the thyroid tissue leads to leakage of TH into circulation, resulting in acute thyrotoxicosis that lasts several weeks until thyroid stores are exhausted
|
|
|
what is the most common cause of hypothyroidism?
|
Hashimoto thyroiditis
|
|
|
is Hashimoto thyroiditis associated with hyper- or hypo-thyroidism?
|
hypothyroidism
|
|
|
what is Hashimoto thyroiditis?
|
Autoimmune disorder involving antibodies against several thyroid components, leading to destructive inflammation of the thyroid gland
|
|
|
what is myxedema?
|
deposition of mucopolysaccharides in the skin, usually resulting from hypothyroidism, and causing swelling of affected areas
|
|
|
what is the most common autoimmune disorder in women?
|
Autoimmune thyroid disease (AITD)
|
|
|
what is the most frequent cause of thyroid dysfunction?
|
Autoimmune thyroid disease (AITD)
|
|
|
what is Autoimmune Thyroid Disease (AIDT)
|
* Most common autoimmune disorder in women
* Most frequent cause of thyroid dysfunction |
|
|
what is hypothyroid cretinism?
|
* Condition resulting from congenital or nutritional hypothyroidism that severely impacts the growth and development in the young
* Can be at the level of the hypothalamus, pituitary, or TH synthesis * Untreated, it leads to poor CNS and bone development |
|
|
how does iodine demand change with pregnancy?
|
During pregnancy, demand for iodine is significantly higher that that of nonpregnant women
* Increased demand results from increased TH production, fetal thyroid use of iodine, and increased renal iodine clearance |
|
|
how does hypothyroidism impact cerebellar development?
|
negatively impacts purkinje cell differentiation
|
|
|
what are the multiple endocrine neoplasia syndromes?
|
* Autosomal dominant inheritance disorders
* MEN 1 (Wermer's syndrome) * MEN2a (Sipple syndrome) * MEN2b (other mutations in RET gene) |
|
|
what is Wermer's syndrome?
|
* Multiple endocrine neoplasia syndrome 1 (MEN1) in which there is a mutation in the MENIN tumor suppressor gene
* Primary hyperparathyroidism * Pancreatic tumors * Pituitary tumors |
|
|
what is sipple syndrome?
|
* MEN2a
* Mutation in RET gene * Medullary carcinoma of thyroid * Pheochromocytoma * Primary hyperparathyroidism * Hirschsprung's megacolon |
|
|
what drives ACTH and cortisol?
|
pulsatile and circadian CRH release
|
|
|
through what receptor does ACTH primarily act to elevate glucocorticosteroidogenesis?
|
melanocortin-2 receptor
|
|
|
what pathway is used by melanocortin-2 receptor upon binding of ACTH?
|
cAMP/PKA pathway
|
|
|
what is the major site of ACTH and AngII action?
|
formation of delta-5-prognenolone
|
|
|
what is the rate limiting part of the biosynthesis of adrenal steroids?
|
StAR expression
|
|
|
how does hydrocortisone/cortisol impact permeabilityof capillaries?
|
decreases permeability
|
|
|
what is the impact of cortisol/hydrocortisone on leukocyte migration?
|
decreases leukocyte migration
|
|
|
what is the impact of cortisol/hydrocortisone on T cell function?
|
supresses t-cell function
|
|
|
what syndrome describes the body's short-term and long-term reactions to stress?
|
general adaptation syndrome
|
|
|
what type of receptors are used by cortisol to regulate transcription of various genes?
|
homodimeric nuclear receptors
|
|
|
who was the first to identify the possible link between stress and disease?
|
Hans Selye
|
|
|
what serves as the substrate for placental estrogen synthesis and may also play a role in pubertal onset?
|
dehydroepiandrosterone (DHEA)
|
|
|
how does aldosterone impact sodium and potassium movement in the renal tubular epithelial cells?
|
aldosterone increases sodium resorption and potassium excretion
|
|
|
what is the other name for pseudohyperaldosteronism?
|
Liddle's syndrome
|
|
|
what causes Liddle's syndrome?
|
constitutive activating mutation in the Na+ channel
|
|
|
what would result from consitutively activating the mineralocorticoid receptor?
|
sever early-onset hypertension
|
|
|
what is the inactivated form of cortisol?
|
cortisone
|
|
|
what converts cortisol to cortisone in the renal tubule cells?
|
11-beta-hydroxy steroid dehydrogenase 2
|
|
|
what is the role of 11-beta-hyroxy steroid dehydrogenase 2?
|
conversion of cortisol to cortisone
|
|
|
what enzyme is inhibited by a chemical in licorice?
|
11-beta-hydroxy steroid dehydrogenase
|
|
|
inactivation of what enzyme leads to apparent mineralocorticoid excess as a result of a compoent in licorice?
|
11-beta steroid hydroxy dehydrogenase
|
|
|
another name for adrenal hypocorticoidism
|
Addison's disease
|
|
|
what is the difference between primary and secondary hypocorticoidism?
|
* Primary hypocorticoidism: adrenal gland is the source of the problem (high ACTH)
* Secondary hypocorticoidism: problem is in pituitary gland (low ACTH) |
|
|
what would be indicated by an abnormal Metyrapone test?
|
secondary adrenocortical insufficiency
|
|
|
what type of adrenocortical insufficiency would be indicated by elevated plasma ACTH?
|
primary adrenocortical insufficiency
|
|
|
what type of adrenocortical insufficiency would be indicated by normal or low plasma ACTH levels?
|
secondary adrenocortical insufficiency
|
|
|
what is the first test for suspected adrenocortical insufficiency?
|
rapid ACTH stimulation test
|
|
|
how is the rapid ACTH stimulation test administered and interpreted?
|
* ACTH is administered and the cortisol produced by the patient in response to that ACTH is measured.
* Abnormal result indicates adrenocortical insufficiency of some type * Normal result will exclude primary adrenocortical insufficiency (bc the ACTH acts on the normal adrenal to make cortisol, which rules out primary adrenal insufficiency) |
|
|
what drug blocks 11-b-hydroxylase?
|
metyrapone
|
|
|
secretions from what gland contain citric acid, acid phosphatase, calcium, and zinc, and enzymes that cause liquefication of ejaculate coagulum?
|
prostate gland
|
|
|
what is the function of prostate-specific antigen with respect to sperm?
|
PSA helps to activate sperm motility by inactivating semenogelin
|
|
|
what is the composition of semen?
|
seminal plasma
semen |
|
|
secretion from what gland is rich in mucoproteins to help lubricate the distal urethra?
|
cowper's gland
|
|
|
absense of fructuose from seminal vesicle fluid is used to diagnose what condition?
|
congenital bilateral absence of vas deferens
|
|
|
secretions from what gland are rich in fructose and prostaglandins, enzymes that cuase coagulation of ejaculate, and semenogelin?
|
seminal vesicles
|
|
|
what gland secretes semenogelin?
|
seminal vesicles
|
|
|
what is the function of semenogelin?
|
sperm motility inhibitor
|
|
|
what is the sperm motility inhibitor secreted by the seminal vesicles?
|
semenogelin
|
|
|
what is the normal intratesticular temperature?
|
34*C
|
|
|
what condition results fom abnormality with pampiniform plexus, can interfer with cooling of testis, and is more commonly found on the left side?
|
varicocele
|
|
|
on which side is varicocele more common and why?
|
Varicocele is more common on the left side, where the pampiniform plexus drains to the left renal vein
|
|
|
how is 60% of androgens transported in the blood?
|
bound to sex hormone-binding globulin (SHBG)
|
|
|
what is sex hormone-binding globulin?
|
glycosylated dimeric protein that is synthesized in the liver, that is homologous to androgen-binding protein secreted by Sertoli cells, that binds to androgens to transport them in the blood
|
|
|
what increases serum concentrations of sex hormone-binding protein?
|
estrogen
thyroxine cirrhosis |
|
|
what decreases serum concentrations of sex hormone-binding protein?
|
exogenous androgens
glucocorticoids GH |
|
|
what hormone is trophic to leydig cells, and increases testosterone?
|
LH
|
|
|
what is the impact of circulating testosterone on LH secretion?
|
testosterone feeds back to inhibit LH secretion
|
|
|
what is the impact of testosterone on FSH secretions?
|
testosterone will only impact FSH secretion in vrey high doses
|
|
|
what is trophic to sertoli cells and increases inhibin B?
|
FSH
|
|
|
what is the impact of inhibin B on FSH secretion?
|
inhibin B feeds back to selectively inhibit FSH secretion
|
|
|
why is damage to sertoli cells associated with marked elevation of FSH?
|
decreased inhibin B secretion by the sertoli cells (inhibin feeds back to inhibit FSH secretion)
|
|
|
what parasympathetic pathways are ultilized in the process of erection?
|
nonadrenergic, noncholinergic pathways (NANC) that release nitric oxide (NO)
|
|
|
what is detumesence?
|
the process of subsiding from erection
|
|
|
what pathway mediates detumescence?
|
sympathetic pathways that release norepinephrine and stimulate alpha-1 adrenergic pathways, leading to contraction of vascular smooth muscle cells
|
|
|
what are the categories of causes of impotence?
|
(1) Neurogenic
(2) Endocrinologic (3) Psychogenic (4) Vasculogenic (5) Drug-induced (6) Miscellaneous |
|
|
what process is defined as the sperm gaining the capacity to penetrate and fertilize the oocyte in vivo?
|
capacitation
|
|
|
what characterizes capacitation?
|
(1) hyperactivated motility
(2) ability to bind zona pellucida (3) ability to undergo acrosome reaction |
|
|
what part of the sperm contains hyaluronidase and proteolytic enzymes?
|
acrosome
|
|
|
what digestive enzymes are contained within the acrosome?
|
hyaluronidase
proteolytic enzymes |
|
|
what type of drug is proplthiouracil and what is it used to treat?
|
antithyroid drug used to treat hyperthyroidism (ie. plummer syndrome)
|
|
|
what type of drug is methimazole and what is it used to treat?
|
antithyroid drug used to treat hyperthyroidism (ie plummer syndrome)
|
|
|
what is a pheochromocytoma?
|
* A catecholamine-secreting neuroendocrine tumor * Arises from the adrenal medulla (chromaffin cells) or from extra-adrenal chromaffin tissue that failed to involute after birth
|
|
|
hormone secreted by zona glomerulosa?
|
aldosterone
|
|
|
hormone secreted by zona fasciculata?
|
glucocorticoids
|
|
|
hormone secreted by zona reticularis?
|
androgens
|
|
|
where gland synthesizes and secretes catecholamines?
|
adrenal medulla
|
|
|
what is the effect of high plasma glucocorticoid levels on CRH secretion by the hypothalamus?
|
high plasma glucocorticoid levels will inhibit CRH secretion from the hypothalamus
|
|
|
what secretes ACTH?
|
anterior pituitary
|
|
|
what secretes CRH?
|
hypothalamus
|
|
|
what are corticotrophs?
|
anterior pituitary cells that secrete ACTH in response to CRH from the hypothalamus
|
|
|
what is the precursor protein present in pituitary corticotrophs, from which various biactive peptides are made by alternate splicing?
|
pro opiomelanocortin (POMC)
|
|
|
what does POMP stand for?
|
pro-opiomelanocortin
|
|
|
what protein is used to take up cholesterol into the mitochondria during steroidogenesis?
|
StAR protein
|
|
|
what is the precursor-intermediate to all steroids in the mitochondria?
|
pregnenolone
|
|
|
what is the main globulin used to transport cortisol in the blood?
|
transcortin
|
|
|
what is transcortin?
|
globulin used to transport cortisol in the blood
|
|
|
what enzyme is critical for steroid synthesis and is also known as the side chain cleavage enzyme?
|
cytochrome p450
|
|
|
within a steroidogenic cell, where is pregnenolone made into progesterone?
|
SER
|
|
|
what is the regulatory site of ACTH and angiotensin II on steroidogenesis?
|
ACTH and Angiotensin II regulate synthesis of Cytochrom p450, which is used in the synthesis of pregnenolone from cholesterol
|
|
|
what are the intermediates in cortisol synthesis from cholesterol?
|
(1) Cholesterol
(2) d5-pregnenolone (3) Progesterone or 17-a-hydroxypregnenolone (4) 17-a-hydroxyprogesterone (5) 11-deoxycortisol (6) Cortisol |
|
|
what reaction is catalyzed by 11-beta-hydroxylase?
|
11-deoxycortisol --> Cortisol
|
|
|
what reaction is catalyzed by 21-hydroxylase?
|
17-alpha-hydroxyprogesterone --> 11-deoxycortisol
|
|
|
steroidogenic reaction catalyzed by cytochrome p450 (side-chain cleavage enzyme)?
|
cholesterol --> delta-5-pregnenolone
|
|
|
what is the rate limiting step of steroid synthesis?
|
cholesterol transfer within the mitochondria involving the StAR protein
|
|
|
what does StAR stand for?
|
steroidogenic acute regulatory protein
|
|
|
how does cortisol impact proteinolysis?
|
cortisol promotes proteinolysis to liberate amino acids for gluconeogenesis
|
|
|
how is gluconeogenesis impacted by cortisol?
|
cortisol promotes gluconeogenesis
|
|
|
why would you lose muscle mass and fat in Cushing's disease?
|
the chroncly high cortisol levels present in patients with Cushing's disease causes breakdown of muscle protein and fat stores to generate free amino acids and fatty acids for gluconeogenesis
|
|
|
how does cortisol impact the action of insulin?
|
* Cortisol impedes the action of insulin by blocking GLUT4 transporters on tissues like muscle tissue in order to keep glucose in the blood
|
|
|
what is the cortisol response to hypoglycemia?
|
in response to low blood glucose, cortisol levels rise to promote gluconeogenesis and inhibit GLUT4 transporters, thereby increasing and stabilizing blood glucose levels within 1-2 hours
|
|
|
mechanism of action of cortisol-based medications?
|
* Anti-inflammatory
* Stabilize lysosomal membranes * Decrease capillary permeability * Decrease leukocyte migration * Suppress T cell function (ie. for autoimmune disease) |
|
|
why would corticosteroids be useful in treating an autoimmune disease?
|
corticosteroids suppress T cell function and decrease leukocyte migration
|
|
|
what does dalton's law describe?
|
total pressure of a mixture of gases is the sum of the individual partial pressures of the gases
|
|
|
what law states that the total pressure of a mixture of gasses is the sum of the individual partial pressures of the gases?
|
Dalton's law
|
|
|
what is total barometric pressure at sea level?
|
760mmHg
|
|
|
at sea level, what is the partial pressure of oxygen in dry air?
|
about 160 mmHg
|
|
|
at sea level, what percent of dry air is oxygen?
|
20.95%
|
|
|
at sea level, what percent of wet air is oxygen?
|
19.65%
|
|
|
at sea level, what is the partial pressure of oxygen in wet air?
|
about 150 mmHg
|
|
|
at sea level, what percent of dry air is CO2?
|
0.03 %
|
|
|
at sea level, what percent of wet air is CO2?
|
0.03 %
|
|
|
at sea level, what percent of wet air is water?
|
6.18%
|
|
|
mathematically, what is Dalton's law?
|
P_I = (P_B - P_H2O)*F
P_I = partial pressure of a gas in trachea P_B = Barometric pressure P_H2O = water vapor presure at 37*C F = fractional concentration of the gas (as a decimal) |
|
|
what does Henry's law state?
|
the concentration of gas dissolved in water (or plasma) is proportional to its partial pressure in the gas phase
|
|
|
what law states that the concentration of gas dissolved in water (or plasma) is proportional to its partial pressure in the gas phase?
|
Henry's Law
|
|
|
what are the two factors that determine how much gas can be dissolved in a fluid?
|
(1) Partial pressure of the gas
(2) Solubility of the gas |
|
|
mathematically, what is Henry's Law?
|
Cx=(Px)*(Solubility)
Cx = concentration of dissolved gas in fluid (ml/dL) Px = Partial pressure of the gas (mmHg) Solubility = solubility of the gas in the fluid (mL of gas /dL of blood /mmHg partial pressure) |
|
|
what is the normal concentration of dissolved oxygen in plasma?
|
0.3 mL/dL
|
|
|
how does the solubility of CO2 compare with that of oxygen?
|
solubility of CO2 is much higher than that of O2
|
|
|
why is it that gasses with low solubilities do not easily diffuse out of alveoli?
|
From the alveoli, gases must dissolve into water (or surfactant) prior to diffusion, thus gasses with low solubility (such as helium and nitrogen) do not easily diffuse out of the alveoli
|
|
|
what does Fick's law state?
|
* Volume of gas diffused per unit time is proportional to surface area and the difference in partial pressures (pressure gradient)
* Volume of gas diffused per unit time is inversely proportional to the thickness of the membrane |
|
|
how does membrane thickness affect the volume of gas diffused across the membrane per unit time?
|
thicker membranes result in slower diffusion of gases
|
|
|
how does surface area of a membrane affect the volume of gas diffused across the membrane per unit time?
|
increasing surface area increases rate of diffusion of gas
|
|
|
what law describes the variables that influence the rate of diffusion of gases across a membrane?
|
Fick's Law
|
|
|
mathematically, what is Fick's Law?
|
Vx = (D*A*dP)/(dX)
Vx = volume of gas diffused per unit time D = diffusion coefficient of the gas A = surface area dP = difference in partial pressures dX = thickness of membrane |
|
|
name three changes that would each decrease the volume of gas diffused across a membrane in a given unit of time
|
(1) Reduced pressure gradient across the membrane
(2) Reduced surface area of the membrane (3) Increased thickness of the membrane |
|
|
what clinical measurement estimates the factors that determine the volume of gas diffused across a membrane per unit time?
|
Diffusion capacity (D_L)
|
|
|
how is equilibrium of gas diffusion affected by increasing the membrane thickness?
|
equilibrium is not affected; membrane thickness only affects the rate at which the gases move towards equilibrium
|
|
|
how is it possible for a decreased diffusion capacity to NOT result in hypoxemia and hypercapnia?
|
there is a very large reserve diffusion capacity, so decreasing it does not always necessarily result in hypoxemia and hypercapnia
|
|
|
what are the partial pressures of oxygen and CO2 in mixed venous blood?
|
P_O2 = 40 mmHg
P_CO2 = 46 mmHg |
|
|
what is the partial pressure of oxygen in mixed venous blood?
|
40mmHg
|
|
|
what is the partial pressure of CO2 in mixed venous blood?
|
46 mmHg
|
|
|
what is the partial pressure of oxygen in systemic arterial blood?
|
100mmHg
|
|
|
what is the partial pressure of CO2 in systemic arterial blood?
|
40 mmHg
|
|
|
why is the normal A-a gradient either zero or very low?
|
* There is normally no (or a very low) A-a gradient because there is normally plenty of time for complete equilibration of oxygen diffusion in the pulmonary capillary
|
|
|
Normally, how much time is required for equilibration of oxygen diffusion in the pulmonary capillary?
|
about 0.3 seconds
|
|
|
normally, for how much time is an erythrocyte in the pulmonary capillary?
|
about 1 second
|
|
|
normally, how much time is required for equilibration of oxygen diffusion in the pulmonary capillary and how much time is the RBC in the capillary?
|
the RBC is normally in the capillary for 1 second, but equilibrium takes only 0.3 seconds
|
|
|
normally, how deoxygenated (in percentage) is hemoglobin in mixed venous blood?
|
Hb in mixed venous blood is normally 75% deoxygenated
|
|
|
why does the oxygen saturation level of hemoglobin in the blood not factor into the diffusion of oxygen to dissolve in plasma
|
only free, unbound gases influence diffusion
|
|
|
why is oxygen transport normally perfusion-limited rather than diffusion limited?
|
* At rest under normal conditions, capillary P_O2 rapidly equilibrates with alveolar P_O2, so there is no limit to our ability for oxygen to diffuse into the plasma. Therefore, we can saturate as much plasma as we can perfuse through the pulmonary capillaries
|
|
|
normally, what limits oxygen transport?
|
oxygen transport is normally perfusion-limited
|
|
|
why might hypoxemia only be uncovered in a patient when the patient exercises?
|
exercise utilizes the "buffer zone" of diffusion capacity that exists due to rapid equilibration, and diffusion is no longer able to equilibrate gases
|
|
|
what measurement would suggest diffusion-limitation?
|
a significant A-a gradient
|
|
|
what is suggested by a significant A-a gradient?
|
Diffusion-limitation
|
|
|
what type of gases would normally have high A-a gradients and why?
|
gases with low solubilities will have high A-a gradients (and therefore be diffusion-limited) because these gases do not easily dissolve in surfactant, which is required for the gases to diffuse into plasma
|
|
|
name three common gasses with low solubilities (numbers optional)
|
Nitrogen (N2) = 0.00123 mL/dL
Helium (He) = 0.00085 mL/dL Carbon monoxide (CO) = 0.00184 mL/dL |
|
|
is carbon monoxide diffusion-limited or perfusion-limited?
|
diffusion-limited
|
|
|
is NO diffusion-limited or perfusion-limited?
|
perfusion-limited
|
|
|
what is the classic example of a condition that reduces diffusion capacity and can therefore produce an A-a gradient and cause hypoxemia?
|
fibrosis disease
|
|
|
why does fibrosis lead to A-a gradient?
|
According to Fick's law, the increased membrane thickness present in fibrotic pulmonary tissue will lower the volume of gas diffused across the alveolar membranes in a given amount of time. This means equilibrium may not be achieved in the pulmonary capillaries
|
|
|
at a plasma PO2 of 100 mmHg, what is the normal percent saturation of Hb with oxygen?
|
at plasma PO2 of 100mmHg, normal Hb is 98% saturated with oxygen
|
|
|
what is the % saturation of hemoglobin with O2 at PO2 of 100mmHg?
|
Hb is 100% saturated with O2 at PO2 = 100mmHg
|
|
|
at PO2 of 40mmHg, what is the % saturation of hemoglobin with oxygen?
|
Hb is 75% saturated with O2 at PO2 = 40 mmHg
|
|
|
at PO2 of 25 mmHg, what is the % saturation of hemoglobin with oxygen?
|
Hb is 50% saturated with O2 at PO2 = 25 mmHg
|
|
|
at PO2 = 60 mmHg, what is the saturation % of hemoglobin with oxygen?
|
Hb is 90% saturated with O2 at PO2 = 60 mmHg
|
|
|
at PO2 = 30 mmHg, what is the saturation % of hemoglobin with oxygen?
|
Hb is 60% saturated with O2 at PO2 = 30 mmHg
|
|
|
what are the 3 important PaO2-HbSaturation points to know?
|
(1) 30 mmHg = 60% HbSat
(2) 40 mmHg = 75% Hb sat (3) 60 mmHg = 90% HbSat |
|
|
at what PO2 (and corresponding % Hb saturation) does deoxyhemoglobin begin producing the bluish tint of cyanosis?
|
PO2 = 40 mmHg
% Hb Saturation = 75% |
|
|
what is the Hb concentration in blood?
|
15g/dL (15 grams Hb per dL of blood)
|
|
|
what is the oxygen capacity of Hb?
|
1.34 mL/g (1.34 mL oxygen per gram of Hb)
|
|
|
what volume of oxygen can be carried by hemoglobin in the blood?
|
20.1 mL oxygen/dL of blood
|
|
|
how is it determed that 20.1 mL oxygen can be carried per dL of blood?
|
* 1.34 mL of oxygen can be carried per gram of Hb
* There is 15g Hb per dL of blood **Therefore: (1.34 mL O2 / g Hb)*(15 g Hb / dL blood) = 20.1 mL O2 / dL of blood |
|
|
normal PAO2
|
100mmHg
|
|
|
normal PaO2
|
90mmHg
|
|
|
normal A-a gradient for oxygen
|
0 - 10 mmHg
|
|
|
normal PaCO2
|
40mmHg
|
|
|
normal P50 for oxygen
|
25 - 30 mmHg
|
|
|
normal PvO2
|
40 mmHg
|
|
|
normal SaO2
|
98%
|
|
|
normal SvO2
|
75%
|
|
|
normal CaO2
|
20 mL/dL
|
|
|
normal CvO2
|
15 mL/dL
|
|
|
normal a-vO2 difference
|
5 mL/dL
|
|
|
normal Q
|
5 L/min
|
|
|
normal O2 delivery per minute
|
1000 mL/min = 1L/min
|
|
|
what is described by the Bohr effect?
|
the oxygen binding affinity of Hb is inversely proportional to the acidity of blood and the concentration of carbon dioxide in the blood
|
|
|
what direction does the Hemoglobin-O2 dissociation curve shift with increased metabolic demand?
|
high metabolic demand shifts Hb-O2 dissociation curve to the right (Bohr Shift)
|
|
|
list 4 changes that will shift the Hb-O2 dissociation curve to the right
|
(1) Increased CO2
(2) Increased [H+] (3) Increased temperature (4) Increased 2,3-BPG |
|
|
list 4 changes that will shift the Hb-O2 dissociation curve to the left
|
(1) Decreased CO2
(2) Decreased [H+] (3) Decreased temperature (4) Decreased 2,3-BPG |
|
|
how does 2,3-BPG affect affinity of Hb for O2?
|
2,3-BPG decreases affinity of Hb for O2, thereby promoting release of O2 to tissues
|
|
|
what measurement is generally not taken by old types of pulse oximeters?
|
carboxyhemoglobin levels
|
|
|
before pulse oximeters could check for carboxyhemoglobin, what technique was used to check for carboxyhemoglobin?
|
arterial blood gas
|
|
|
what 4 measurements are made by modern pulse oximeters?
|
(1) O2 Saturation
(2) Hemoglobin (3) Carboxyhemoglobin (4) Oxyhemoglobin |
|
|
how does Hb affinity for CO compare to Hb affinity for O2?
|
Hb affinity for CO is 250x greater than Hb affinity for O2
|
|
|
in what two ways does CO inhibit oxygen delivery to tissues by Hb?
|
(1) Hb binds CO with 250x greater affinity than Hb binds O2, so CO will bind preferentially
(2) When CO binds Hb, it traps any O2 already present and prevents that previously-bound O2 from being delivered to tissues |
|
|
how does 100% O2 affect half life of CO?
|
100% O2 will competitively inhibit CO and decrease its half-life from 4 hours to less than 1 hour
|
|
|
what is the PAO2 that results from placing a patient on 100% O2?
|
PAO2 = 660 mmHg when on 100% oxygen
|
|
|
what is the PaO2 that results form placing a patient on 100% O2?
|
PaO2 = 600mmHg when on 100% O2
|
|
|
what A-a gradient results from placing patient on 100% oxygen?
|
A-a gradient is 60 mmHg when on pure oxygen
|
|
|
why does increasing barometric pressure in hyperbaric chamber help increase dissolved oxygen in patients?
|
* Dalton's Law states that, for dry air, the partial pressure of a gas is directly proportional to two factors: fractional concentration of the gas and barometric pressure.
* Increasing barometric pressure to 3 atm will increase PaO2 to 1800 mmHg and will therefore increase dissolved oxygen to 5-6 mL/dL |
|
|
at 3 atm, what is the PaO2?
|
1800 mmHg
|
|
|
at 3 atm and a PaO2 of 1800 mmHg, what will the dissolved oxygen be?
|
5-6 mL / dL
|
|
|
what are the 3 modes of CO2 transport?
|
(1) Dissolved CO2
(2) CO2 bound to Hb (3) Bicarb |
|
|
Describe the mechanism of how CO2 can be transported as bicarb
|
(1) CO2 from tissues diffuses into plasma and into RBC's
(2) CO2 combines with water via carbonic anhydrase enzyme to form H2CO3 (carbonic acid) (3) Carbonic acid dissociates to H+ and HCO3- (bicarb) (4) Chloride shift: bicarb diffuses to plasma and chloride diffuses into RBC |
|
|
how does carbonic anhydrase reaction lead to the Bohr effect?
|
(1) Carbonic anhydrase reaction forms carbonic acid from CO2 produced by tissues and water.
(2) Carbonic acid dissociates to give bicarb and H+ (3) H+ reduces affinity of Hb for O2, thereby facilitating unloading of oxygen to tissues |
|
|
what is the chloride shift?
|
* Diffusion of HCO3- out of RBC's into plasma with the diffusion of Cl- into RBC's from the plasma
* Allows CO2 transport in plasma in the form of bicarb |
|
|
relative to the amount of O2 carried by plasma, how much CO2 does plasma carry?
|
Plasma carries at least twice as much CO2 than O2
|
|
|
what is normal arterial bicarb level?
|
about 24 mM
|
|
|
what percent of CO2 is transported as bicarb?
|
90%
|
|
|
what is the main way CO2 is transported in blood?
|
as bicarb
|
|
|
by what amount will total CO2 exceed bicarbonate concentration?
|
total CO2 will exceed bicarbonate concentration by 1 - 1.5 mEq/L
|
|
|
how would diuretics influence venous CO2 measurement?
|
diuretics would increase venous CO2 levels
|
|
|
3 components of conducting zone
|
(1) Trachea
(2) Bronchi (3) Bronchioles |
|
|
3 components of respiratory zone
|
(1) Respiratory bronchioles
(2) Alveolar ducts (3) Alveolar sacs |
|
|
what is bronchiectasis?
|
irreversible upper airway dilation
|
|
|
what is defined as irreversible upper airway dilation?
|
bronchiectasis
|
|
|
what is the most common presentation of bronchiectasis?
|
chronic cough producing mucopurulent sputum
|
|
|
what is mucupurulent sputum?
|
sputum containing mucus and pus
|
|
|
what is the goal of treatments for bronchiectasis?
|
reduce and control infections
|
|
|
condition of complete mirror image reversal of the thoracic and abdominal organs
|
situs inversus
|
|
|
what condition accounts for 50% of bronchiectasis cases?
|
cystic fibrosis
|
|
|
two drugs that antagonize the muscarinic ACh receptors on bronchiolar smooth muscle to induce bronchodilation
|
(1) Ipatropium
(2) Tiotropium |
|
|
two drugs that are agonists for the beta-2-adrenergic receptors on bronchiolar smooth muscle and induce bronchodilation
|
(1) Albuterol
(2) Salmeterol |
|
|
what is salmeterol?
|
beta-2-agonist (used as bronchodilator)
|
|
|
what is albuterol?
|
beta-2-agonist (used as bronchodilator)
|
|
|
what is ipatropium?
|
muscarinic ACh receptor antagonist (used as bronchodilator)
|
|
|
what is tiotropium?
|
muscarinic ACh receptor antagonist (used as bronchodilator)
|
|
|
what cells synthesize and secrete surfactant?
|
type II pneumocytes
|
|
|
what is Functional Residual Capacity (FRC)?
|
the amount of air that remains in lungs after quiet expiration
|
|
|
what is inspiratory reserve volume (IRV)?
|
the amount of extra air that can be forcefully inspired above the normal tidal volume
|
|
|
what is tidal volume (TV)?
|
volume of air moved into or out of lungs during quiet breathing
|
|
|
what is vital capacity (VC)?
|
volume of air breathed out after the deepest inhalation
|
|
|
volume of air breathed out after the deepest inhalation
|
vital capacity (VC)
|
|
|
volume of air moved into or out of lungs during quiet breathing
|
tidal volume
|
|
|
volume of air remaining in the lungs after a maximal exhalation
|
residual volume
|
|
|
what is total lung capacity (TLC)?
|
volume in the lungs at maximal inflation. TLC is the sum of VC and RV
|
|
|
what is inspiratory capacity (IC)?
|
sum of inspiratory reserve volume (IRV) and tidal volume (TV)
|
|
|
what term refers to the amount of air you would have in your lungs after a normal, quiet expiration?
|
functional residual capacity
|
|
|
draw a chart diagramming lung volumes and capacities
|
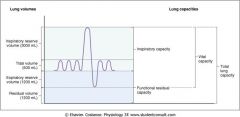
|
|
|
what technique is used to determine most lung volumes?
|
spirometry
|
|
|
what is the fuctional residual capacity for a normal sized human?
|
about 1.2 liters
|
|
|
what term refers to the amount of air you can forcefully breath in after you have already quietly inspired?
|
inspiratory reserve volume
|
|
|
what is the formula to calculate Minute Ventilation?
|
(Minute Ventilation) = (Tidal Volume)*(Respiration Rate)
|
|
|
what is the formula for total lung capacity (TLC)?
|
TLC = VC + RV
TLC = vital capacity + residual volume |
|
|
what lung volume cannot be measured directly by spirometry?
|
residual volume
|
|
|
how does residual volume change with obstructive pulmonary diseases?
|
residual volume increases
|
|
|
how does residual volume change in patients with restrictive pulmonary disease?
|
residual volume decreases
|
|
|
how does residual volume in a patient with emphysema compare to the residual volume in a patient with pulmonary fibrosis, and why?
|
* RV in pt. w/emphysema = high RV because there is difficulty expiring air and the damaged airways compress easily and trap more air.
* RV in pt. w/fibrosis = low RV because the stiff fibrotic lungs have low compliance but high elasticity, so there is a strong recoil with expiration that lowers RV. |
|
|
obstructive pulmonary disease characterized by destruction of alveolar tissue, loss of elastic recoil, and increased lung compliance
|
emphysema
|
|
|
describe the primary changes that occur in patients with emphysema
|
destruction of alveolar tissue, loss of elastic recoil, and increased lung compliance leading to abnormal dilation of terminal airspaces with destruction of alveolar septa and increased expiratory work
|
|
|
how does expiratory work change in a patient with emphysema and why?
|
emphysema increases expiratory work because the disease process decreases elasticity and therefore elastic recoil
|
|
|
what changes occur to the mucus producing glands in patients with bronchitis?
|
hypertrophy and hyperplasia of the mucus producing glands occurs in patients with bronchitis, leading to excessive tracheobronchial mucus production with resultant chronic productive cough
|
|
|
in a patient with bronchitis, what leads to hypoxemia?
|
alveolar ventilation/perfusion (V/Q) is mismatched
|
|
|
what are the 2 key features of interstitial lung diseases?
|
(1) Fibrosis: resulting in stiff, non-compliant lungs
(2) Inflammation of the alveolar walls and tissue |
|
|
are interstitial lung diseases obstructive or restrictive?
|
restrictive
|
|
|
what type of cough is generally present in patients with interstitial lung disease?
|
nonproductive cough
|
|
|
how is total lung capacity affected by interstitial lung disease and why?
|
TLC is decreased because inspiratory muscles can't easily expand the lungs due to the decreased compliance
|
|
|
how is RV affected by interstitial lung disease and why?
|
RV will decrease because interstitial lung disease is restrictive and will increase elastic recoil, making it easier for expiratory muscles to move air out of the lungs at low volumes
|
|
|
what is the formula for alveolar ventilation (V_A)?
|
V_A = [(Tidal Volume) - (Dead Space Ventilation)]*(Resp. Rate)
|
|
|
in words, define alveolar ventilation
|
Alveolar ventilation is the amount of fresh air that participates in gas exchange
|
|
|
what term refers to the amount of fresh air that participates in gas exchange?
|
alveolar ventilation
|
|
|
what is dead space ventilation (V_D)?
|
volume of air reaching areas of the lung that are not perfused (consists of both anatomic dead space and alveolar dead space)
|
|
|
what term refers any gas that is inspired but that does not participate in gas exchange?
|
dead space ventilation
|
|
|
what is tidal volume for a normal sized person?
|
500 mL
|
|
|
how would a pulmonary embolism affect anatomic dead space and why?
|
pulmonary embolism will increase amount of anatomic dead space because the embolism will prevent perfusion of some alveoli. The air in these alveoli will therefore not participate in gas exchange, effectively making these alveoli into dead space
|
|
|
what term refers to the volume of fresh air that reaches the alveoli?
|
alveolar ventilation
|
|
|
what is physiological dead space?
|
anatomic dead space + alveolar dead space
|
|
|
from what regions of the lungs does CO2 in expired air come?
|
CO2 in expired air comes from regions of the lung that are both ventilated and perfused
|
|
|
why must expired CO2 come from areas of the lung that are both ventilated and perfused?
|
Air is essentially free of CO2. Therefore, if there is CO2 in expired air, that CO2 had to come from an area participating in gas exchange (perfused area), and because it is being exhaled, the CO2 had to come from an area being ventilated.
|
|
|
why is CO2 measured from the end tidal gas?
|
CO2 in expired air comes from areas of the lungs that are ventilated and perfused. Of the 500mL tidal volume of air that is quietly exhaled, the first 150mL is from the conducting airways. Therefore, CO2 is present only in the last 350mL of expired air because it comes from respiratory zones.
|
|
|
from what part of expired air is CO2 measured?
|
end-tidal gas
|
|
|
how much CO2 is present in dead space air?
|
none
|
|
|
what is suggested by a reduction in [CO2] in expired air?
|
reduced [CO2] in expired air indicates increased dead space ventilation
|
|
|
if a question gives you expired CO2, what formula will you think about using and what will it calculate?
|
Bohr equation to calculate dead space volume
|
|
|
in words, what is the bohr equation?
|
An equation used to determine the dead space ventilation (V_D) based on the tidal volume and expired CO2 levels
|
|
|
mathematically, what is the Bohr Equation?
|
(V_D) = (V_T)*[(PaCO2 - PECO2)/PaCO2]
|
|
|
why would increased dead space ventilation reduce the concentration of CO2 in expired air?
|
dead space air is practically free of CO2, therefore any increase in dead space ventilation will "dilute" the CO2 that is present in normally expired air
|
|
|
why would an increase in dead space indicate a ventilation-perfusion (V/Q) mismatch?
|
increased dead space (indicated by decreased [CO2] in expired air) means that there is increased regions in the lungs that are not participating in gas exchange. This would occur if an area was being ventilated without perfusion, such as in a patient with a pulmonary embolism
|
|
|
what expired CO2 level indicates pulmonary embolism and why?
|
Low expired CO2 indicates pulmonary embolism because the embolism increases dead space (not participating in gas exchange due to lack of perfusion)
|
|
|
what is tidal alveolar ventilation for a normal sized person?
|
350 mL
|
|
|
what is normal alveolar ventilation for normal sized person?
|
4.3 L/min
|
|
|
what is the normal average V_CO2 per minute?
|
200 mL/min
|
|
|
for a given level of CO2 production, how is the partial pressure of alveolar CO2 (PACO2) related to alveolar ventilation, and why?
|
* For a given level of CO2 production, PACO2 is inversely proportional to alveolar ventilation
* This is because if CO2 production is constant, but you increase the ventilation of your alveoli, you would be diluting the CO2 in your alveoli by bringing in more oxygen (remember that air is practically free of CO2) |
|
|
what is the proportionality between alveolar ventilation and the partial pressure of CO2 in the alveoli?
|
V_A is inversely proportional to PACO2
|
|
|
what determines alveolar partial pressures of oxygen and CO2?
|
alveolar ventilation
|
|
|
if normal alveolar partial pressure of CO2 is 40 mmHg and you then double alveolar ventilation (holding CO2 production constant), what will the new alveolar partial pressure of CO2 be?
|
20 mmHg (because V_A and PACO2 are inversely proportional at a constant CO2 production)
|
|
|
why would doubling alveolar ventilation result in respiratory alkalosis?
|
doubling alveolar ventilation will cut alveolar pCO2 in half, causing hypocapnia. Because CO2 increases acidity of the blood, lowering CO2 will make the blood more alkaline
|
|
|
what is respiratory alkalosis?
|
increased pH of blood resulting from increased alveolar ventilation
|
|
|
as alveolar ventilation increases, what happens to PAO2 relative to PIO2?
|
as alveolar ventilation increases, PAO2 approaches PIO2
|
|
|
what predicts PAO2 using PaCO2?
|
alveolar gas equation
|
|
|
what equation will be used to calculate the A-a gradient and to determine mechanisms of hypoxemia?
|
alveolar gas equation
|
|
|
what three facts are needed to use the Alveolar Gas Equation?
|
(1) Inspired O2 (determined using Dalton's Law to be 150mmHg at sea level on room air)
(2) PaCO2 = PACO2 (determined using blood gas level) (3) R = respiratory exchange ratio = VCO2/VO2 |
|
|
what is the respiratory exchange ratio and its normal value?
|
The ratio of CO2 produced (VCO2) over the O2 consumed (VO2)
R = VCO2 / VO2 = 0.8 mL/min |
|
|
what is the alveolar gas equation (mathematically, with numbers)?
|
PAO2 = PIO2 - (PACO2/R)
PAO2 = 150 - (1.25*PaCO2) |
|
|
inhalation is limited by reduced compliance of lung while forced exhalation is augmented by enhanced elastic recoil
|
restrictive disesae
|
|
|
exhalation is limited by increased smooth muscle tone (asthma), by augmented bronchial secretions (bronchitis), and/or by loss of elastic recoil and loss of small airway tethering (emphysema)
|
obstructive disease
|
|
|
what is the normal FEV1/FVC?
|
75% - 80%
|
|
|
what measure indicates the volume of gas exhaled in one second?
|
FEV1
|
|
|
how is FEV1 changed in patient with restrictive pulmonary disease?
|
it is high, bc there is high recoil augmenting exhalation
|
|
|
how is FEV1 changed in patient with obstructive disease?
|
FEV1 is decreased because exhalation is inhibited by obstruction, lack of elasticity, and loss of tethering
|
|
|
what can reduce FEV1?
|
(1) Decreased TLC (fibrosis)
(2) Airway obstruction (3) Loss of elastic recoil (4) Muscle weakness |
|
|
3 unique features of asthma?
|
(1) Inflammation of airways
(2) Paroxismal airway narrowing (3) Hyper-responsiveness of airway smooth muscle |
|
|
treatments for asthma
|
(1) Beta-2 agonists
(2) M3 receptor antagonists (3) Corticosteroids |
|
|
what is the bronchial provocation test?
|
tests asthmatic response to histamine or methacholine challenge, to determine if airways are hyper-responsive
|
|
|
in patients with asthma, how does FEV1 change after bronchodilator treatment?
|
FEV1 improves (increases) by about 20% following administration of albuterol (beta-2 agonist) or ipatropium (muscarinic blocker)
|
|
|
what is fluticasone?
|
inhaled corticosteroid to treat obstructive pulmonary diseases
|
|
|
what limits expiratory flow rates?
|
dynamic compression
|
|
|
what 4 changes are seen on flow-vollume loop for patient with COPD?
|
(1) Reduced FVC
(2) Expiratory dip (3) Reduced Peak Flow Rate (particularly with asthma) (4) Decreased FEV1/FVC ratio (not clearly seen on Flow-Volume loops) |
|
|
what is expiratory dip on flow volume loop with COPD patient?
|
the part of the flow-volume loop at which flow rate drops very rapidly, occurs at a much earlier point in the expiratory portion of flow-volume loop
|
|
|
4 changes to flow-volume loops for pt. with restrictive disease?
|
(1) FEV1/FVC is normal or elevated
(2) Decreased FVC (3) Narrow or steep overall shape (4) Peak flow rate may be normal or reduced |
|
|
graphically compare expiratory flow-loops in obstructive and restrictive disease to normal lung
|
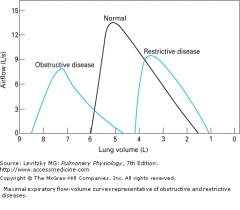
|
|
|
how does total lung capacity in patient with COPD compare to normal?
|
TLC is increased in patient with COPD
|
|
|
on flow-volume loop, what indicates extra-thoracic obstruction?
|
the most notable change occurs to the inspiration effort portion of the flow-volume loop, because the mass is pulled inward during inhalation
|
|
|
on flow-volume loop, what indicates intra-thoracic obstruction?
|
most notable change to flow-volume loop occurs in the expiratory effort portion of the F-V loop, because positive pressure pushes the mass into airway during exhalation
|
|
|
what is measured in plethysmography?
|
small pressure changes with changes in volume
|
|
|
what are the 6 PFT's?
|
(1) Spirometry
(2) Residual volume and FRC (3) Diffusing capacity (4) Blood gas (5) Maximal voluntary ventilation (muscle strength) (6) Lung mechanics |
|
|
4 goals of PFT's?
|
(1) Assess patient presenting with dyspnea
(2) Assess impairment for "disability" (3) Follow course of disease progression or treatment (4) Pre-operative assessment |
|
|
describe the variability of PFT's
|
* High inter-subject variability
* Low intra-subject variability |
|
|
what is left following exhalation of forced vital capacity?
|
residual volume
|
|
|
what is the equation for systemic vascular resistance?
|

|
|
|
what is the equation for pulmonary vascular resistance?
|

P_PA = pulmonary artery pressure
P_LA = left atrial pressure |
|
|
what measurement is made when the Swan-Ganz catheter balloon is DEFLATED?
|
Pulmonary artery pressure
|
|
|
what measurement is taken when the Swan-Ganz catheter balloon is INFLATED?
|
left atrial pressure (wedge pressure)
|
|
|
how is thermal diluttion cardiac output measurement performed?
|
cold saline is injected into the right atrium, and the temp change is measured in the pulmonary artery near the balloon of the Swan-Ganz catheter
|
|
|
4 signs and symptoms of pulmonary hypertension?
|
(1) Fatigue and exertional syncope
(2) Pulmonic valve regurgitation (3) RV Hypertrophe (4) Signs of Cor Pulmonale |
|
|
what is Cor Pulmonale and what are the symptoms?
|
Cor pulmonale = RV systolic heart failure
(1) RV dilation (2) Dyspnea (3) JVD (4) Abdominal distension and Ascites (5) Peripheral edema |
|
|
what arterial PO2 is the cutoff for hypoxia
|
hypoxia is PaO2 < 60 mmHg
|
|
|
how do you measure cardiac output with a right side catheterization?
|
thermal dilution cardiac output
|
|
|
5 potential causes of hypoxemia?
|
(1) High Altitude
(2) Hypoventilation (3) Fibrosis (4) V/Q defect (5) R --> L Shunt |
|
|
draw table comparing the 5 causes of hypoxemia and their associated PaO2, A-a gradients, and whether or not O2 administration is helpful to the patient
|
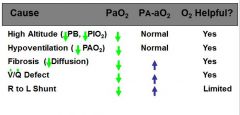
|
|
|
in the standing position, where are ventilation and perfusion greatest in the lung?
|
base of the lung
|
|
|
when standing, where in the lung is the V/Q ratio the greatest?
|
apex
|
|
|
when standing, where in the lung is ventilation the greatest?
|
base of the lung
|
|
|
when standing, where in the lung is perfusion the greatest?
|
base of the lung
|
|
|
when moving from apex to base of the lung, does ventilation or perfusion change with greater magnitude?
|
ventilation and perfusion both increase from apex to base (greatest at base and lowest at apex), but the change in blood flow is greater than the change in ventilation, resulting in a high V/Q at the apex and a low V/Q at the base
|
|
|
how do PaO2 andPaCO2 compare between base and apex of lungs?
|
APEX: PaO2 is high and PaCO2 is low (efficient V/Q)
BASE: PaO2 is low and PaCO2 is high (inefficient V/Q) |
|
|
where is intrapleural pressure lowest?
|
near the apex
|
|
|
why do alveoli in the apex of the lungs have greater resting volumes than alveoli in the base of the lungs?
|
* At the apex of the lungs, intrapleural pressure is the most negative and you get a greater transpulmonary pressure
* Greater transpulmonary pressure leads to alveoli with greater resting volume |
|
|
what is transpulmonary pressure and what does it tell us?
|
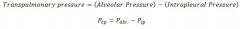
* The difference between alveolar pressure and intrapleural pressure
* Transpulmonary pressure tells us how open the lungs are (greater transpulmonary pressure = more open) |
|
|
give two reasons why blood flow is greatest through pulmonary capillaries at the base of the lungs
|
(1) Gravity: gravity leads to increased hydrostatic pressures at the base of the lung
(2) Large resting alveolar volumes at the apex of the lungs can compress the pulmonary capillaries towards the apex, reducing perfusion more and more as alveoli get larger |
|
|
what is the normal V/Q ratio at the apex while standing?
|
V/Q at apex while standing = 3
|
|
|
what is the normal V/Q at base of the lung while standing?
|
V/Q at base of lung while standing = 0.6
|
|
|
is the mycobacteria responsible for tuberculosis aerobic or anaerobic?
|
aerobic
|
|
|
why does mycobacterium that causes tuberculosis tend to localize at the apex of the lung?
|
the mycobacterium is aerobic and therefore localizes to the apex of the lung, where PAO2 is greatest
|
|
|
why is PAO2 greatest at the apex of the lungs?
|
the V/Q ratio is the greatest at the base of the lungs, and therefore the alveolar PO2 approaches atmospheric levels
|
|
|
on a chest radiograph, where would you expect to see tuberculosis?
|
near the apex, where PAO2 is highest
|
|
|
where in the lung is compliance the lowest and why?
|
* Compliance is lowest at the apex due to the more negative starting intrapleural pressure
* Because the starting intrapulmonary pressure is lowest at the apex, the alveoli at the apex start out in an overinflated state. ---> For the same change in intrapleural pressure, these apex-alveoli cannot inflate as much as alveoli at the base (dP/dV decreased at apex) |
|
|
where in the lungs is compliance greatest?
|
at the base
|
|
|
describe zone 1 of the lungs
|
* Pressures: Alveolar > arterial > venous
* Blood flow may approach/reach zero (abnormally), when alveolar pressure is increased (positive pressure ventilation) or arterial pressure is reduced (hemorrhage) |
|
|
describe zone 2 of the lungs
|
* Pressures: Arterial > Alveolar > Venous
* Blood flow is driven by the difference between arterial pressure and alveolar pressure **Therefore, because arterial pressure increases towards the base (due to gravity/hydrostatic pressure), blood flow is greater at the bottom of zone 2 |
|
|
describe zone 3 of the lungs
|
* Pressures: Arterial > Venous > Alveolar
* Blood flow depends on arterial-venous pressure difference, which is constant throughout the zone * Resistance to blood flow decreases lower in this zone (towards base of lung) because the arterial pressure (and therefore transmural pressure) increases towards the base, causing distension of pulmonary capillaries |
|
|
how and why does blood flow vary throughout zone 2 of the lung?
|
In zone 2, blood flow through the lung is driven by the difference between arterial pressure and alveolar pressure. Arterial pressure increases as you move lower in the lungs, and therefore blood flow increases as you move lower within zone 2 of the lung
|
|
|
where in the lungs is venous pressure greatest?
|
the base of the lungs (zone 3)
|
|
|
what is the V/Q ratio of a shunt and why?
|
V/Q= zero, because ventilation is zero due to obstruction
|
|
|
what is the V/Q ratio of a pulmonary embolism and why?
|
V/Q = infinity, because blood flow is zero
|
|
|
how does V/Q influence oxygenation?
|
V/Q is a measure of how effectively you can ventilate a given amount of perfused blood. Therefore, oxygenation will increase when V/Q ratio increases.
|
|
|
two conditions of low V/Q ratio
|
(1) Shunt (complete obstruction)
(2) Venous Admixture (stenotic airway) |
|
|
two conditions with high V/Q ratio
|
(1) Dead-space ventilation (pulmonary embolism)
(2) Wasted ventilation (stenotic pulmonary capillary) |
|
|
obstructive disease is indicated by a FEV1/FVC below what level?
|
FEV1/FVC below 70% indicates obstructive pulmonary disease
|
|
|
what are the 3 major components of asthma?
|
(1) Paroxysmal narrowing of bronchial airways
(2) Airway inflammation (3) Hyper-responsiveness |
|
|
what portion of the flow-volume loop is effort-independent," and why?
|
late descending portion of the expiratory flow-volume loop is "effort-independent" due to dynamic compression of airways
|
|
|
describe the "effort-independence" of the late descending portion of the expiratory flow-volume loop
|
as the patient exhales with increasing force, the airways compress proportionally to the increased pressure, keeping the expiration rate constant during the late descending portion of the expiratory flow-volume loop
|
|
|
during forced expiration, above what point in the airway does the airway dynamically compress?
|
* In the airway, there is a pressure gradient that decreases as you get closer to the atmosphere.
--> Above the point at which airway pressure equals intrapleural pressure, airways compress |
|
|
what limits expiratory flow rates?
|
dynamic compression
|
|
|
what are the 4 features of spirometry in a patient with obstructive lung disease?
|
(1) Reduced FVC
(2) Expiratory "dip" (3) Reduced Peak Flow (particularly with asthma) (4) Decreased FEV1/FVC (not clearly seen on flow-volume loops) |
|
|
what are the 4 features of spirometry in a patient with restrictive disease?
|
(1) FEV1/FVC = normal or increased
(2) Decreased FVC (3) Narrow steep pattern (4) Peak flow rate may be normal or reduced |
|
|
Compare expiratory flow loops in obstructive and restrictive disease to normal expiratory flow loops (drawing)
|

|
|
|
how can you differentiate extra-thoracic obstruction from intra-thoracic obstruction based on the flow-volume loops
|
* Extra-thoracic obstruction: prominent restriction of forced inspiratory flow occurs (most noticeable on inspiratory curve)
* Intra-thoracic obstruction: prominent restriction of forced expiratory flow |
|
|
what portion of flow-volume loop is most affected by intra-thoracic obstruction?
|
F-V loop shows prominent restriction of forced expiratory flow
|
|
|
what portion of the F-V loop is most affected by an extra-thoracic obstruction?
|
produces prominent restriction of forced inspiratory flow
|
|
|
what happens to extra-thoracic obstruction during breathing?
|
negative pressure pulls the mass into airways during inspiration
|
|
|
what happens to an intrathoracic obstruction during breathing?
|
positive pressure pushes the mass into airway during exhalation
|
|
|
how does air movement through the airways produce breath sounds?
|
vibrations in the walls of the airways associated with turbulent air movement through airways
|
|
|
where is turbulent airflow most likely to occur?
|
regions of the lung where velocity of air flow is greatest and there is an abnormal airway structure
|
|
|
how is sound affected by air or fluid?
|
sound is diminished by air or fluid
|
|
|
what are the three types of normal breath sounds?
|
(1) Tracheal breath sound
(2) Vesicular breath sound (3) Bronchial breath sound |
|
|
what part of breathing is longer in vesicular breathing, inspiration or expiration?
|
inspiration is longer in vesicular breathing
|
|
|
what part of tracheal breathing is longer, inspiration or expiration?
|
expiration is longer in tracheal breathing
|
|
|
where is vesicular breathing normally heard?
|
over most of the lungs
|
|
|
what normal breath sound is hollow and nonmusical, and is clearly heard in both phases of the respiratory cycle?
|
tracheal breath sound
|
|
|
what normal breath sound is soft and nonmusical, and is clearly heard in both phases of the respiratory cycle?
|
vesicular OR bronchial
|
|
|
what generates vesicular breath sound?
|
* Inspiratory component: generated within the lobar and segmental airways
* Expiratory component: generated from more central sources |
|
|
where are the three normal breath sounds heard?
|
(1) Tracheal: heard over the trachea
(2) Vesicular: heard over most of the lungs (3) Bronchial: heard over the manubrium |
|
|
what breath sound is alteredif upper-airway patency is altered, and how is it affected?
|
tracheal breath sound (heard directly over the trachea) can become more noisy or musical if upper-airway patency is altered
|
|
|
what two general factors can diminish vesicular breath sounds?
|
(1) Factors affecting sound generation
(2) Factors affecting sound transmission |
|
|
what are the 7 adventitious breath sounds?
|
(1) Wheezes
(2) Rhonchi (3) Fine Crackles (4) Coarse Crackles (5) Stridor (6) Pleural friction rub (7) Squawk |
|
|
what breath sound can be described as continuous high-pitched musical sound, resulting from high velocity air flow through narrowed or obstructed airways?
|
wheezes
|
|
|
what causes wheezes?
|
high velocity air flow through narrowed or obstructed airways
|
|
|
what is suggested by localized wheezing?
|
airway narrowing or blockage, such as foreign body or tumor
|
|
|
what is suggested by widespread wheezes?
|
generalized airway narrowing and airflow limitation, as in asthma and COPD
|
|
|
what breath sounds can be described as musical, low-pitched breath sounds, similar to snoring?
|
rhonchi
|
|
|
what condition is suggested by rhonchi?
|
rupture of fluid films and abnormal airway collapsibility
|
|
|
describe crackles (in general)
|
short, explosive., non-musical sounds heard on inspiration and sometimes expiration
|
|
|
what breath sound can be described as being similar to the sound made by separating strips of velcro?
|
fine crackles
|
|
|
what is the likely cause of fine crackles?
|
sudden inspiratory opening of small airways held closed by surface forces during the previous expiration
|
|
|
what breath sounds are caused by sudden inspiratory opening of small airways held closed by surface forces during previous expiration?
|
fine crackles
|
|
|
what breath sound is caused by boluses of gas passing through airways as they open and close intermittently?
|
coarse crackles
|
|
|
what causes coarse crackles?
|
boluses of gas passing through airways as they open and close intermittently
|
|
|
nonmusical, short, explosive breath sounds heard on mid-to-late inspiration and occasionally on expiration
|
fine crackles
|
|
|
these breath sounds are unrelated to secretions, are unaffected by cough, are gravity-dependent, and are not transmitted to the mouth
|
fine crackles
|
|
|
these breath sounds can be the earliest sign of disease, even before detection of changes on radiology
|
fine crackles
|
|
|
nonmusical, short, explosive breath sounds heard on early inspiration, throughout expiration, and are affected by coughing and are transmitted to the mouth
|
coarse crackles
|
|
|
what breath sounds indicate intermittent airway opening and may be related to secretions
|
coarse crackles
|
|
|
harsh, loud, high-pitched whistline or blowing sound of constant pitch
|
stridor
|
|
|
what is indicated by stridor
|
significant upper airway obstruction
|
|
|
what breath sounds are indicative of severe upper airway obstruction?
|
stridor
|
|
|
sudden onset of what breath sound is a medical emergency?
|
stridor
|
|
|
what breath sound may be observed after traumatic intubation or as a result of recurrent laryngeal nerve damage?
|
stridor
|
|
|
what breath sound may be heard at a distance without a stethoscope?
|
stridor
|
|
|
nonmusical, explosive, usually biphasic breathsounds, typically heard over basal regions?
|
pleural friction rub
|
|
|
what is indicated by pleural friction rub?
|
pleural inflammation or pleural tumors
|
|
|
what breath sound is associated with pleural inflammation or pleural tumors?
|
pleural friction rub
|
|
|
what breath sound can be described as a mixed sound with short musical components, accompanied or preceeded by crackles?
|
squawk
|
|
|
what is associated with squawks?
|
conditions affecting distal airways (pneumonia and other interstitial lung diseases)
|

