![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is heat? |
Heat is a form of energy |
|
|
|
What is temperature |
The temperature of a substance is a measure of the mean kinetic energy of its particles |
|
|
|
What is heat measured in |
Heat is measured in joules |
|
|
|
What is temperature measured in |
Temperature is measured in degrees Celsius |
|
|
|
Name another unit that temperature is measured in |
Another unit of temperature is Kelvin |
|
|
|
Name three methods of heat transfer |
Convection conduction radiation |
|
|
|
What is kinetic theory |
Kinetic theory is when you explain or describe quantities such as pressure temperature and temperature in terms of the motion of these particles. |
|
|
|
Explain how heat is transferred by convection |
Transfer of heat energy through a moving liquid or gas. Normal particles rise above cooler ones to make a convection current |
|
|
|
Explain how heat is transferred by conduction |
Transfer of heat through a material by transferring kinetic energy from one particle to another |
|
|
|
Explain how heat is transferred through radiation |
Infrared radiation spreads out from the source to surrounding areas or materials. It doesn't involve particles |
|
|
|
What is -273 degrees c in Kelvin |
0 (absolute zero) |
|
|
|
What is pressure what is the equation and what is its unit? |
Pressure is the force acting per unit area. Unit is Pascals(Pa) and it's equation is pressure=Force/Area |
|
|
|
Give another measurement for pressure |
Another measurement for pressure is Nm-² |
|
|
|
What is the kinetic model of gases |
The kinetic model describes the random movement of particles in a gas. The particles collide with the walls of a container and exert a force. This creates a pressure since pressure = force / area |
|
|
|
What does the kinetic model of gases describe |
The kinetic model describes the random movement of particles in a gas |
|
|
|
At what temperature is there no pressure |
The temperature to give no pressure is - 273 degrees c or 0 Kelvin/absolute 0 |
|
|
|
Complete the sentence heat will flow from .... objects to .... surroundings. The greater the .... difference with the ...., the .... the rate of ..... .... |
Hot, colder, temperature, surroundings, greater, heat loss |
|
|
|
What is specific heat capacity |
Specific heat capacity is the heat energy required to increase the temperature of 1kg of a substance by 1 degrees Celsius |
|
|
|
What is the formula and measurement for specific heat capacity |
Eh=cm∆T and Jkg-¹°C-¹ |
|
|
|
What is absolute zero |
Absolute 0 is the temperature at which gas particles stop moving. This results in the gas having no volume and no pressure. Absolute 0 is 0 k |
|
|
|
What is specific latent heat |
Terrific latent heat is the heat energy required to change the state of 1kg of a substance. It is measured in Jkg-¹ |
|
|
|
What is specific latent heat of Fusion |
Latent heat of fusion involves changing solid to liquid or vice versa |
|
|
|
What is specific latent heat of vaporization |
Latent heat of vaporization involves changing liquid to gas or vice versa |
|
|
|
What happens to the temperature when a substance changes state |
Temperature remains constant |
|
|
|
What quantity of energy used to heat up a substance will be given out when the substance cools |
The same quantity of energy used to heat up a substance will be given out when the substance cools |
|
|
|
What is the equation used to calculate heat energy |
Power=Energy/time |
|
|
|
What is double the temperature of 20 degrees c |
Double the temperature 20 degrees c is 313 degrees Celsius. |
In order to figure this out you have to convert it to kelvin. 20 degrees c = 293 k. You double this which makes it 586 k. To return to celsius subtract 273 which gives you an answer of 313 degrees c |
|
|
Sketch a graph of temperature and pressure when temperature is in degrees Celsius |
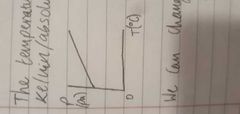
|
|
|
|
Sketch a graph of pressure and temperature when temperature is measured in Kelvin |

|
|
|
|
Give the equation of pressure and temperature |
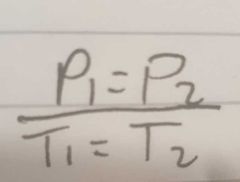
|
|
|
|
A bike has tires inflated to 275 kPa on a cold day at 10 degrees Celsius. Calculate the the pressure if the temperature is 20 degrees Celsius |
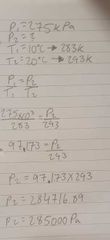
|
|
|
|
Use the kinetic model of gases to explain what happens to the pressure as the temperature decreases. |
As the temperature decreases the pressure. This is because the particles are slowing down and losing kinetic energy. They will collide the walls less frequently and with a smaller force. This decreases pressure as pressure = force / area |
|
|
|
What is the equation for weight (this is how you would find force) |
Weight=mass×gravitational... |
|
|
|
Use the kinetic model of gases to explain what happens to the pressure as the volume decreases |
As the volume decreases the particles collide with the walls more often so the pressure increases (Boyles law). |
|
|
|
Sketch a graph of pressure and volume when volume is 1/V(ml-¹) |
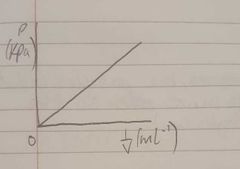
|
|
|
|
A graph of volume and pressure when volume is measured in V(ml) |
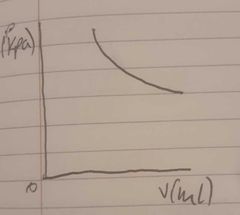
|
|
|
|
What is the equation for pressure and volume |
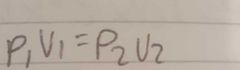
|
|
|
|
Use the kinetic model of gases to explain what happens to the volume as the temperature increases |
When temperature increases the particles of the gas gain kinetic energy and collide with the container walls more frequently at a greater speed. This results in a greater force being exerted on the the container walls as p = F/A. The volume of the gas must increase to maintain a constant pressure. |
|
|
|
What is the equation of temperature and volume |
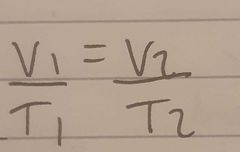
|
|
|
|
What is the general gas law equation |
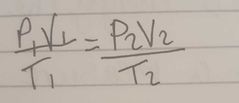
|
|
|
|
What is the equation for latent heat |
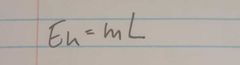
|
|
|
|
Draw a diagram of the changes of state including what it's called when one state changes from one to the next and draw arrows to show the energy change |

|
|
|
|
How do you figure out which thing has the lowest heat capacity |
Whichever one has the largest temperature change requires the smallest amount of energy to increase by 1 degrees c so has a lower heat capacity. |
|
|
|
What measures pressure |
Pressure is measured with a bourdon gauge or a pressure sensor connected to a computer |
|
|

A 100w electric heater is used to heat 20 g of a solid material. Calculate the heat energy needed to melt the solid |
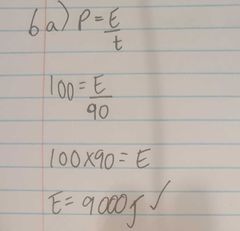
|
|
|

100w electric heater is used to heat 20 grams of a solid material as shown in the graph. Calculate the specific latent heat of fusion |
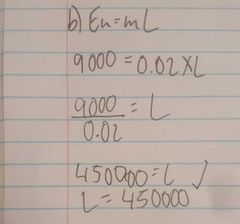
|
|
|

100 watt electric heater is used to heat 20 g of a solid material as shown in the graph. Calculate the heat energy needed to warm the liquid. |
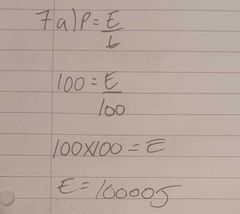
|
|
|

A 100w electric heater is used to heat 20 g of a solid material as shown in the graph. Calculate the specific heat capacity of the liquid. |
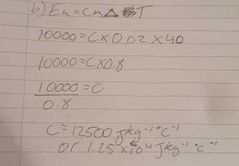
|
|

