![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
Transient Equilibrium
|
Half life of parent is not much longer than that of the daughter
A2/A1 = T1/ (T1-T2) |
|
|
mean life
|
1/¥ or 1.44T1/2
|
|
|
1 amu
|
1.66 ×10^-27 kg
|
|
|
electron amu
|
.00055 amu
|
|
|
proton amu
|
1.00727 amu
|
|
|
neutron amu
|
1.00866 amu
|
|
|
Avegadros number
|
6.022×10^23 atoms Na
|
|
|
#e/g
|
Na^2/Am
|
|
|
#g/atom
|
am/Na
|
|
|
keV
|
10^3eV
|
|
|
MeV
|
10^6eV
|
|
|
Specific Activity
|
SA = A/original mass
|
|
|
N
|
N=Noe^-¥ t
|
|
|
A
|
A=¥ N
|
|
|
1Bq =1dps
|
= 2.7×10^-11 Ci
|
|
|
1Ci
|
3.7×10^10 dps
|
|
|
Radiation
|
changed or unchanged particles that transfer energy
|
|
|
Secular Equilibrium
|
half life of parent is much longer than daughter. A2= A1
|
|
|
Uranium Series
|
A = 4n+2
|
|
|
Actinium Series
|
A = 4n+3
|
|
|
Thorium Series
|
A = 4n+0
|
|
|
Alpha Particle Decay
|
Z (atomic #): -2
A (mass #): -4 |
|
|
Beta Particle Decay
|
Z: +1
A: 0 |
|
|
Negatron Emission
|
Z: +1
A: 0 0/-1B + v + energy |
|
|
Positron
|
Z: -1
A: 0 competes with positron converts protons -> neurton electron gets sucked in from k shell characteristics xrays |
|
|
Internal Conversion
|
Z: 0
A: 0 High Z isotopes |
|
|
Bombarding Particle
|
Alpha
endonergic reaction |
|
|
Isomeric Transition
|
Metastable state (excited state)
augers e |
|
|
Deuteron Bombardment
|
combination of proton and neutron
(heavy hydrogen) STRIPPING |
|
|
Neutron Bombardment
|
Alpha particle
n and gamma = most common!! Thermal reaction |
|
|
Fission
|
bombard high atomic number by neutrons
|
|
|
Fusion
|
low mass nuclei to combine to make 1
hard to combine |
|
|
X ray tube
|
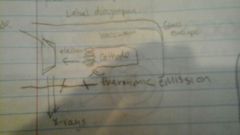
|

