![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
78 Cards in this Set
- Front
- Back
|
Ideal Gas Law |
PV= nRT=NkT |
|
|
v_rms (root-mean-squared velocity) |
sqrt[3kT/m] |
|
|
U_thermal |
N*f*kT/2 where f is degrees of freedom |
|
|
First Law of Themodynamics |
▲U = Q + W |
|
|
"quasistatic" |
a volume change process slow enough that the gas is continually in equilibrium during the process |
|
|
Quasistatic Work |
W= – P▲V for constant pressure, or |
|
|
isothermal |
▲T=0 —>▲U = 0 . Often this process is slow enough to treat quasistatically for calculating work. |
|
|
Adiabatic |
Q=0, no Heat —>▲U = W |
|
|
Adiabatic exponent (\gamma) |
\gamma = (f+2)/f where f is the degrees of freedom |
|
|
heat capacity |
heat per degree temperature increase, |
|
|
Heat capacity at constant volume |
constant volume implies zero work thus, |
|
|
Heat capacity at constant pressure |
C_p = ([▲U - (-P▲V)] /▲T)_p |
|
|
Microstate |
One possible state |
|
|
Macrostate |
the number of unique states, not counting degeneracies |
|
|
Multiplicity |
the number of degenerate Microstates corresponding to a given Macrostate |
|
|
Paramagnet |
a material which is magnetically active and aligns parallel to any external B-field |
|
|
Einstein Solid |
a model of solids in which each atom is 3 seperate yet identical oscillators with quantized energy |
|
|
Fundamental assumption of Stat Mech |
In an isolated system in thermal equilibrium, all accessible microstates are equally probable. |
|
|
Stirling's Approximation (including the pi, his only contribution) |
ln(N!)= NlnN - N + ½ln(πN) or |
|
|
Second Law of Thermodynamics |
"Multiplicity tends to increase." |
|
|
Irreversible and Reversible |
Irreversible processes create new Entropy. Isentropic (▲S = 0) implies a Reversible process because no entropy was created, so reversing the process doesn't violate 2nd Law |
|
|
Entropy |
S ≡ k ln Ω S= -(∂G/∂T)_P,N |
|
|
Temperature |
1/T ≡ (∂S/∂U)_N,V |
|
|
Pressure |
P ≡= T (∂S/∂V)_U,N
|
|
|
Chemical Potential ( µ ) |
µ ≡ – T (∂S/∂N)_U,V |
|
|
change in Entropy |
dS = dU/T = Q/T for a quasistatic process (or when W=0) |
|
|
Third Law of Thermodynamics |
C_v –> 0 as T –> 0 |
|
|
Magnetization |
M = - U/B where B is the magnitude of the B-field |
|
|
Quasistatic Heat |
Q = Tds for a quasistatic process |
|
|
Thermodynamic Identity (IMPORTANT) |
dU = TdS - PdV + µdN |
|
|
Efficiency of an Engine ( e ) |
e = W/ Q_h where Q_h is the heat absorbed. More useful though, using W = Q_h - Q_c,
|
|
|
Work in an Engine |
Q_h=W+Q_c with both Q's taken to be positive |
|
|
Carnot Cycle |
A cycle of two adiabats and two isotherms that achieves maximum theoretical value of efficiency, e = 1 – T_c/T_h |
|
|
Coefficient of Performance (COP) |
COP = Q_c/W = 1/(Q_h/Q_c – 1) . Used to rate Refrigeration processes. |
|
|
Curie's Law |
M ∝ 1/T which holds at high T limits for all paramagnets. |
|
|
Rankine Cycle |
Standard cycle utilized in Steam Engines |
|
|
Otto Cycle |
4 and 2 Strokes Engines use this cycle where a gas is adiabatically compressed, ignited by a spark(plug) pushing the piston during expansion, waste is expelled and new fuel injected. Rinse repeat |
|
|
Diesel Cycle |
Compress air to temperatures hot enough such that upon injection of fuel, it immediately ignites pushing the piston. Generally tuned to maintain near constant pressure throughout the cycle. |
|
|
Throttling |
A process where a gas is forced through a porous plug or a very small valve . Enthalpy remains constant during this process. |
|
|
Exceptions to Throttling |
Throttling processes (Hampson-Linde Cycle) liquefies most gases except Hydrogen and Helium, which will only liquefy if drastically cooled before the throttling process. Intermolecular attraction for these two gases is much too small, collisions dominate INCREASING temperature in this process unless molecules are moving sufficiently slow (read cold). |
|
|
Laser Cooling |
Absolutely badass. |
|
|
Enthalpy, H |
H= U+ PV
|
|
|
Helmholtz free Energy |
F= U- TS
|
|
|
Gibbs Free Energy |
G= U-TS+PV |
|
|
Thermodynamic Potentials (little memory trick) |
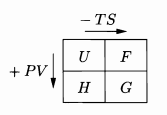
Remember, you can derive all the relevant partial derivative relations from the Thermo Identities by setting stuff to 0! |
|
|
"Free Energy is a Force toward Equilibrium" |
If no particles can enter or leave a system *At constant energy and volume, S tends to increase (maximized by system) *At constant temperature and volume, F tends to decrease (minimized by system) |
|
|
Extensive Quantity |
V, N, S, U, H, F, G, mass |
|
|
Intensive |
T, P, µ, density
|
|
|
Interpretation of µ (Chemical Potential) |
G = Nµ , chemical potential is just the Gibbs free energy per particle! |
|
|
Van Der Waals Equation of state |
a qualitative model for understanding real gases. The first correction, a, accounts for short range attractive forces ("stickiness") while the second correction accounts for for the strong short range repulsive forces (molecules have "finite volume" and gas isn't infinitely compressible) |
|
|
Boltzmann factor |
exp[-ßE] (that's a shitty Beta)
ß= 1/kT |
|
|
Probability in Boltzmann Stats |
P(s) = 1/Z exp[-ßE(s)] |
|
|
Average Energy( E-bar) |
-1/Z ∂Z/∂ß = -∂/∂ßlnZ
U= NE-bar, so depending on Z, this formula can also yield total energy U (this is up to whether you put the N in the power of Z, or multiply it in later) |
|
|
Standard Deviation |
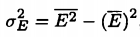
where E^2-bar is given by taking another derivative with respect to beta (given by Johnson) |
|
|
Partition Function for noninteracting, indistinguishable particles |
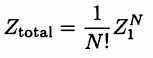
Resolution to Gibb's Paradox, truly indistinguishable particles |
|
|
Gibbs Factor |
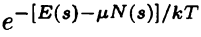
Like Boltzmann, but allows for particle exchange with reservoir. |
|
|
Bosons |
Photons, pions, etc. Integer-spin particles that can occupy the same state. Generally all the boson try to get into the lowest available energy state |
|
|
Fermions |
Electrons, Protons, Neutrons, Neutrinos etc. Half-integer spin particles. These can never occupy the same state due to Pauli Exclusion (well that's what its called at least, which is hardly telling of the physics involved). |
|
|
Fermi-Dirac Distribution |
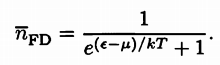
|
|
|
Bose-Einstein Distribution |
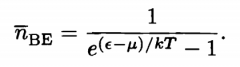
|
|
|
Fermi Energy |
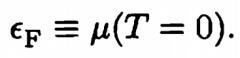
At T=0, n_FD becomes a step function with the cutoff at µ(T=0), which we call the fermi energy. A gas like this is said to be Degenerate
|
|
|
Bulk Modulus |
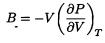
A useful quantity that's easily measured, goes at the change in pressure when the material in compressed, divided by the change in volume:
|
|
|
Density of States |
dn/dε : find surface area of 1/8 sphere in momentum space, multiply by dp, divide by the volume of a single momentum state (pi*h/2L)^3. This essentially tells you how many states there are if you go from p to p+dp |
|
|
Ultraviolet Catastrophe |
Basically before well developed QM, because the number of possible wavelengths in an electromagnetic wave is infinite, shouldn't the total thermal energy produced by all these waves be infinite? Of course not, QM tells us that energy of a harmonic oscillator is quantized, and thus higher energy (shorter wavelengths) get exponentially suppressed. |
|
|
Planck distribution |
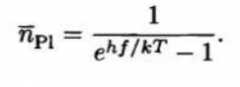
Essentially the Bose-Einstein Distribution with µ=0 and ε=hf. This makes sense because photons are Bosons, and they can be created and destroyed, thus µ must be 0 |
|
|
Photons |
Spin 1 Bosons. The particles of light. Essentially each photon is a quantum harmonic oscillator that moves through space. Photons have 2 polarizations, so make sure to slip in that factor of 2 when you find U or N from the distribution |
|
|
Wien's Law |
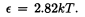
Higher temperatures yield higher energy photons. |
|
|
Stefans' Law |
P/A = σT^4 |
|
|
Blackbody Radiation |
Radiation emmited by a non-reflective object. Completely equivalent to the emission of radiation from a hole in a box of radiation. (Essentially Stefan's Law) |
|
|
Emissivity (e) |
A measure of how reflective a surface is. 1 is a perfect blackbody, 0 is a perfectly reflective surface. Modifies Stefan's Law:
|
|
|
Phonons |
Spin 0 Bosons (unless Transverse). "Particles" of oscillation in a solid. Essentially these are embodiments of the modes of oscillation of coupled oscillators. Waves in solid analogous to waves of light, phonons analogous to photons. |
|
|
Debye Approximation |
Peter Debye basically said that summing over arbitrary shapes of the crystal lattice (which determines the shape of "n-space") is too hard, so let's pretend its a sphere, convert the sum to an integral in spherical cords and bam, its solvable. Magically, this approximation is exact in both low and high temperature limits. |
|
|
Bose-Einstein Condensation (BEC) |
The tendency for bosons below a certain temperature (called the condensation temperature T_c) to aburtly accumulate in the ground-state (lowest energy state). At low temperatures, µ is very small, and ε_0 is very small, so the distribution favors ground state (ridiculously favors). |
|
|
Superfluid |
The superfluid component is an example of a BEC. The superfluid atoms are constantly moving to the lowest available state, and so behave in mind-blowing ways. |
|
|
Curie Temperature |
Temperature at which a ferromagnet's net magnetization becomes zero. |
|
|
Ising Model |
Model of a ferromagnet in which long-range magnetic interaction between dipoles is ignored, and the assumptions that there is a preferred axis of magnetization and that each dipole can only point parallel or antiparallel to it are made. |
|
|
Mean Field Approximation |
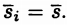
The Approximation here is that at every moment, the alignments of all dipoles is "typical" - no major fluctuations that cause M to be less or more than average |
|
|
Monte-Carlo Simulation |
A computer simulations where a Metropolis algorithm(give each dipole a ton of opportunities to flip) is used in combination with importance sampling (probability to flip weighted on exp(-ΔU/kT) to try and model the behavior of a ferromagnet. Quite successful if done in enough complication. |

