![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
49 Cards in this Set
- Front
- Back
|
If two balls have the same mass but different sizes, which has the larger density |
The smaller one |
|
|
Playdow- Elastic or plastic? |
Plastic |
|
|
Rubberbands |
Elastic |
|
|
With tension, compression and shear which state of matter does so with the most difficulty? |
Solids |
|
|
Why does an apple look red? |
It absorbs all the other colors and reflects red |
|
|
If you have a spectrum made up of descrete bright lines, what kind of spectrum is it? |
Emission spectrum |
|
|
Which element was first discovered in the solar spectrum? |
Helium- First found at the sun |
|
|
What do we call materials that become conductors when disolved in water? |
Ionic conductors- Salt |
|
|
If a gas mixture is at a uniform temperature, the molecules have the same..... |
Avg Kinetic Energy |
|
|
Who modeled the continous spectre of hot objects? |
Planc |
|
|
Who modeled the spectrum of hydrogen? |
Bohr |
|
|
Who came up with a mathematical formula for the hydrogen spectrum? |
Reidburg |
|
|
Who did the alpha particle gold foil experiment-solar system model? |
Rutherford |
|
|
Who did the oil drop experiment? |
Milikan |
|
|
Who did the voltage tube experiments-plum pudding model |
JJ Thompson |
|
|
Who first used the spectrum elements to identify materials? |
Kirchof |
|
|
Zero energy levels- How many colored photons will you have? (How many colored levels) |
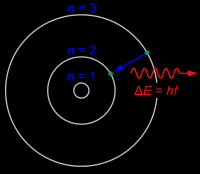
0- If you have 1 level still zero- cant jump (remember the level drawing) |
|
|
An allowed bohr orbit- are they energy fields, particles or standing waves |
Standing waves |
|
|
It is impossible to determine both _____ and ______ of an electron simultaneously. |
Position and momentum |
|
|
Suppose you have a wave pulse, traveling. Do you know the position well or the momentum? |
The position |
|
|
What levels in hydrogen are we considering for the red line? |
3 and 2 |
|
|
Who first succeeded in measuring the speed of light? |
Romer and (hoigen) |
|
|
Order of the electromagnetic spectrum |
radio-microwave-infared-visible light-uv-xray-gamma rays |
|
|
To explain refraction do we need the wave model of light or the particle model? |
Wave |
|
|
To explain the double slit interferance patern do we need the particle or wave model? |
Wave |
|
|
To explain the photo electric effect? Wave or particle> |
Particle |
|
|
Which has more energy? red or blue photon? |
Blue Photon? |
|
|
If light were a particle, would you expect the pattern on a screen to get bigger or smaller with a smaller opening? |
Smaller, if it were acting like a wave- larger |
|
|
If light were a WAVE, and you took a photo, and developed it under low light. Would you see a dim but complete image or random dotts? |
Dim but complete |
|
|
IF light were a wave would electrons be ejected regardless of the color? or would the color matter? |
Color Matters |
|
|
If the gases O2 and CO2 have the same temperature, which will be going faster? |
O2 |
|
|
In which of the following will the surroundings warm up? Just as water evaporates, or just before it begins to snow? |
Just before it begins to snow? |
|
|
If the air current out of your mouth expands? Then the temperature of the air that expands.... |
goes down |
|
|
The more significant reason why it takes longer to cook soup at high elevation is because its colder or lower air pressure? |
lower air pressure |
|
|
How does persperation help you cool off |
the water evaporating |
|
|
The ideal gas law PV=MKT T goes up then Volume |
Volume goes up |
|
|
PV=MKT Temp constant V goes up |
P goes down |
|
|
Photons will be emitted when they what? Rise, drop or accelerate in their orbit? |
When they drop levels |
|
|
What was the major theoretical problem with the solar system model? |
Why dont the electrons radiate? |
|
|
IF you fire bullets from a rickety machinge gun....(indisputable particles) through two slits. How many peaks do you get on the wall? |
Multiple |
|
|
What if you fire electrons through two or more narrow slits? How many wave patterns? |
Multiple |
|
|
True or false. Its physically possible to get two peaks and know which of the two slits the electron travels through? |
False wave function goes through both |
|
|
The energy of a photon h. If it were different, and people acted like wave patterns how would it be different? |
It would be bigger-h |
|
|
Whose equation gives the shapes for orbitals? |
Shrodinger |
|
|
What are the orbital letters and shapes? |
S-sphere P-Dumbell D- Clover F- crazy weird |
|
|
True or false- in the quantum mechanical model the electron was thought to be moving within the shape or on the surface |
False |
|
|
How many p orbital orientations are there in the second shell? |
3 each can have 2 electrons for a total of 6 electrons |
|
|
How many d orbital orientations are there in the third shell? |
5 |
|
|
How many f orbital orientations are there in the fourth shell? |
7 for total of 14 electrons |

