![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
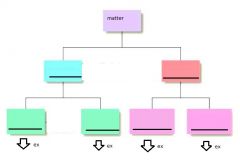
Fill in the blanks and give examples for the last row
|
possible example for; elements: gold
compounds: water homo: air Hetero: granite |
|
|
mass (is/is not) the same as weight
|
is not
|
|
|
What are the four main ideas of the Particle Model?
|
1. All matter is made of particles
2. Particles are always moving 3. There is space between the particles 4. The particles attract each other |
|
|
Note whether the following are positive, negative, or neutral. Answers may be used once, more than once, or not at all.
protons:_______ Neutrons:________ Electrons:_______ |
protons are positive
neutrons are neutral electrons are negative |
|
|
Where are electrons found?
|
circling the neucleus
|
|
|
What is the difference between a physical and chemical change?
|
A physical change does not alter the composition of the material. A chemical change occurs when atoms of different elements are rearranged and ombine in different ways.
Therefore, while objects that have been physicaly changed remain basically the same, objects that have been chemically changed come out different. |
|
|
What are the distinguishing characteristics of solids?
|
their definite shape
definite volume and low compressability |
|
|
What are the distinguishing characteristics of liquids?
|
definite volume
no definite shape low compressibility- while they are close together, they are mobile. |
|
|
What are the characteristics of a gas?
|
indefinite volume
indefinite shape high compressibility The particles are far apart and move quickly |
|
|
What is the 4th state of matter?
|
plasma
|

