![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
56 Cards in this Set
- Front
- Back
|
A ______ is a form of matter that is uniform throughout in chemical composition and physical state.
|
phase
|
|
|
A ________ is the spontaneous conversion of one phase into another and may be studied by techniques that include thermal analysis.
|
phase transition
|
|
|
The thermodynamic analysis of phases is based on the fact that, at equilibrium, the ______ of a substance is the same throughout a sample.
|
chemical potential
|
|
|
The _____ relates the number of variables that may be changed while the phases of a system remain in mutual equilibrium
|
phase rule
|
|
|
What is the equation form of the phase rule?
|
F = C -P + 2
|
|
|
A _________ is a chemically independent constituent of a system
|
component (C)
F = C - P + 2 |
|
|
A solution of sodium chloride has how many constituents? How many components?
|
three constituents: water, sodium ions, and chloride ions
two components: water, and NaCl. Since the quantity of Na and Cl ions are dependent on the quantity of the other, they count as one component |
|
|
In terms of phase behavior, Carbon dioxide's attributes are best explained by _____
|
weak intermolecular forces
|
|
|
In terms of phase behavior, water's attributes are best explained by ____
|
extensive hydrogen bonding
|
|
|
In terms of phase behavior, Helium's attributes are best explained by ______
|
low mass and weak molecular interactions
|
|
|
The _____ of a substance decreases with increasing temperature at a rate determined by its molar entropy.
|
chemical potential
|
|
|
The chemical potential of a substance increases with increasing _____ at a rate determined by its molar volume
|
pressure
|
|
|
When pressure is applied to a ______, it's vapour pressure rises
|
condensed phase
|
|
|
At very low temperatures and provided the pressure is not too low, the _____ phase of a substance has the lowest chemical potential and is therefore the most stable phase
|
solid
|
|
|
The __________ equation is an expression for the slope of a phase boundary.
|
Clapeyron
|
|
|
The __________ is an expression for the slope of a phase boundary.
|
Clapeyron equation
|
|
|
The Clapeyron equation gives an expression for the slope of the solid-liquid phase boundary in terms of the ________
|
enthalpy of fusion
|
|
|
The Clausius-Clapeyron equation is an approximation that relates the slope of the __________ to the enthalpy of vaporization.
|
liquid-vapor boundary
|
|
|
The Clausius-Clapeyron equation is an approximation that relates the slope of the liquid-vapor boundary to the __________.
|
enthalpy of vaporization.
|
|
|
The slope of the ________ is similarly related to the enthalpy of sublimation.
|
solid-vapor boundary
|
|
|
In a simple mixture, µ is uniform at __________
|
equilibrium
|
|
|
What is Raoult's law?
|
For ideal solutions, pressure of the solution equals pressure of pure component times the mole fraction of the component.
|
|
|
dG = __dp ___ dT
|
dG = Vdp - SdT
|
|
|
___ = Vdp - SdT + µ_a dn_a + µ_b dn_b
|
dG
|
|
|
What is the mole fraction of benzene in a mixture of 0.20 moles benzene and 1.80 moles toluene?
|
(.2/2.0) = 0.1
|
|
|
Is partial pressure in vapor phase determined by the number of moles present in liquid solution?
|
Yes.
|
|
|
A liquid mixture is referred to as a(n) _________ when Raoult's Law is obeyed by every component.
|
Ideal solution
|
|
|
True or false: In a mixture, the mole fraction of the vapor phase (Xi) can be different from the mole fraction of the liquid phase.
|
True.
|
|
|
True or false: Ideal solutions are characterized by similar sizes of constituents, and similar intermolecular interactions
|
True
|
|
|
True or false: Ideal solutions are characterized by similar intermolecular interactions and similar functional groups.
|
False: Ideal solutions are characterized by similar sizes of constituents, and similar intermolecular interactions
|
|
|
What is Raoult's Law?
|
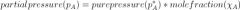
partial pressure (p_A) = pure pressure (p_A^* )
* mole fraction(\chi_A) |
|
|
At 25°C, the density of a 50% (mass) ethanol/water solution is 0.914g per cubic cm. Given that the partial molar volume of water in the solution is 17.4 cubic cm per mol, what is the partial molar volume of the ethanol?
|
56.4 cubic cm per mol
|
|
|
The Gibbs energy of mixing is calculated by forming the difference of the Gibbs energies before and after mixing: the quantity is ______ for perfect gases at the same pressure.
|
negative
|
|
|
The Gibbs energy of mixing is calculated by forming the difference of the Gibbs energies before and after mixing: the quantity is negative for perfect gases at the same ________.
|
pressure
|
|
|
The entropy of mixing of perfect gases initially at the same pressure is _______ and the enthalpy of mixing is zero.
|
positive
|
|
|
The entropy of mixing of perfect gases initially at the same pressure is positive and the enthalpy of mixing is _______.
|
zero
|
|
|
Suppose that 2.0 mol H2 at 2.0 atm and 25°C and 4.0 mol N2 at 3.0 atm and 25°C are mixed at constant volume. Calculate Δ_mix G. What would be the value of Δ_mix G had the pressures been identical initially?
|
-9.7 kJ, -9.5 kJ
|
|
|
Raoult's law provides a relation between the ______ of a substance and its _______ in a mixture
|
1) vapor pressure 2) mole fraction
|
|
|
_______ is the basis of the definition of an ideal solution.
|
Raoult's law
|
|
|
Henry's law provides a relation between the vapor pressure of a ______ and its mole fraction in a mixture.
|
solute
|
|
|
_______ is the basis of the definition of an ideal-dilute solution.
|
Henry's law
|
|
|
Henry's law provides a relation between the vapor pressure of a ______ and its _________ in a mixture.
|
1) solute 2) mole fraction
|
|
|
The Gibbs energy of mixing of two liquids to form an ideal solution is calculated in the same way as for two perfect gases. The enthalpy of mixing is _______ and the Gibbs energy is due entirely to the entropy of mixing.
|
zero
|
|
|
The Gibbs energy of mixing of two liquids to form an ideal solution is calculated in the same way as for two perfect gases. The enthalpy of mixing is zero and the Gibbs energy is due entirely to the _______
|
entropy of mixing.
|
|
|
A regular solution is one in which the entropy of mixing is the same as for an ideal solution but the enthalpy of mixing is ______
|
non-zero
|
|
|
A regular solution is one in which the ____________ is the same as for an ideal solution but the enthalpy of mixing is non-zero
|
entropy of mixing
|
|
|
A(n) _______ is a solution in which the entropy of mixing is the same as for an ideal solution but the enthalpy of mixing is non-zero
|
regular solution
|
|
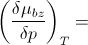
In the vapor phase of benzene, if the benzene vapor is treated as ideal gas, then (∂µ/∂p)_T = __?__
|
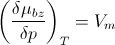
V_m
|
|
|
The reaction Gibbs energy, Δ_rG, is defined as the slope of the graph of the Gibbs
energy plotted against the _______: |
extent of reaction
|
|
|
How does one calculate the equilibrium constant (K)?
|
RT ln K = -Δ_rG^bar
|
|
|
The ratio P_b/P_a is known as the reaction quotient, and is symbolized by ____
|
Q
|
|
|
_________ is a quantity indicating the overall extent of RXN
|
extent of reaction (ξ)(xi)
|
|
|
What is the ratio of (dG)/(dξ)?
|
slope of graph of G vs ξ
|
|
|
efficiency of an engine = __?___
|
[work performed(w)] / [heat absorbed from hot source(q_h)]
|
|
|
What are 4 processes in Carnot cycles?
|
1) Reversible isothermal expansion 2) Reversible adiabatic expansion (no energy leaves the system as heat, so ∆S = 0) 3) Reversible isothermal compression (energy is released as heat to the cold sink; the change in entropy is q/T) 4) Reversible adiabatic compression (no energy enters the system as heat, so ∆S=0)
|
|
|
What is the entropy change for an irreversible adiabatic expansion for an ideal gas?
|
since "irreversible" suggests a spontaneous process, we can assume that ∆S is greater than zero
|

