![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
95 Cards in this Set
- Front
- Back
|
What is a Bronsted Lowry Acid? |
A Species with a tendency to lose a proton(donates it) |
|
|
What is a Bronsted Lowry base? |
A species with a tendency to gain a proton(accepts it) |
|
|
Is it the same as Lewis theory? |
No, the Lewis theory concerns electron pairs. |
|
|
How is it expressed? |
Acid *double arrows* H+ + conjugate base (acid without H+) |
|
|
When does the acid yield a H+? |
When a conjugate base is present or when it can form in order to accept H+. |
|
|
Do Acids and Bases have to be neutral? |
No, they can be ions. |
|
|
Give the 2 types of acid and define them? |
Strong acid- strong electrolytes in general completely dissociate in solution and hence donate all of their protons. Weak acid- weak electrolytes do not completely dissociate in solution. |
|
|
Show a general acid-base eqm? |
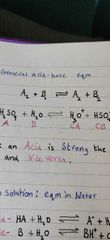
|
|
|
What if an acid is strong what effect? |
The conjugate base is weak. |
|
|
Write down the expression of Acid eqm with water and base eqm with water? |
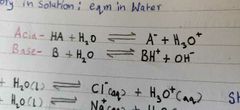
|
|
|
What is the ionic product of water? |
When 2 molecules of water dissociate with one acting as the acid and one as the base. |
|
|
What is significant about ionic product of water? |
Even the most purified water possesses residual conductivity due to the eqm. |
|
|
What does the Ionic product of water indicate? |
That water is amphiprotic which means it can gain or lose a proton so it can act as both acid and base. It is an autoprotolysis reaction which occurs when a proton is transferred between 2 identical molecules. |
|
|
Express the ionic product of water in terms of Keq? |
Products/reactants, you then eliminate a water from top and bottom, and then replace them with respect to activity. |
|
|
Describe the degree of ionisation for Ionic product of water? |
It is very small and therefore equal to 1 as the activity of a pure liquid is 1. Therefore keq will acc be [H+] [OH-] |
|
|
Why is the ionic product of water defined Kw? |
[H2O] is constant in pure water and dilute aqueous solutions as it is large so the ionic product of water is defined as such. It is equal to 1x 10^-14. |
|
|
What is the concentration of H+ and OH- in pure water? |
1x10^-7 as they are equal and maintain a charge balance. If Kw is less than that for H+ then the solution is basic and vice versa. |
|
|
What is pH? |
Hydrogen ion exponent, you know it equals to lol. |
|
|
Can pH values fall outside the 1-14 range |
Yes, for acids and bases stronger than 1M in concentration. |
|
|
Express Kw in terms of pH? |
-logKw= -log [H+] + -log [OH-] 14= pH + pOH |
|
|
How do you express weak acid and base dissociation constants? |
Products over reactants. Also the bigger the ka or kb(the weaker the acid basically) the smaller the pka or pkb. |
|
|
What are salts divided into? |
4 according to their acid/base derivation:
Strong acid and base- KCl Strong acid weak base- NH3Cl Weak acid strong base- Sodium Ethanoate Weak acid weak base- ammonium methanoate |
|
|
Explain parameter of Strong acid and strong base salt? |
1. Break them down to their respective oppositely charged parts. 2. Match them with H+ and OH- from water 3. Now you know whether they are a strong acid and strong base 4. They form a neutral solution |
|
|
Explain the parameters of weak acid and strong base? |
Same steps as before to identify whether they are weak or strong. There is an excess of OH- as H+ associates with the weak acid anions, and the solution is alkaline. |
|
|
Explain the parameters of S acid and W base? |
Same as the last one, this time solution is acidic. |
|
|
Explain the parameters of W acid and W base? |
Both will interact with water and the solutions pH depends on the dissociation constants of both. Ka=kb means neutral and so on. |
|
|
What is a buffer solution? |
An acidic(or basic) buffer consists of a W acid(or base) mixed with one of its salts(conjugate). It resists changes in pH when small amounts of acid or base are added. |
|
|
Why is a buffer solution able to do that? |
Ration between concentration of HA and A- does not vary much. [H+]= [acid]/[salt] x ka |
|
|
What is Henderson-Hasselbalch equation? |
pH= pKa + log ( [salt]/[acid]) And for a mixture containing equal amounts of HA and A- pH is equal to pKa. |
|
|
What is a Half neutralised buffer solution? |
Follows Le chateliers principle. |
|
|
What is electrolysis? |
Chemical effect of passing an electric current through a solution. |
|
|
What is an electrolyte? |
A solute which when dissolved in a suitable solvent yields a solution which conducts electricity. |
|
|
What is an ion? |
An atom,molecule or fragment bearing a residual electric charge. |
|
|
What is an electrode? Give the 2 types? |
1 of 2 or more conducting pathways from a source of current to a solution. Anode- electrode towards which the anions migrate. Cathode- electrode towards which the cations migrate. |
|
|
What is Ohm's law? |
Pd in volts across a conductor is = current flowing through the conductor x resistance. Kgm-1S-3A-2 |
|
|
What is Potential difference? |
Work done when 1 coulomb of charge passes through the points. V= W/Q |
|
|
What is a coulomb? |
Amount of charge transported by a constant current of one ampere in one second. 1C= 6.242 x 10 to the 18 protons. |
|
|
What is resistivity? |
How easily a material allows current through.
P= R X A/L Units are horseshoe meters
Current rises as A rises and drops as L Rises |
|
|
What is Conductance? |
The inverse of resistance. G= 1/R units are S |
|
|
What is conductivity? |
Conductance multiplied by cell constant. S x m-1 units |
|
|
What does conductivity depend on? |
Number of ions in solution, but not proportional to concentration. The same applies for conductance. |
|
|
What is molar conductivity? |
Conductivity of a solution/ concentration Units are SM2Mol-1 |
|
|
How to draw a molar conductivity graph? And what is it used for? |
Molar conductivity of Y-axis. Square root C on x axis. Used to distinguish between weak(goes down curved) and strong (linear decrease) electrolytes |
|
|
What is ion migration? |
Movement by an electric field. |
|
|
What is ion mobility? |
How electrolytes move through the solvent. Either by diffusion or migration. |
|
|
What are the types of interactions? |
Ion-Ion interactions and ion-solvent interactions. |
|
|
What is Ion-Ion interactions? |
Intiates from electrostatic interactions between ions of opposing charges which can produce 2 things: 1. Ion pairing 2. Ionic atmosphere |
|
|
What is ion pairing? |
It occurs transiently meaning as soon as they are within range and they form neautral species and is strongest for small,higher charged ions and higher concentrations. |
|
|
What is ionic atmosphere? |
Constantly in flux meaning that on avg an ion of a certain charge will have more ions around it of the opposite charge forming a cloud of oppositely charged species. |
|
|
Does the ionic atmosphere stay like that? |
No, when a charge is applied they begin to move to the oppositely charged electrode. |
|
|
What is ion solvent interaction? |
Ion-dipole interactions where water is the key solvent, hydration shells are formed due to the charged nature of ions and classified as either primary or secondary shells. |
|
|
How do ion-ion Interactions fair with a strong electrolyte? |
In an infinitely dilute solution there would be no ion-ion interactions to affect ion mobility. This is limiting molar conductivity. |
|
|
How do Ion-solvent interactions fair with strong electrolytes? |
An electrophretic effect occurs meaning the symmetry effect between the moving ion and the oppositely charged ions acting as a dragnet. The solvent molecules also get dragged but in the opposite direction causing friction and then ions slow down. |
|
|
What is the kohlrausch law? and when is it used? |
Molar conductivity= limiting molar conductivity - (experimental constant x root conc) Used for strong electrolytes at moderate concentrations. |
|
|
What did Kohl discover? |
That at RT the Limiting MC values for pairs of salts having a common ion was approx. 0. He also said MC was the sum of contributions in terms of the number of cations/anions and limiting MC of each pair. |
|
|
Why is Kohl's law useful? |

Helps us estimate limiting MC values for weak electrolytes, and this is done utilising their salts which would be considered strong electrolytes. |
|
|
What is the Arrhenius Ionisation theory? |
Solutions of electrolytes exist in eqm between undissociated solute molecules and fully dissociated ions. For weak electrolytes the Degree of dissociation= molar conductivity/ limiting molar conductivity. |
|
|
What are the 2 extremes in dissociation? |
Highly concentrated acidic solution, little water present hardly any dissociates. Infinitely dilute- fully dissociates as both LMC and MC are equal (weak electrolytes decrease rapidly with concentration). |
|
|
What are the Ostwald equations? |
Eqm constant= (MC squared x C) / LMC(LMC-MC) |
|
|
What would happen if electrolytes spontaneously ionise? |
Attraction of opposite charges would make them immediately recombine. |
|
|
What is Er? |
Dielectric constant, the factor by which the electric charge between 2 point charges is reduced relative to that in a vacuum. |
|
|
What counterbalances the result in the overall speed? |
2 factors, electric force of the applied field and frictional force. |
|
|
What is the applied electric field? |
Fa (accelaration force) = Z (charge number) e (charge on electron) E (field strength) |
|
|
What is Stokes law? |
Fr (frictional viscous drag) = 6pie n ( medium viscosity) aV (radius of the ion) d (drift velocity) |
|
|
What 2 factors govern conduction? |
2 factors when there's no inter-ionic interactions and they are: 1. Concentration of mobile charges 2. Mobility of charge carriers |
|
|
What is the Grotthuss mechanism? |
The mobility of H+ and OH- ions in water at RT is incredibly fast, H+ is specially fast is strongly solvated solutions. |
|
|
What is this high Mobility due to? |
Proton transfer between hydronium and H+ in water and vice versa with hydroxyl and OH- This is what we call the grotthuss mechanism. |
|
|
What happens at very low t? |
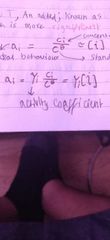
Quantum tunneling effect occurs and it is more significant for lighter,smaller ions. |
|
|
What is an activity coefficient? |
A measure of non-ideality of mixing. |
|
|
What do activity coefficients depend on? |
Size of ion Composition of the solution Temperature As the solute is more dilute it becomes 1 |
|
|
What is the case in a non-ideal solution? |
Due to interactions of the solute with other species which becoming likely to reduce its availability which is not the case at very high concentrations. |
|
|
What does the debye-huckel theory describe? |
Behaviour of strong electrolytes in solution. |
|
|
What does the DH theory presume? |
Strong electrolytes completely dissociate into ion. Non-ideality is due to electrostatic interactions |
|
|
What is the main problem with DH theory? |
Estimating the extra free energy that arised from the interactions. |
|
|
What does an electrochemical cell consist of? |
2 or more electrodes(metallic) immersed in an electrolyte (it may be in solution,liquid or solid) |
|
|
What is the anode? |
Negative terminal of a galvanic cell(oxidation occurs here) |
|
|
What is a galavanic cell? |
A cell that produces electricity as a result of a spontaneous reaction. |
|
|
What is an electrolytic cell? |
A cell that requires an external source of electricity to drive a non-spontaneous reaction. |
|
|
What does an electrode compartment consist of? |
One electrode and its accompanying electrolyte or both electrodes if they have the same electrolyte. A single electrode compartment with one electrode consists a 'half-cell'. |
|
|
What happens if the electrolytes differ? |
The 2 compartments are joined by a semi-permeable membrane or salt bridge. |
|
|
How are the components in a cell? |
Either in physical or electrical contact or both. |
|
|
How are these differences indicated? |
If they are in direct contact we use a | separator And if separated by a semi permeable membrane we use a 3 dotted line And if separated by a salt bridge then a || line is used |
|
|
What is that cell notation known as? |
Cell diagram with left hand side showing oxidation and right hand side showing reduction. |
|
|
Briefly state different electrodes? |
Ion-ion electrodes- half reactions which involve only ionic species. Electrons must be supplied and removed from the system by a solid electrical conductor. Usually Pt as it is inert. Pt is written at the start of the cell diagram. And no phase changes are denoted by a comma. |
|
|
Give some important points about cell diagrams? |
Substance losing electrons is written closest to the metal electrode. The most oxidised species are written furthest away from the metal electrode. |
|
|
Another type of electrode? |
Gas which occur for half reactions which involve a gas. Pt is used here too. |
|
|
How is a general cell diagram written? |
Starting from the left(anode): 1. Reactive/inert metal electrode 2. Solution of metal ions/redox couple 3. Salt bridge 4. Same as 2 5. Same as 1 |
|
|
How do cells work? |
Driving force in a cell series arises from a decrease in free energy. This comes from: 1. A chemical reaction 2. Physical change |
|
|
What is electromotive force? |
Voltage developed by a source of electrical energy. |
|
|
What is a liquid junction potential? |
When a difference in [ ] and mobility of ions exists at an interface between 2 ionic solutions. |
|
|
How is a LJP minimised? |
By a salt bridge that uses a salt like KCl where the mobilities of each proponents are almost the same. |
|
|
What does the EmF of a cell depend on? |
The two ion concentrations x and y The molalities (amount of substance in a specified mass of solvent) of the 2 solutions are 1 mol kg-1 |
|
|
How is the pd of a cell written? |
E= Er-EL |
|
|
Briefly explain the calomel electrode? |
Hg(l) | Hg2Cl2 (s) | Cl-(sat) The 2 mercuries are covered with a saturated KCl solution. It is slow and very T sensitive. |
|
|
Key features of standard potential? |
Always positive, always written as reduction potential, not affected by changes in stoichiometry. |

