![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
58 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Relative Mass of electron |
1/1840 |
|
|
|
Isotope |
Isotopes are atoms with the same number of protons, but different numbers of neutrons. |
|
|
|
Stages of Time of Flight Mass Spectrometer |
Ionisation Area Acceleration Area Ion drift Area Detection Area |
|
|
|
Electron Impact |
•A vaporised sample is injected at low pressure •An electron gun fires high energy electrons at the sample •This knocks out an outer electron •Forming positive ions with different charges E.g. Ti (g)Ti+ (g)+ e– |
|
|
|
Electrospray ionisation |
The sample is dissolved in a volatile, polar solvent • injected through a fine hypodermic needle giving a fine mist or aerosol • the tip of needle has high voltage • at the tip of the needle the sample molecule, M, gains a proton, H+, from the solvent forming MH+ • M(g) + H+ MH+(g) • The solvent evaporates away while the MH+ ions move towards a negative plate |
|
|
|
Detection |
The ions reach the detector and generate a small current, which is fed to a computer for analysis. The current is produced by electrons transferring from the detector to the positive ions. The size of the current is proportional to the abundance of the species |
|
|
|
Electronegativity |
is the relative tendency of an atom in a covalent bond in a molecule to attract electrons in a covalent bond to itself. |
|
|
|
Hydrogen bonding |
It occurs in compounds that have a hydrogen atom attached to one of the three most electronegative atoms of nitrogen, oxygen and fluorine, which must have an available lone pair of electrons. |
|
|
|
Enthalpy Change |
is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. |
|
|
|
Standard state |
100 kPa pressure • 298 K (room temperature or 25oC) • Solutions at 1mol dm-3 • all substances should have their normal state at 298K |
|
|
|
Calorimetric Method |
washes the equipment (cup and pipettes etc) with the solutions to be used dry the cup after washing put polystyrene cup in a beaker for insulation and support Measure out desired volumes of solutions with volumetric pipettes and transfer to insulated cup clamp thermometer into place making sure the thermometer bulb is immersed in solution measure the initial temperatures of the solution or both solutions if 2 are used. Do this every minute for 2-3 minutes At minute 3 transfer second reagent to cup. If a solid reagent is used then add the solution to the cup first and then add the solid weighed out on a balance. If using a solid reagent then use ‘before and after’ weighing method stirs mixture (ensures that all of the solution is at the same temperature) Record temperature every minute after addition for several minutes |
Measuring enthalpy |
|
|
Errors in calorimeter method |
energy transfer from surroundings (usually loss) • approximation in specific heat capacity of solution. The method assumes all solutions have the heat capacity of water. • neglecting the specific heat capacity of the calorimeter- we ignore any energy absorbed by the apparatus. • reaction or dissolving may be incomplete or slow. • density of solution is taken to be the same as water. |
|
|
|
Mean Bond Energy |
The mean bond energy is the enthalpy needed to break the covalent bond into gaseous atoms, averaged over different molecules. |
|
|
|
Two features of dynamic equilibrium |
Forward and backward reactions are occurring at equal rates. The concentrations of reactants and products stays constant |
|
|
|
Perfect Ionic Model |
Theoretical lattice enthalpies assumes a perfect ionic model where the ions are 100% ionic and spherical and the attractions are purely electrostatic. |
|
|
|
Typical Method for Measurement of the change in volume of a gas |
Measure 50 cm3 of the 1.0 mol dm–3 hydrochloric acid and add to conical flask. • Set up the gas syringe in the stand • Weigh 0.20 g of magnesium. • Add the magnesium ribbon to the conical flask, place the bung firmly into the top of the flask and start the timer. • Record the volume of hydrogen gas collected every 15 seconds for 3 minutes. |
|
|
|
rate-determining step |
The slowest step |
|
|
|
What changes Kp? |
Temperature |
|
|
|
Why use a high resistance voltmeter? |
The voltmeter needs to be of very high resistance to stop the current from flowing in the circuit. In this state it is possible to measure the maximum possible potential difference (E). The reactions will not be occurring because the very high resistance voltmeter stops the current from flowing. |
In electrode Potential |
|
|
Salt bridge |
The salt bridge is used to connect up the circuit. The free moving ions conduct the charge. A salt bridge is usually made from a piece of filter paper (or material) soaked in a salt solution, usually potassium nitrate. The salt should be unreactive with the electrodes and electrode solutions. A wire is not used because the metal wire would set up its own electrode system with the solutions. |
|
|
|
Measuring the electrode potential of a cell |
It is not possible to measure the absolute potential of a half electrode on its own. It is only possible to measure the potential difference between two electrodes. • To measure it, it has to be connected to another half-cell of known potential, and the potential difference between the two half-cells measured. • by convention we can assign a relative potential to each electrode by linking it to a reference electrode (hydrogen electrode), which is given a potential of zero Volts |
|
|
|
Components of a standard hydrogen electrode. |
: 1. Hydrogen gas at pressure of 100kPa 2. Solution containing the hydrogen ion at 1.0 mol dm-3 (solution is usually 1M HCl) 3. Temperature at 298K 4. Platinum electrode |
|
|
|
Standard condition in electrode Potential |
•All ion solutions at 1 mol dm-3 •temperature 298K •gases at 100kPa pressure •No current flowing |
|
|
|
Advantages of Fuel cells over conventional petrol or diesel-powered vehicles |
(i) less pollution and less CO2. (Pure hydrogen emits only water whilst hydrogen-rich fuels produce only small amounts of air pollutants and CO2). (ii) greater efficiency |
|
|
|
Limitations of hydrogen fuel cells |
-expensive -storing and transporting hydrogen, in terms of safety, feasibility of a pressurised liquid and a limited life cycle of a solid ‘adsorber’ or ‘absorber’ -limited lifetime (requiring regular replacement and disposal) and high production costs, -use of toxic chemicals in their production |
4 points are on card |
|
|
Fuel cell reaction in alkali |
Alkaline Conditions 4e- + 4H2O2H2 +4OH- E=-0.83V 4e- + 2H2O +O2 4OH- E=+0.4V Overall reaction 2H2 + O2 2H2O E=1.23V |
|
|
|
Constructing a pH curve |
Calibrate meter first by measuring known pH of a buffer solution. This is necessary because pH meters can lose accuracy on storage. Transfer 25cm3 of acid to a conical flask with a volumetric pipette Measure initial pH of the acid with a pH meter Add alkali in small amounts (2cm3) noting the volume added Stir mixture to equalise the pH Measure and record the pH to 1 d.p. Repeat steps 3-5 but when approaching endpoint add in smaller volumes of alkali Add until alkali in excess Can also improve accuracy by maintaining constant temperature |
|
|
|
Indicators |
Only use phenolphthalein in titrations with strong bases but not weak bases- Colour change: colourless acid pink alkali Use methyl orange with titrations with strong acids but not weak acids Colour change: red acid yellow alkali (orange end point) |
Names Colours Uses |
|
|
2 bonding pairs |
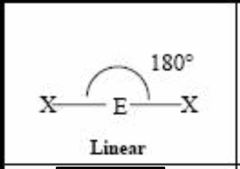
Back (Definition) |
|
|
|
3 bonding pairs |

Back (Definition) |
|
|
|
3 bonding pairs |

Back (Definition) |
|
|
|
2 bonding pairs 1 lone pair |

Back (Definition) |
|
|
|
4 bonding pairs |
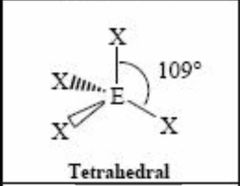
Back (Definition) |
|
|
|
4 bonding pairs |

Back (Definition) |
|
|
|
3 bonding pairs 1 lone pair |

Back (Definition) |
|
|
|
2 bonding pairs 2 lone pairs |

Back (Definition) |
|
|
|
5 bonding pairs |

Back (Definition) |
|
|
|
5 bonding pairs |
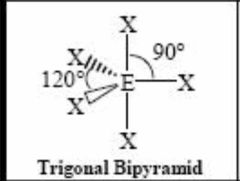
Back (Definition) |
|
|
|
4 bonding pairs 1 lone pair |

Back (Definition) |
|
|
|
5 bonding pairs |
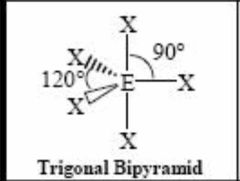
Back (Definition) |
|
|
|
4 bonding pairs 1 lone pair |

Back (Definition) |
|
|
|
2 lone pairs 3 bonding pairs |
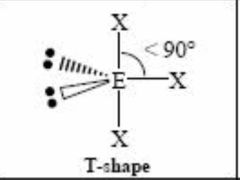
Back (Definition) |
|
|
|
3 lone pairs 2 bonding pairs |

Back (Definition) |
|
|
|
6 bonding pairs |

Back (Definition) |
|
|
|
1 lone pair 5 bonding pairs |

Back (Definition) |
|
|
|
4 bonding pairs 2 lone pairs |
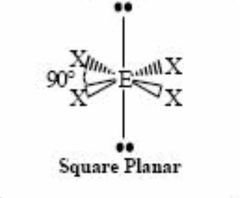
Back (Definition) |
|
|
|
3 bonding pairs 3 lone pairs |

Back (Definition) |
|
|
|
4 lone pairs 2 bonding pairs |

Back (Definition) |
|
|
|
Lithium Cell Equations |
Li+ + CoO2 + e - —> Li+[CoO2]- Li ——> Li+ + e- |
|
|
|
In conventional representation of half cells where do the reduced species go? |
Outside of the cell |
|
|
|
In electrode Potentials what side is oxidation/negative? |
Left |
|
|
|
Give 3 reasons why you may never see a particular reaction happen? |
-The activation energy is too high -The rate is too slow -The E cell value is negative |
|
|
|
Conventional representation for standard electrode Potential for the Ag+/Ag electrode |

Back (Definition) |
|
|
|
Suggests why the recharging of a lithium cell may lead to release of carbon dioxide into the atmosphere |
Electricity for recharging the cell may come from power stations burning (fossil) fuel |
|
|
|
Universal Indicator Colours for pHs |

Back (Definition) |
|
|
|
I2 and starch |
Makes blue (Clock reaction) |
|
|
|
How Do Van Der Waals Arise? |
-Electron movement in first molecule / temporary dipole - Induces a dipole in another molecule -(induced-temporary) attraction orδ+ attracts δ- in different/adjacent molecules |
|
|
|
Why is Silicon Dioxide (and other macromolecules) insoluble? |
-Macromolecular -Covalent bonding -Water cannot (supply enough energy to) break the covalent bonds / lattice |
|

