![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
115 Cards in this Set
- Front
- Back
|
What is the most important fuel for the brain?
|
glucose
|
|
|
What pathways are in place to ensure sufficient glucose to the brain?
|
Complex regulatory pathways
|
|
|
Brain glucose uptake occurs at the same rate during what 2 periods?
|
absorptive and post-absorptive periods
|
|
|
Glucose is not altered by what type of diabetes?
|
type 2
|
|
|
Insulin is among the best studied of ___ hormones
|
polypeptide
|
|
|
Insulin was the first protein for which the complete ___ sequence was determined?
|
amino aid sequence
|
|
|
What was the first hormone to be molecularly cloned?
|
Insulin
|
|
|
Insulin is released from what cells?
|
pancreatic β cells
|
|
|
Insulin is released from pancreatic β cells in response to what?
|
response to myriad stimuli including elevated concentrations of blood glucose
|
|
|
providing hormone replacement (when deficient) or agents that enhance hormone action (when resistance occurs) is a common strategy for what?
|
hyperglycemia
|
|
|
In case of insulin, hormone replacement therapy is fairly sophisticated yet complications still exist. What is an example of this?
|
Islet transplantation (It is an experimental treatment for type 1 diabetes mellitus)
|
|
|
Over-production of insulin is usually associated with what?
|
insulin resistance or pre-diabetes, insulin secreting tumors (insulinoma) are rare but do exist
|
|
|
Earliest known record of the diabetes disease mentioned when?
|
3rd Dynasty Egyptian papyrus; mentions polyuria as symptom (1552 BC)
|
|
|
What was diabetes described as in (Arateus, 1st Century AD)?
|
‘the melting down of flesh and limbs into urine’
|
|
|
Insulin, the miracle cure treated Leonard Thompson in what year?
|
1922
|
|
|
Who discovered insulin?
|
(Banting and McLeod)
|
|
|
How many people world wide have diabetes?
|
285 million people
|
|
|
In 2005 how many people died from diabetes?
|
1.1 million
|
|
|
Almost 80% of diabetes deaths occur in income based countries?
|
low- and middle-income
|
|
|
Almost half of diabetes deaths occur in people under the age of what?
|
70
|
|
|
55% of diabetes deaths are in men or women?
|
women
|
|
|
WHO projects that diabetes deaths will double between when?
|
2005 and 2030.
|
|
|
Number of Americans with Diabetes Projected to double or triple by when?
|
double or triple by 2050
|
|
|
What activities can prevent or delay the onset of diabetes.
|
Healthy diet,
regular physical activity, maintaining a normal body weight and avoiding tobacco use can |
|
|
Insulin resistance/Metabolic syndrome is most often associated with what?
|
obesity
|
|
|
Insulin resistance/Metabolic syndrome can also occur in what 2 other cases?
|
high stress, immunosuppression
|
|
|
Normal pregnancy physiology is a state of _____ insulin resistance
|
maternal
|
|
|
normal amounts of insulin are ineffective in lowering plasma glucose concentrations. What disease is this known as?
|
Hyperinsulinemia
|
|
|
Hyperinsulinemia has impacts on what?
|
multiple tissues/organs
|
|
|
Metabolic syndrome increases risk of developing what 2 diseases?
|
diabetes and cardiovascular disease
|
|
|
Insulin is synthesized and secreted from β cells in where?
|
the islets of Langerhans in the endocrine pancreas
|
|
|
The islet of Langerhans has 4 cell types which are what?
|
α (glucagon)
Β (insulin) Δ (somatostatin) F (pancreatic polypeptide) |
|
|
Insulin is synthesized as what? How many amino acids is it composed of?
|
as pre-proinsulin (110 amino acids)
|
|
|
What kind of peptide is cleaved during transport through rough ER to yield pro-insulin?
|
24 amino acid N-terminal signal peptide
|
|
|
What 3 other events yield insulin?
|
Protein folding,
di-sulfide bond formation and further cleavage events |
|
|
What subunits are in chains of insulin insulin with 1 intra-subunit and 2 inter-subunit disulfide bonds?
|
α (21 amino acids) and β (30 amino acids)
|
|
|
Insulin is member of family of related peptides called what?
|
IGFs
|
|
|
IGFs (IGF1 and 2) are produced in many cells and mediate effects of what?
|
growth hormone and other factors
|
|
|
Insulin and IGF receptors are also related and insulin can bind to IGF receptors with what kind of affinity?
|
LOW affinity
|
|
|
Insulin secretion is what kind of phase?
|
bi-phasic
|
|
|
Insulin secretion is stimulated when?
|
when β cells sense high glucose [ ]
|
|
|
How are B-cells excited? What does this regulate?
|
electrically excitable, and this regulates insulin secretion
|
|
|
Β-cells are electrically excitable, and this regulates insulin secretion. Ion channels, pumps and transporters combine to regulate what 2 components?
|
intracellular Ca2+ and membrane potential
|
|
|
What channels set the resting membrane potential?
|
KATP channels
|
|
|
Besides glucose, what are 5 other stimulatory factors?
|
1)Dietary amino acids (ALA, GLY, ARG)
2)Acetylcholine (vagus nerve) 3)Gastrointestinal hormones released from enteroendocrine cells of intestinal mucosa 4)GIP (glucose-dependent insulinotropic peptide) 5) Fatty acids |
|
|
Glucose-stimulated insulin secretion
Step 1) Glucose is taken up by what cells? Via what? |
pancreatic β cells via GLUT2 (glucose transporter)
|
|
|
Glucose-stimulated insulin secretion
Step 2) Glucose is than ____ by ___ |
phosphorylated by glucokinase → glucose “sensor”
|
|
|
Glucose-stimulated insulin secretion
Step 3) Glucose is further ____ via ___ ____? What does this result in? |
metabolized via mitochondrial respiration → ATP generation
|
|
|
Glucose-stimulated insulin secretion
Step 4) ↑ [ATP] results in what 4 factors? |
→ block of K+ channels
→ membrane depolarization → influx of Ca2+ → pulsatile insulin exocytosis (secretion) |
|
|
Glucose-stimulated insulin secretion
Step 5) ___ ____ > stimulus for insulin secretion vs. IV due to stimulatory effects of ___ hormones |
Oral glucose >
GI hormones |
|
|
Biphasic insulin secretion in response to high glucose concentrations
|
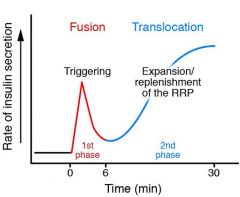
|
|
|
Draw a graph demonstrating release of insulin that occurs in respone to an IV glucose load in normal patients and diabetic patients.
|
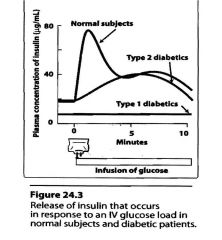
|
|
|
Insulin in the liver inhibits what kind of production? Stimulates up take of what?
|
hepatic glucose production (decreases gluconeogenesisand glycogenolysis)
Hepatic glucose |
|
|
Insulin in the muscle stimulates uptake of what? It inhibits flow of ____ precursors to the liver?
|
Glucose uptake
Flow of gluconeogenic precursors |
|
|
Insulin in adipose tissue stimulates the uptake of what?
Inhibits the flow of what precursor to liver? What does it reduce? |
glucose uptake (amount is small compared to the muscle)
Gluconeogenic precursor to liver (glycerol) Reduces energy substrate for hepatic glucogenesis (nonesterfied fatty acids) |
|
|
Glucagon:______-____hormone to insulin
|
Counter-regulatory
|
|
|
Glucagon is what kind of chain?
|
29 aa single chain polypeptide
|
|
|
Glucagon is synthesized from what?
|
pre-pro glucagon (180 aa)
|
|
|
What cells in islets of Langerhans are there?
|
alpha
|
|
|
Glucagon is an agonist for what?
|
GPCR that signals via Gs
|
|
|
Glucagon secretion is regulated by what 4 factors?
|
dietary glucose,
insulin, amino acids fatty acids |
|
|
Glucagon in the liver increases what 2 components?
|
gluconeogenesis and glycogenolysis
|
|
|
Glucagon in adipose tissue increases what?
|
↑ lipolysis (hydrolysis of lipids)
|
|
|
Glucagon in the GI tract results in what?
|
relaxation of smooth muscle
|
|
|
Glucagon in the heart increases what?
|
force of contraction
|
|
|
Signaling pathways for insulin culminate in regulation of what 4 components?
|
Gene expression
Glucose uptake, glycogen synthesis lipogenesis—”metabolic effects” |
|
|
Insulin signaling pathways diverge where?
|
at IRS-1
|
|
|
Proteins associate with P-TYR residues via what domains?
|
SH-2 domains
|
|
|
What signaling events are involved in “metabolic” signaling by insulin?
|
PI-3Kinase signaling
|
|
|
Insulin signaling Part 1: Regulation of gene expression via MAP kinase pathway
Step 1: Insulin receptor binds what? What does it undergo? Where does it undergo this? |
Insulin receptor binds insulin
Undergoes autophosphorylation On its carboxyl-terminal Tyr residues |
|
|
Insulin signaling Part 1: Regulation of gene expression via MAP kinase pathway
Step 2) Insulin receptor does what? What does it do this to? Where is that components located? |
Insulin receptor phorylates IRS-1 on its Tyr residues
|
|
|
Insulin signaling Part 1: Regulation of gene expression via MAP kinase pathway
Step 3) What binds to what? SOS binds to ____ then ___? What does this cause release and binding of? |
SH2 domain of Grb2 binds to )-Tyr of IRS-1.
Sos binds to Grb2, then to Ras causing GDP release and GTP binding to Ras |
|
|
Insulin signaling Part 1: Regulation of gene expression via MAP kinase pathway
Step 4) What binds and whats is activated as a result? |
Activated Ras binds and activates Raf-1
|
|
|
Insulin signaling Part 1: Regulation of gene expression via MAP kinase pathway
Step 5) What phosphorylates on what? What is then activated? What then phosphorylates what? What results in this? |
Raf-1 phosphorylates MEK on two Ser resides activating it.
MEK phosphorylates ERK on a THR and a TYR residue activating it |
|
|
Insulin signaling Part 1: Regulation of gene expression via MAP kinase pathway
Step 6) What moves into the nucleus? What does it phosphorylate? What results in this? |
ERK moves into the nucleus
and phosphorylates nuclear transcription factors such as ELK1, activating them |
|
|
Insulin signaling Part 1: Regulation of gene expression via MAP kinase pathway
Step 7) What joins what? To stimulate what? And translation of what? Needed for what? |
Phosphorylated ELK1 joins SRF
to stimulate the transcription and translation of a set of genes needed for cell division. |
|
|
Insulin activation of phosphodiesterase causes break-down of what?
|
cAMP
|
|
|
Regulation of hepatic glucose production (gluconeogenesis):
Insulin activation of phosphodiesterase causes break-down of cAMP → oppose effects of _____ to activate ___ ___ _ |
PKA to activate phosphorylase kinase B (via phosphorylation)
|
|
|
Regulation of hepatic glucose production (gluconeogenesis):
Insulin indirectly activates |
PP-1
|
|
|
PP-1 does what to glycogen phosphorylase?
|
de-phosphorylates
|
|
|
Hepatic vs. central effects of insulin on regulation of hepatic glucose production: Direct effects of insulin on HGP have been observed in what 2 models?
|
vitro and with animal models
|
|
|
What regulates HGP and is required for direct effect?
|
Hypothalamic insulin signaling via PI3Kinase
|
|
|
PI3K inhibitors administered ICV block what?
|
insulin regulation of HGO
|
|
|
Insulin receptor is what kind of receptor?
|
receptor tyrosine kinase (RTK
|
|
|
IR is a receptor tyrosine kinase (RTK) which regulates what?
|
regulates gene expression and metabolic pathways
|
|
|
IR is a receptor tyrosine kinase (RTK) which regulates gene expression and metabolic pathways by what kind of signaling?
|
complex signaling events emanating from phosphorylation of IRS-1
|
|
|
IR is a prototype for other RTKs including what 2 receptors?
|
EGF and PDGF receptors
|
|
|
The binding of adapter proteins such as GRB2 to P-Tyr residues (via SH2 domains) represents what kind of important mechanism that promotes what?
|
for promoting protein-protein interactions initiated by RTKs
|
|
|
Dysregulation of insulin signaling pathways is one of the hallmarks of what kind of diabetes?
|
Type 2
|
|
|
What Causes of Hyperglycemia in NIDDM?
|
Increased glucose production in liver
Receptor + post receptor defect in Peripheral tissues Impaired insulin secretion on Pancreas |
|
|
Activation of glycogen synthase by insulin
Step 1) What happens to IRS-1? What does it activate? How does it activate it? What is than converted? |
IRS-1, phosphorylated by the insulin receptor
activates PI-3k by binding to its SH2 domain PI-3K converts PIP2 to PIP3 |
|
|
Activation of glycogen synthase by insulin
Step 2) PKB bound to ___ is ____ by ___? This results in in what? |
PKB bound to PIP3 is phosphorylated by PDK1.
Thus activated , PKB phosphorylates GSK3 on a SER residue, inactivating it |
|
|
Activation of glycogen synthase by insulin.
Step 3) What can't GSK3 do? What remains active? |
GSK3 inactivated by phosphorylation cannot convert glycogen synthase (GS) to its inactive form by phosphorylation, so GS remains active
|
|
|
Activation of glycogen synthase by insulin.
Step 4) Synthesis ____ from ___ is accelerated? |
synthesis of glycogen from glucose is accelerated
|
|
|
Activation of glycogen synthase by insulin.
Step 5) PKB stimulates what? From where to where? What does this increase? |
PKb stimulates movement of glucose transporter GLUT4
from internal membrane vesicles to the plasma membrane increasing the uptake of glucose |
|
|
During Activation of glycogen synthase by insulin, Transmission of the signal is mediated by what 2 Kinases?
|
PI-3 kinase (PI-3K) and protein kinase B (PKB).
|
|
|
During the Epinephrine cascade. Epinephrine triggers a series of reactions in what? What does this result in?
|
hepatocytes in which catalysts activate catalysts, resulting in great amplification of the signal.
|
|
|
Binding of a small number of molecules of epinephrine to specific β-adrenergic receptors on the cell surface activates what?
|
adenylyl cyclase
|
|
|
Cross talk between the insulin receptor and the β2-adrenergic receptor (or other GPCR).
When INS-R is activated by insulin binding, its Tyr kinase directly phosphorylates what 2 components? |
the β2-adrenergic receptor (right side) on two Tyr residues (Tyr350 and Tyr364) near its carboxyl terminus
and indirectly (through activation of protein kinase B (PKB)) causes phosphorylation of two Ser residues in the same region |
|
|
Cross talk between the insulin receptor and the β2-adrenergic receptor (or other GPCR).
The effect of these phosphorylations is internalization of the ___ receptor? Reducing the respons to the ___ ___? |
adrenergic receptor,
the adrenergic stimulus |
|
|
Cross talk between the insulin receptor and the β2-adrenergic receptor (or other GPCR).
Alternatively (left side), INS-R–catalyzed phosphorylation of a GPCR (an adrenergic or other receptor) on a carboxylterminal Tyr creates the point of what? What serves as the adapater protein? |
nucleation for activating the MAPK cascade
with Grb2 serving as the adaptor protein |
|
|
Elevated fasting plasma glucose concentrations are a hallmark of what?
|
T2DM
|
|
|
During the night (basal/fasting conditions) the liver produces glucose to support the needs of the brain. In T2DM the ↑ in basal HGO closely correlates with what?
|
fasting hyperglycemia
|
|
|
Following glucose ingestion, insulin is secreted into the ___ ____ and carried to the ___? What is suppressed?
|
portal vein and carried to the liver, where it suppresses hepatic glucose output.
|
|
|
If the liver is insulin-resistant, it will continue to produce what?
|
glucose →marked hyperglycemia
|
|
|
Hyperglycemia is also a potent inhibitor of what?
|
HGO
|
|
|
the livers of T2DM patients are also markedly _____-_____
|
glucose-resistant
|
|
|
In T2DM, dysregulation of key regulatory enzymes involved ___ and ___ appear to be part of the underlying mechanism
|
gluconeogenesis and glycolysis
|
|
|
What may also play a major regulatory role?
|
Central insulin resistance
|
|
|
Effect of diabetes on life expectancy vs non diabetics:
Type 1 Type 2 |
Type 1 on average ↓ 20 yrs
Type 2 on average ↓ 30-50% vs. non diabetics |
|
|
What 3 factors can greatly improve outcomes for diabetic patients?
|
Early diagnosis, careful glucose monitoring and control
|
|
|
What is a Critical biomarker for glucose control?
|
Glycosylated Hemoglobin (HbA1c):
|
|
|
What another factor tat is important in management of diabetes? ( aside from healthy living)
|
A biomarker of average glucose control over long term
|
|
|
A non-enzymatic rxn occurs between ___ and _ __ _ in hemoglobin (hb)?
|
glucose and 1° amino groups
|
|
|
Concentrations of HbA1c in blood is directly related to what?
|
to blood glucose concentrations over the life cycle of the RBC (120 days)
|

