![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
56 Cards in this Set
- Front
- Back
|
Resistance of bacteria to antibiotics
a. Mechanisms b. General side-effect profile |
a. Mechanisms
1. Mutation (Bacteria undergo spontaneous mutation at a frequency of 1\10^16) 2. Adaptation (Several ways - alter the uptake of the drug by changes in their lipopolysaccharide coat, improve a transport system that removes the drug from the cell, increase metabolism of the drug) 3. Gene transfer (Through plasmids and transposons.) (Plasmids are extrachromosomal genetic elements (RNA or DNA) that are transferred from bacteria to bacteria by conjugation (through contact by means of specialized pili) and transduction (viral)) (Transposons\jumping genes are segments of DNA that can migrate from one plasmid to another within the same bacterium, to a bacterial chromosome, or to a bacteriophage. Mediated by insertion sequences) b. General side-effect profile 1. Allergic 2. Toxic 3. Idiosyncratic (Reactions that are not related to immune responses or known drug properties) (Ie hemolysis that occurs in G-6P DH deficient patients after treatment with sulfonamides, peripheral neuropathy after isoniazid administration in genetically slow acetylators) (1-3 apply to all drugs) 4. Related to changes in the normal body flora (Unique to antibiotics) (Usually refer to the changes that occur in the GI tract. Elimination of normal body flora -> overgrowth of new microbial populations - Superinfection. Most often clostridium difficile. Clostridium difficile produce a toxin that causes a disorder called pseudomembranous enterocolitis) (Stringer) |
|
|
Antimicrobials - Inhibitors of cell wall synthesis
|
Beta-lactams
1. Penicillins 2. Cephalosporins 3. Carbapenems 4. Monobactams (Aztreonam) Other inhibitors 1. Vancomycin 2. Bacitracin 3. Fosfomycin 4. Daptomycin (Stringer) |
|
|
Antimicrobials - Inhibitors of cell wall synthesis
a. Describe the final step in the synthesis of the bacterial cell wall b. Characteristic feature of all Beta-lactm antibiotics c. What is the most common mode of drug resistance to beta-lactam antibiotics |
a. The final step in the synthesis of the bacterial cell wall
I. Cross-linking of adjacent peptidoglycan strands by transpeptidation II. The mediating enzymes are called penicillin-binding proteins (PBPs) (Penicillins and cephalosporins are structurally similar to the termianl portion of the peptidoglycan strands and can compete for and bind to PBPs -> structurally weak cell wall, oddly shaped bacteria, death) b. They all contain a beta-lactam ring in their structure. (Some bacteria inactivate the beta-lactam antibiotics by an enzyme (beta-lactamase) that opens the beta-lactam ring.) c. Plasmid transfer of the genetic code for the beta-lactamase enzyme (Specific enzyme for penicillins - penicillinase, specific enzyme for cephalosporins - cephalosporinase) (We can give beta-lactamase inhibitors such as clavulanic acid or sulbactam to inhibit the deactivation or we can modify it -> later generation drugs) (Stringer) |
|
|
Penicillins - Four groups with most used members
|
1. Natural
I. Penicillin G (Narrow spectrum - gram+) II. Penicillin V (Penicillinase sensitive) III. Benzathine penicillin G (G and Vs) 2. Penicillinase resistant I. Methicillin II. Cloxacilin III. Dicloxacillin IV. Oxacillin (Narrow spectrum - gram+, synthesized to be penicillinase resistant, -oxacillins) 3. Aminopenicillins I. Amoxicillin II. Ampicillin (Start with am-, broad spectrum - some gram- activity also, penicillinase sensitive) 4. Extended spectrum penicillins I. Azlocillin II. Carbenicillin III. Mezlocillin IV. Piperacillin V. Ticarcillin (Actiavte agaisnt pseudomonas, relatively ineffective against G+) (Except methicillin and nafcillin, all the rest are in the 4th group) (All penicillins end in -cillin) (Stringer) |
|
|
Penicillins
a. Excretion - How, influenced by which drug b. Classification of allergic reactions of penicillins |
a. Excreted by tubular secretion, tubular secretion can be blocked by probenecid.
(Probenecid is a uricosuric agent used for gout, it can be administered to prolong the action of the penicillins) b. Allergic penicillin reaction 1. Immediate I. < 20 minutes II. S&S: Pruritus, paresthesia, wheezing, choking, fever, edema, urticaria\hives, hypotension -> shock -> LOC -> death III. IgE mediated 2. Accelerated I. Within 1-72 hours II. Mainly urticaria\hives 3. Delayed I. Within > 72 hours II. Mainly skin rash (Stringer) |
|
|
Cephalosporins
a. Common name b. Organization - 4 groups |
a. Almost all begin with cef- or ceph-
b. Cephalosporins 1. First generation I. Cefazolin II. Cephalexin (Narrow spectrum similar to broad spectrum penicillins, sensitive to beta-lactamase) 2. Second generation I. Cefaclor II. Cefamandole III. Cefoxitin (Increased activity against G-, increased stability) 3. Third generation I. Cefotaxime II. Cectazidime III. Ceftriaxone (Even broader in spectrum and more resistant to beta-lactamase) 4. Fourth generation I. Cefepime II. Cefpirome (G+ and G- activity - especially against Pseudomonas aeruginosa, include G- with multiple-drug resistant patterns) (Stringer) |
|
|
Cephalosproins
a. Which penetrate the CNS and can be used to treat meningitis b. Which other antibiotic class do they exhibit cross-allergy with c. What are the mechanism for their hypocoaguable effect |
a. The third generation and some of the second generation.
(3rd - cefotaxime, ceftazidime, ceftriaxone) b. Penicillins c. Antivitamin K effects (Some can cause a disulfiram-like reaction because they block alcohol oxidation, causing acetaldehyde to accumulate) (Stringer) |
|
|
Antibiotics - Cell wall synthesis inhibitors - beta-lactams - Carbapenems
a. Members b. Which of them is a broad spectrum beta-lactam antibiotic, which drug is it always given with, and why |
a. Imipenem, ertapenem, meropenem
(All administered IV) c. Imipenem I. Always given with cilastatin because cilastatin inhibit a renal dipeptidase (on the luminal PCT brush border) which metabolize imipenem to a toxic inactive metabolite (Stringer) |
|
|
Antibiotics - Cell wall synthesis inhibitors - beta-lactams
a. Aztreonam - group b. Aztreonam - speectrum |
a. Monobactam
(Only member) b. Good for aerobic G- including pseudomonas, ineffective against G+ organisms. (Its highly resistant to the action of beta-lactamases) (Stringer) |
|
|
Antibiotics - Cell wall synthesis inhibitors - others
a. Vancomycin - Mechanism, use, adverse effect b. Fosfomycin - Mechanism, use c. Daptomycin - Mechanism, use |
a. Vancomycin
I. Glycopolypeptide that inhibit cell wall synthesis by preventing polymerization of the linear peptidoglycans II. Only effective against G+ (Very poor oral absorption) III. Dose-related ototoxicity (-> Tinnitus, high-tone deafness, hearing loss, deafness (?)) (Teicoplanin is a new related drug) b. Fosfomycin I. Inhibits enolpyruvyl transferase - one of the first steps in the synthesis of peptidoglycan for the cell wall II. For uncomplicated UTIs c. Daptomycin I. Lipopeptide, bind to the bacteria membrane and cause depolarization which cause death (Not a cell wall synthesis inhibitor, but acts like one) II. spectrum of activity similar to vancomycin (Stringer) |
|
|
Antibiotics - Protein synthesis inhibitors
a. General mechanism of action b. General route of resistance c. Classes |
a. Bind to an intracellular protein (ribosomal subunit) and inhibit it.
b. Blockage of the movement of the drug into the cell by the bacteria c. Classes 1. Aminoglycosides (bactericidal) 2. Tetracyclines 3. Macrolide (ie erythromycin) 4. Ketolides 5. Streptogramins 6. Oxalizodinones 7. Chloramphenicol 8. Clindamycin (Related to the chemical structure) (Stringer) |
|
|
Antibiotics - Protein synthesis inhibitors - Aminoglycosides
a. Members b. Spectrum c. Synergistic effect with, why d. Side effects |
a. Aminoglycosides
1. Gentamicin 2. Tobramycin 3. Amikacin 4. Kanamycin 5. Streptomycin (All end in -mycin\-micin except amikacin. However, so does clindamycin and the macrolides (erythromycin, clarithromycin..) b. Broad-spectrum, but anaerobics are generally resistant to them. (Because most of the aerobs use an oxygen-dependent transport system to bring the aminoglycosides into the cell, which the anaerobs don't have) c. Penicillins. I. Penicillins cause cell wall abnormalities that allow the aminoglycosides to gain entry into the bacteria. d. Side effects 1. Ototoxicity (Cochlear and vestibular -> Tinnitus, deafness, vertigo, gait unsteadiness, high-frequency hearing loss) 2. Nephrotoxicity (Related to the rapid uptake of the drug by PCT cells, they are then killed. Acute nephrotoxicity is reversible) 3. Neuromuscular toxicity (Blockade of presynaptic release of Ach) (Small therapeutic window) (Stringer) |
|
|
Antibiotics - Protein synthesis inhibitors - Tetracyclines
a. Members b. Spectrum c. Side effects |
a. Members
1. Tetracyclien 2. Doxycycline 3. Chlortetracycline (All end in -cycline) b. Broad-spectrum 1. G+ and G- facultative organisms and anaerobs 2. Rickettsial disease - Rocky Mountain spotted fever 3. Chlamydial disesae 4. Lyme disease (Spirochetes) 5. Mycoplasma pneumonia c. Side effects 1. Staining of teeth 2. Retardation of bone growth (Incorporate into bone and teeth -> Not recommended in children and pregnant women) 3. Photosensitivity (Stringer) |
|
|
Antibiotics - Protein synthesis inhibitors - Macrolides
a. Members b. Spectrum |
a. Members
1. Erythromycin 2. Azithromycin 3. Clarithromyicn (All end in -omycin, most in -thromycin) b. Spectrum 1. Mycoplasma infections 2. Pneumonia 3. Legionnaires' disease (Legionella pneumophila) 4. Chlamydial infections 5. Diptheria 6. Pertussis (Mnemonic for which Erythromycin is the drug of choice - Legionnaires Camp on My Border -> Legionella, Campylobactera, Mycoplasma, Bordella) (Ketolides is a new group derived from macrolides. The prototype is Telithromycin. Bacteria that have developed resistance to macrolides are often still sensitive to ketolides) (Stringer) c. |
|
|
Antibiotics - Protein synthesis inhibitors - Chloramphenicol
a. Spectrum b. Side effects |
a. Spectrum
I. Most aerobic and anaerobic bacteria except Pseudomonas aeruginosa. b. Side effects 1. Bone marrow depression 2. Idiosyncartic aplastic anemia (1\40 000, Usually fatal!) 3. Gray baby syndrmoe (Often fatal, caused by transplacental toxic effects) (Stringer) |
|
|
Antibiotics - Protein synthesis inhibitors - Clindamycin
a. Spectrum b. Side effects |
a. Spectrum
I. Activity against anaerobes, drug of choice for anaerobic GI infections b. Side effects I. Pseudomembranous enterocolitis (Because clostridium difficile is resistant against it) (Lincosamides - Clindamycin and lincomycin. Lincomycin is rarely used.) (Stringer) |
|
|
Antibiotics - Folate antagonists
a. Difference in bacteria and humans in folate uptake b. Synthesis of folic acid in bacteria - Substrates, Pathway |
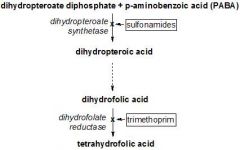
a. Uptake
I. Humans cannot make it (-> vitamin), so it can only be absorbed II. Bacteria cannot absorb it, they must make it b. Synthesis of folic acid in bacteria I. Need PABA (para-aminobenzoic acid, pteridine, and glutamate) 1. PABA + Pteridine --Dihydropteroate reductase--> 2. Pteroic acid --> 3. Folic acid --> 4. Dihydrofolate --Dihydrofolate reductase--> 5. Tetrahydrofolate |
|
|
Antibiotics - Folate antagonists
a. Members b. Other uses of folate antagonists c. Spectrum |
a. Members
1. Sulfamethoxazole 2. Trimethoprim 3. Cotrimoxazole - Combination of 1 and 2 (Other sulfonamides - Sulfacetamide, sulfadiazine, sulfapyridine, sulfasalazine) b. Other use 1. Sulfasalazine is used for inflammatory bowel disease 2. Methotrexate is used against neoplasms, psoriasis, rheumatoid arthritis (Inhibit dihydrofolate reductase) 3. Pyrimethamine is used as an antimalarial agent that is effective against plasmodium falciperum and against toxoplamsosis (Inhibits dihydrofolate reductase) c. Spectrum I. Broad-spectrum agents - G+, G- (UTIs, Pneumocystis carinii pneumonitis..) (Stringer) |
|
|
Antibiotics - Quinolones
a. Members b. Mechanism c. Spectrum |
a. Members
1. Ciprofloxacin 2. Enoxacin 3. Levofloxacin 4. Norfloxacin 5. Ofloxacin (-xacin) b. Mechanism I. Inhibit DNA synthesis by inhibiting DNA gyrase (DNA gyrase is the bacterial enzyme that is resposible for unwinding and supercoiling of the DNA. This is the only class of antibacterials that ihnibits DNA replication (more common strategy for antivirals and anticancer drugs)) b. Spectrum I. Broad-spectrum, especially against G- (Used to treat urogenital (UTI, prostatitis), respiratory, and GI infections caused by various G- organisms) (Stringer) |
|
|
Drugs used in tuberculosis and leprosy
a. First-line drugs b. Isoniazid - Mechanism c. Isoniazid - Use d. Isoniazid - Side effects |
a. First-line drugs
1. Isoniazid 2. Pyrazinamide 3. Rifampin 4. Ethambutol 5. Streptomycin (One regimen consists of 2 months of isoniazid, rifampin and pyrazinamide, followed by 4 months of isoniazid and rifampin. The long duration is due to the slow growing nature of these microorganisms) b. Isoniazid - Mechanism I. Inhibit the synthesis of mycolic acids that are unique to the mycobacteria, these are a constituent of the bacteria's envelope. c. Isoniazid - Use I. Drug of choice for chemoprophylaxis in recent convertors (Chemoprophylaxis - prohylaxis by the use of chemicals\drugs) (If a person has had negative TB tests (purified protein derivative (PPD) test in the past, and then 1 year later the test is positive, that person is said to be a recent convertor. The current recommendations is that the person be placed on isoniazid for 6-12 months as long as there are no evidence of clinical disease (ie X-ray)) d. Isoniazid - Side effects 1. Hepatotoxicity (Isoniazid-induced liver dysfunction can occur in 10-20% of patients, but is reversible in most cases) 2. Peripheral neuropathy (<- Pyridoxine deficiency, isoniazid forms a chemical combination with pyridoxine, can be overcomed by pyridoxine supplementation) (There are genetically fast and slow acetylators of isoniazid. Slow acetylators have a longer half-life) (Stringer) |
|
|
Drugs used in Tuberculosis and Leprosy - Rifampin
a. Mechanism b. Spectrum c. Metabolism d. Side effects |
a. Mechanism
I. Inhibits RNA synthesis by forming a stable complex with the beta-subunit of DNA-dependent RNA polymerase. (Develop resistance by a single mutation in the beta-subunit) b. Spectrum 1. Mycobacterium tuberculosis 2. Mycobacterium leprae 2. Some G+ and G- bacteria c. Metabolized in the liver, induce microsomal P-450 enzymes. d. Side effects 1. Hepatitis and jaundice 2. Induce liver P-450 enzymes 3. Can color urine, feces, saliva, sweat, and tears red-orange (Rifabutin is an analogue that has some activity against rifampin-resistant Mycobacterium tuberculosis) (Stringer) |
|
|
Drugs used in Tuberculosis and Leprosy - Pyrazinamide
a. Mechanism b. Spectrum c. Side effects |
a. Uknown
b. Mycobacterium tuberculosis (Particularly effective against intracellular organisms) c. Side-effects 1. Hyperuricemia 2. Hepatotoxicity (Stringer) |
|
|
Drugs used in Tuberculosis and Leprosy - Ethambutol
a. Mechanism b. Spectrum c. Side effects |
a. Mechanism - unknwon
b. Spectrum - Mycobacterium tuberculosis c. Side effects 1. Optic neuritis -> Loss of central vision and impaired red-green discrimination 2. Hyperuricemia (From induced decreased renal clearance of urate) (Stringer) |
|
|
Antifungal drugs - Polyene antifungals
a. Members b. Mechanism c. Which one is most commonly used for serious disseminated yeast and fungal infections, particularly in immunocompromised patients d. What is the most common and serious side-effect of Amphotericin B |
a. Members
1. Amphotericin B 2. Nystatin b. Bind and disrupt ergosterol, the principal fungal membrane sterol. -> Cause disruption of membrane function and allow leakage of electrolytes followed by death. (In humans its cholesterol) c. Amphotericin B (Nystatin is too toxic for systemic use. Its use is limited to topical treatment for candida albicans) d. Nephrotoxicity (Dose and duration dependent) (Also chills, fever and tachypnea after use) (Stringer) |
|
|
Antifungal drugs - Azole antifungals
a. Members b. Spectrum c. Mechanism |
a. Members (-azole)
1. Imidazole (2N in azole ring) I. Topical and systemic: Ketoconazole Miconazole II. Topical: Butoconazole Clotrimazole Econazole 2. Triazoles (3N) I. Systemic Fluconazole Itraconazole Voriconazole b. Spectrum I. Broad-spectrum fungistatic agents c. Mechanism I. Inhibit 13-alpha-demethylase which is a part of the fungal ergosterol synthetic pathway (Imidazoles are primarily topical, triazoles are active systemically. The triazoles have fewer side effects, better drug distribution, and fewer drug interactions than imidazoles) (Stringer) |
|
|
Antifungal drugs - Others
a. Caspofungin - Group, Spectrum, Mechanism b. Terbinafine - Mcehanism, spectrum c. Griseofulvin |
a. Caspofungin
I. Echinocandin group II. Vs invasive aspergillosis and candidasis III. Non-competitively inhibits beta-(1,3)-D-glucan, a unique cell wall component of the fungi b. Terbinafine I. Prevent ergosterol synthesis by inhibiting squalene epoxidase -> intracellular squalene accumulation II. Vs skin and nail fungi (But given orally..) (Tolnafte have similar mechanism) c. Griseofulvin I. Bind to keratin in keratin precursor cells and make them more resistant to fungal infections (A dermatophyte infection can only be cured when the infected skin, nail, or hair is replaced by the new keratin-containing griseofulvin) II. Vs superficial fungal infections - skin, hair (Stringer) |
|
|
Anti-HIV drugs - Reverse transcriptase inhibitors
a. Members - 3 Groups b. Mechanism |
a. Reverse transcriptase inhibitors
1. Nucleosides I. Abacavir II. Didanosine III. Lamivudine 2. Nonnucleoside I. Amprenavir II. Delavirdine III. Efavirenz 3. Nucleotides I. Adefovir II. Tenofovir (Mutation of the RT enzyme is very rapid, its therefore used at least two RT inhibitors simultaneously to slow the emergence of resistant viruses) b. Mechanism I. Inhibit the formation of viral DNA from RNA by RT II. Nucleotides and nucleosides is integrated into the DNA strand and cause early termination (Stringer) |
|
|
Anti-HIV drugs
a. Protease inhibitors - Members b. Protease inhibitors - Mechanism c. Enfuvirtide - Member of which group, Mechanism of action d. What is currently assumed to be the most effective treatment for HIV |
a. Protease inhibitors - Members
1. Amprenavir 2. Indinavir 3. Nelfinavir 4. Ritonavir b. Protease inhibitors - Mechanism I. Interfere with the processing of the viral protein -> Prevent formation of new viral particles (The HIV protease enzyme is involved in maturation of the newly forming viral particle.) (Many side effects, including changes in fat deposition and metabolic abnormalities) c. Enfuvirtide I. Belong to fusion inhibitors II. Inhibit fusion of the virus with the cell by being an analog of the HIV protein that mediates fusion. (-> When enfuvirtide binds in place of the HIV protein it traps the viral particle in a conformation that prevents its fusion with the cell) c. Triple-therapy\cocktail with two RT inhibitors and one protease inhibitor simultaneously. (Stringer) |
|
|
Antiviral drugs used in influenza
a. Neuraminidase inhibitors - Members b. Which is used for the prevention and treatment of influenza type A infections c. Neuraminidase inhibitors - Mechanism |
a. Neuraminidase inhibitors
1. Amantadine (Also used for Parkinson's -> Anticholinergic, antiglutamatergic, probably also release dopamine in striatum (Blumenfeld)) 2. Rimantadine 3. Oseltamivir b. Amantadine (Shortens the duration of symptoms by half if begun within 48 hours of the onset of illness) c. Neuraminidase inhibitors - Mechanism I. Neuraminidase is an enzyme located on the surface of the virus that breaks a bond between the virus and proteins on the cell surface II. Inhibition -> Inhibit breakage of this bond and release of newly formed viral particles from the cell (Competitive inhibitor) (Blumenfeld) |
|
|
Antiviral drugs
a. Drugs used to treat patients with herpes infection b. Drugs used to treat patients with RSV infection |
a. Drugs used to treat patients with herpes infection
1. Acyclovir (Drug of choice, must be activated by triple phosphorylation to be active (is a nucleoside), inhibits the herpes virus DNA polymerase) 2. Famciclovir 3. Ganciclovir 4. Vidarabine (ara-A) 5. Cidofovir b. Drugs used to treat patients with RSV infection 1. Ribavirin (used to treat RSV in infants and young children, aerosol) 2. Palivizumab (Humanized monoclonal antibody against a glycoprotein on the surface of the virus, it is given as an injection at the start of RSV season in high-risk children to provide passive immunity) (Blumenfeld) |
|
|
Protozoa, Which disease is caused by
a. Entamoeba histolytica b. Balantidium coli c. Trichomonas vaginalis d. Giardia lamblia e. Leishmania f. Trypanosoma brucei g. Trypanosoma cruzi |
a. Entamoeba histolytica -> Amebiasis
(Diarrhea) b. Balantidium coli -> Balantidial dysentery c. Trichomonas vaginalis -> Trichomoniasis (Genital infection) d. Giardia lamblia -> Giardiasis (Diarrhea, c. and d. is most common in US and both are treated with metronidazole) e. Leishmania -> Leishmaniasis (Three types) f. Trypanosoma brucei -> African sleeping disease g. Trypanaosoma cruzi -> Chagas' disease (South American) (Blumenfeld) |
|
|
Antiprotozoal drugs
a. Metronidazole - Spectrum b. Metronidazole - Mechanism c. Metronidazole - Side effects d. Other antiprotozoal drugs |
a. Metronidazole - Spectrum
1. Trichomoniasis and giardiasis (The most common protozoal infections in US) 2. Anerobic bacteria (+ Other protozoal infections) b. Metronidazole - Mechanism 1. It penetrates only protozoal and bacterial cell walls (Cant enter mammalian cells) 2. Must be activated by nitroreductase (Found only in anaerobic organisms) 3. Reduced metronidazole inhibits DNA replication by causing breaks and inhibiting repair of the DNA c. Metronidazole - Side effects 1. N&V 2. Diarrhea 3. Turn urine dark or red-brown 4. Metallic taste in the mouth 5. Disulfiram-like reaction when taken with alcohol (: Abdominal cramping, vomiting, flushing, headache) (Blumenfeld) |
|
|
Antimalarial agents
a. Of the 50 different species of plasmodium, which four are infectious to humans b. Which two can persist in the liver c. What is the most prevalent d. What is the most serious and lethal form |
a. The four plasmodium species infectious to humans
1. Plasmodium malariae 2. Plasmodium vivax 3. Plasmodium ovale 4. Plasmodium falciparum b. Plasmodium vivax and ovale (-> Only patients infected with these species can relapse) c. Plasmodium vivax d. Plasmodium falciparum (Stringer) c. |
|
|
Antimalarial agents
a. Which part of the cycle is thought to give rise to symptoms, What are drugs that eliminate this form called b. What is the drug of choice for all malarial infections except those strains that are resistant c. What are the drugs of choice for oral use d. Which agent is effective against the liver form (exoerythrocytic) and kills gametocytes e. Which agent is used for prophylaxis for travelers entering areas malaria is endemic |
a. The erythrocytic form, Suppressive or Schizonticidal agents
(Schizont - A sporozon trophozite (vegetative form)) b. Chloroquine (Becomes concentrated in melanin-containing structures, in high doses this can lead to corneal deposits and blindness (and skin problems)) d. Primaquine (Often used for prophylaxis or prevention of relapse due to its effectiveness against the liver phase) (Can cause hemolytic anemia in G-6P DH deficient patients) e. Chloroquine (However, only for areas where chloroquine-sensitive malaria is endemic, resistance is becoming a big problem) (Stringer) c. Quinine and quinidine (<- Cinchona tree, Overdose -> Cinchonism - Sweating, N&V&D, Ringing in the ears, impaired hearing, and blurred vision) |
|
|
Anticancer drugs - Organization of drugs - 2 groups
|
1. Cytotoxic drugs
(Drugs which block cell replication) I. Alkylating agents - Nitrogen mustards and nitrosoureas II. Antimetabolites - Folate antagonists, purine and pyrimidine analogues (Antimetabolite - A substance that competes with, replaces, or antagonizes a particular metabolite) III. Antibiotics and other natural products - Anthracyclines, vinca alkaloids IV. Antibodies - To improve specificity V. Kinase inhibitors - Imatinib, Gefitinib VI. Proteasome inhibitors - Bortezomib VII. Other cytotoxic drugs 2. Hormonal agents (Drugs for hormone-sensitive tumors - Prostate, breast) (Stringer) |
|
|
Cytotoxic drugs - Alkylating agents
a. Mechanism b. Cycle specific c. Members - 3 Groups |
a. Works by adding an alkyl group to DNA
(All of these drugs introduce alkyl groups into nucleophilic sites with covalent bonds, the degree of DNA alkylation correlates with the cytotoxicity of the drug) b. No (-> Prone to cause local tissue necrosis and damage) c. Alkylating agents 1. Nitrogen mustards I. Chlorambucil II. Cyclophosphamide III. Ifosfamide IV. Mechlorethamine 2. Nitrogen mustards (-mustine) I. Carmustine II. Lomustine (2. is lipid soluble -> cross BBB -> used for brain tumors) 3. Other alkylating agents I. Busulphan II. Carboplatin III. Cisplatin IV. Dacarbazine (Stringer) |
|
|
Cytotoxic drugs - Antimetabolites
a. General mechanism b. Members - 3 Groups |
a. Compete for binding sites on enzymes or can be incorporated into DNA or RNA.
b. Antimetabolites 1. Folate antagonists I. Methotrexate 2. Purine analogues I. Cladribine II. Mercaptopurine (6-mercaptopurine) III. Thioguanine 3. Pyrimidine analogues I. Capecitabine (Prodrug for 5-FU) II. Fluorouracil (5-FU) III. Cytarabine (Cytosine arabinoside, ara-C) (Arabinoside - a ribonucleoside in which the sugar is arabinose) (Stringer) |
|
|
Cytotoxic drugs - Antimetabolites - Methotrexate
a. Mechanism b. How is cellular resistance achieved c. What is leucovorin used for d. Use |
a. Methotrexate - Mechanism
1. Competitively inhibits dihydrofolate reductase -> 2. Inhibit thymidylate synthase -> (deoxyuridine monophosphate -> thymidine monophosphate, use tetrahydrofolate) 3. Inhibit DNA synthesis b. Cellular resistance I. Cellular uptake of methotrexate is by a carrier-mediated active transport II. Cellular resistance is presumably caused by decreased transport into the cell (This can be overcome by using high doses) c. It provides reduced folate to ''rescue'' normal cells from the action of methotrexate ("Leucovorin rescue" during cancer treatment) d. Methotrexate spectrum 1. A whole variety of cancers (Can be given intrathecally) 2. Psoriasis 3. Severe RA (Stringer) |
|
|
Anticancer drugs
a. Log kill b. Parts of the cell cycle c. General and important specific side effects |
a. Log kill
I. The concept that anticancer drugs kill a constant fraction of cells instead of an absolute number II. Example of first-order kinetics (There is a linear relationship between the concentration of an anticancer drug and the number of surviving cells, when the number of cells is plotted on a log scale) (A drug that reduces a tumor cell load from 10^8 cells to 10^5 cells is said to have achieved a 3 log kill) b. Cell cycle I. --> G1 --> (G0 go in and out of G1) II. S --> (DNA synthesis) III. G2 --> IV. M --> V. G1 -->... c. Side effects 1. Bone marrow toxicity (<- Destruction of proliferating hematopoietic stem cells, -> pancytopenia) I. Increased risk of infection II. Increased risk of bleeding 2. Gastrointestinal toxicity I. N&V (<- Central effect of almost all the cancer chemotherapy drugs, stimulate chemoreceptor trigger zone) II. Direct damage on proliferating mucosa (-> Ulcer formation anywhere in the GI tract - Mouth, esophagus, stomach..) 3. Hair loss (<- Damage rapidly dividing cells of the hair follicles) (Especially with cyclophosphamide, doxorubicin, vincristin, methotrexate, and dactinomycin) 4. Cisplatin and higher-dose methotrexate -> Renal tubular damage 5. Cyclophosphamide -> Hemorrhagic cystitis 6. Anthracyclines -> Cardiotoxicity (with D-rubicins - doxyrubicin, daunorubicin) (Arrhythmias, decreased function, myofibrillar degeneration, focal necrosis of myocytes. Presumed to be from free radical generation and lipid peroxidation) 7. Bleomycin -> Pulmonary fibrosis (Can be fatal) 8. Vincristine -> Nervous system toxicity (The only anticancer drug that has a dose-limiting neurotoxicity) (Stringer) |
|
|
Anticancer drugs
a. How is the effects of bone marrow toxicity minimized b. How is N&V minimized |
a. By using growth factors
1. Filgrastim -> Accelerate recovery of neutrophils (filGRASTIM - GRAnulocyte colony-STIMulating factor) 2. Sargramostim -> Accelerate general bone marrow population (sarGRAMoSTIM - GRAnulocyte-Macrophage colony-STIMulating factor) 3. Thrombopoietin -> Platelets 4. Erythropoietin -> RBCs b. Antiemetics for chemotherapy 1. Serotonin antagonists (5-HT3) - Ondansetron (-setrons: dolasetron, granisetron, palonosetron) 2. Substance P neurokinin 1 receptor antagonist - Aprepitant (Neurokinin 1 receptors are present in the emesis center in the medulla) 3. Glucocorticoids (Triple combo is used) (Blumenfeld) |
|
|
Anticancer drugs - Antibiotics and other natural products - Organization
|
1. Anthracyclines
I. Daunorubicin (Daunomycin) II. Doxorubicin III. Epirubicin IV. Idarubicin 2. Other antibiotics I. Bleomycin II. Dactinomycin (Actinomycin D) III. Mitomycin (Mitomycin C) IV. Plicamycin (Mithramycin) 3. Vinca alkaloids I. Vinblastine II. Vincristine III. Vinorelbine 4. Other natural products I. Docetaxel II. Paclitaxel III. Etoposide IV. Teniposide (These -sides are topoisomerase inhibitors) (Stringer) |
|
|
Anticancer drugs - Antibiotics and other natural products
a. Which have cardiotoxicity b. Which can cause fatal pulmonary fibrosis c. Which can be used to treat life-threatening hypercalcemia associated with malignancy d. Which binds to tubulin and disrupt the spindle apparatus during cell division e. For which drug are the neurologic toxicity dose-limiting f. Which drug works by preventing depolymerization of microtubules |
a. The anthracyclines
(Include arrhythmias, decreased function, myofibrillar degeneration, and focal necrosis of myocytes. It has been postulated that the free damage is caused by free radical generation and lipid peroxidation. Dexrazoxane help to protect against this by chelating intracellular iron so the iron cant react with superoxide anions and hydrogen peroxide to produce the highly toxic free radicals) b. Bleomycin c. Plicamycin (mithramycin) (By inhibiting resorption of bone by osteoclasts) d. The vinca alkaloids (Vincristine, vinblastine, vinorelbine) e. Vincristine (The bone marrow toxicity is limiting for the other two vinca alkaloids (which both have b in them - vinoBlastine and vinorelBine) f. Paclitaxel (Paclitaxel is isolated from the rare Pacific yew and quantities are very limited, docetaxel is synthesized from a precursor isolated from the more available European yew tree) (Stringer) |
|
|
Radial nerve lesion
a. Synonym a. S&S |
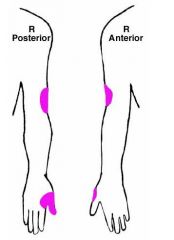
a. Saturday night palsy
(<- Often results from sleeping drunk with one arm over the chair all night, compromising the radial nerve in the spiral groove of the humerus) b. S&S 1. Weakness of elbow and wrist extension I. Wrist drop 2. Poor extension of fingers at MCP joints 3. Decreased triceps reflex 4. Sensory deficit in colored area (Goldberg) |
|
|
Median nerve lesion
a. S&S |
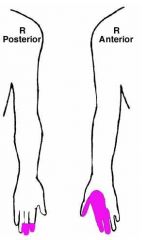
a. S&S
1. Weakness of I. Wrist flexion II. Thumb flexion III. Index, and middle finger flexion IV. Forearm pronation V. Flexor carpi radialis 2. Atrophy of the thenar eminence 3. Sensory deficits in the colored areas (Goldberg) |
|
|
Ulnar nerve lesion
a. Synonym b. S&S |
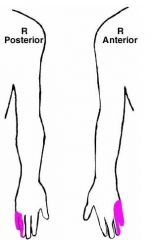
a. Partial\Ulnar claw hand
(Ring and small finger) b. S&S 1. Weakness of I. Flexion of wrist II. Flexion of ring and small finger III. Opposition of little finger IV. Flexor carpi ulnaris V. Abduction and adduction of fingers 2. Atrophy of the hypothenar eminence 3. Sensory deficit in colored areas (Goldberg) |
|
|
Musculocutaneous nerve lesion
a. S&S |
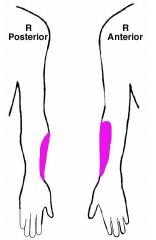
a. S&S
1. Weakness of I. Elbow flexion II. Forearm supination 2. Absent biceps reflex 3. Sensory deficit in colored areas (Goldberg) |
|
|
Femoral nerve lesion - S&S
|
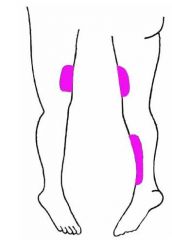
S&S
1. Weakness of knee extension 2. Absent knee jerk 3. Sensory deficit in colored area (Goldberg) |
|
|
Rostral midbrain
1-7 - Structure, result of lesion |
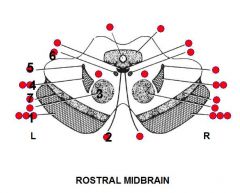
1. Several
I. Corticomesencephalic tract -> Inability to look contralaterally (It contains fibers from frontal and occipital lobes that control lateral conjugate gaze to the opposite environment) II. Corticospinal tract -> Contralateral weakness of body III. Corticobulbar tract -> Contralateral weakness of lower face and in some people tongue (For practical reasons the corticobulbar fibers may be considered to cross over at the level of the nuclei of CN VII and XII) 2. CN III -> I. Weakness of extraocular muscles except lateral rectus and superior oblique -> Eye is down and out II. Pupil dilated and nonresponsive to light III. Ptosis 3. Red nucleus -> Contralateral intention tremor and ataxia (Since it receives input from the contralateral cerebellum which cross in the midbrain) 4. Several structures I. Trigeminal lemniscus -> Contralateral loss of facial sensation II. Medial lemniscus -> Contralateral loss of proprioception and stereognosis 5. Spinothalamic tract -> Contralateral loss of pain-temperature sensation 6. MLF -> Inability to adduct ipsilateral eye on attempted contralateral gaze 7. Substantia nigra -> Contralateral slowness, stiffness, and Parkinson-like tremor (4-6 Hz) (Goldberg) |
|
|
Caudal midbrain
1-8 - Name and effect of lesion |
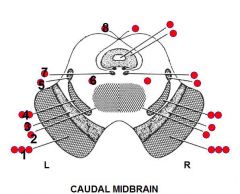
1. Several structures
I. Corticospinal tract -> Contralateral weakness of body II. Corticobulbar tract -> contralateral weakness of lower face and in some people the tongue III. Corticomesencephalic tract -> Inability to initiate contralateral lateral conjugate gaze (<- Contain fibers from frontal and occipital lobes that control lateral conjugate gaze to the opposite environment) 2. Decussation of superior cerebellar peduncle 3. Substantia nigra -> Contralateral slowness, sitffness, and Parkinson-like tremor 4. Several structures I. Trigeminal lemniscus -> Contralateral loss of facial sensation II. Medial lemniscus -> Contralatereal loss of proprioception-stereognosis of body 5. Spinothalamic tract -> Contralateral loss of pain-temperature sensation of body 6. Reticular formation -> I. Ipsilateral Horner's syndrome (Horner's syndrome can arise from a lesion anywhere from the hypothalamus to sympathetic plexus on ICA) (Brain stem Horner's may be associated with contralateral loss of pain-temperature since fibers lie near spinothalamic tract) 7. MLF -> Inability to adduct ipsilateral eye on attempted gaze to contralateral side 8. Trochlear nerve -> Loss of superior oblique function I. Double vision II. head tilt (Infraduct when eye is adducted, intorsion when eye is abducted) (Goldberg) |
|
|
Rostral pons
1-9 - Name, Effect of lesion |
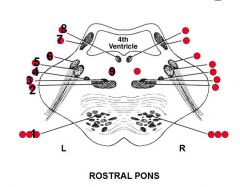
1. Several structures
I. Corticospinal tract -> Contralateral weakness of body II. Corticomesencephalic tract -> Weakness of contralateral lateral conjugate gaze III. Corticobulbar tract -> Weakness of contralateral lower face and in some people weakness of contralateral tongue 2. Medial lemniscus -> Contralateral loss of proprioception and stereognosis 3. Trigeminal lemniscsus -> Contralateral loss of facial sensation 4. Spinothalamic tract -> Contralateral loss of pain-temperature sensation 5. Motor nucleus of CN V -> Ipsilateral weakness of the chewing muscles, especially masseter 6. Main sensory nucleus of CN V -> Ipsilateral loss of facial sensation (Particularly of light touch at this brain stem level) 7. Superior cerebellar peduncle -> Ipsilateral ataxia 8. MLF -> Inability to adduct ipsilateral eye on attempted gaze to contralateral side 9. Reticular formation -> Horner's syndrome ++ (Goldberg) |
|
|
Caudal pons
1-6 - Area, effect of lesion |
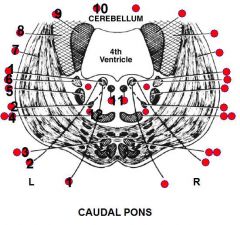
1. CN VI and its nuclei -> Inability of ipsilateral eye to abduct
2. CN VII -> I. Paralysis of upper and lower part of the ipsilateral face II. Hyperacusis 3. Several structures I. Corticospinal tract -> Contralatereal weakness of the extremities II. Corticobulbar tract -> Weakness of contralateral lower face and in some people weakness of the contralateral tongue (For practical considerations, the corticobulbar fibers may be considered to cross over at the level of the nuclei of CN VII and VN XII) 4. Several structures I. Trigeminal lemniscus -> contralateral loss of facial sensation II. Medial lemniscus -> Contralateral loss of proprioception and stereognosis of body 5. Main sensory nucleus of CN V -> Ipsilateral loss of facial sensation (Particularly light touch at this brain stem level) 6. Vestibular nuclei -> Vertigo and absent cold caloric testing on that side (Goldberg) |
|
|
Caudal pons
7-12 - Name, effect of lesion |
7. Middle cerebellar peduncle
(Receives fibers from contralateral pontine nuclei, which in turn receive fibers from cerebrum ipsilateral to the same pontine nuclei) 8. Inferior cerebellar peduncle -> Ipsilateral ataxia (Receives input from the ipsilateral spinocerebellar tract) 9. Superior cerebellar peduncle -> Ipsilateral ataxia (Since it comes from ipsilateral cerebellum, crosses over to the red nucleus via the decussation of the superior cerebellar peduncle at the midline of the midbrain) 10. Cerebellum -> Ipsilateral ataxia 11. MLF -> Inability of ipsilateral eye to adduct on attempted contralateral lateral conjugate gaze 12. Spinothalamic tract -> Ipsilateral loss of pain and temperature sensation (Goldberg) |
|
|
Anticancer drugs - Antibodies - Give the target for
a. Alemtuzumab b. Bevacizumab c. Cetuximab d. Gemtuzumab ozogamicin |
a. Alemtuzumab - CD52
(On mature lymphocytes, used in the treatment of chronic lymphocytic leukemia) b. Bevacizumab - For vascular endothelial growth factor (Angiogenesis inhibitor) c. Cetuximab - Epidermal growth factor receptor (For patients with EGFR-expressing cancers) d. Gemtuzumab - CD33 (Expressed on the surface of leukemic myeloblasts and immature cells of the myelomonocytic line, conjugated with an antitumor antibiotic called calicheamicin. The binding of the antibody to the surface of the cell triggers internalization of the complex. The calicheamicin piece is released and binds to DNA causing the strands to break. Thus, this antibody improves targeting of a cytotoxic agent) (Stringer) |
|
|
Anticancer drugs - Antibodies - Give the targets for
a. Ibritumomab tiuxetan labeled with yttrium 90 b. Tositumomab labeled with I-131 c. Trastuzumab |
a. Ibritumomab tiuxetan labeled with yttrium 90 - CD20
b. Tositumomab labeled with I-131 - CD20 (a. and b. are targeted for CD20 which is a B-cell antigen. Both have a radioactive label. The antibody brings the radiation to the tumor cells to increase specificity of the radiation.) c. Trastuzumab - HER2 (Human epidermal growth factor receptor 2, a protein giving higher aggressiveness to breast cancer) (Stringer) |
|
|
Anticancer drugs - Hormonal agents
a. Mechanism b. Members - 5 groups |
a. Reduce the level of the hormone that is stimulating growth of the tumor or block the receptor for the hormone
b. Members 1. Glucocorticoids 2. Aromatase inhibitors I. Anastrozole II. Exemestane III. Formestane (2. are used to treat estrogen-dependent tumors (breast cancer) resistant to tamoxifen. -ozoles (anastrozole and letrozole) are competitive inhibitors while -mestanes (formestane) bind covalently to the enzyme) 3. Estrogens\Antiestrogens I. Fulvestrant II. Tamoxifen citrate III. Toremifene (Tamoxifen and toremifene are competitive antagonists used in the treatment of breast cancer, fulvestrant works as an estrogen-receptor antagonist and downregulates the estrogen receptor) 4. Androgen receptor antagonists I. Bicalutamide II. Flutamide III. Nilutamide (The -lutamides are competitive testosterone antagonists used to treat prostate cancer) 5. GnRH analogs and antagonists I. Analogs - Gosrelin, Triptorelin II. Antagonists - Abarelix, Leuprolide (Both GnRH analogs and antagonists will decrease serum levels of estrogen and testosterone and are used to treat androgen-dependent prostate cancer) (The hypothalamus normally releases GnRH in a pulsatile fashion, continuous administration of GnRH analogs will suppress release of LH and FSH) (Stringer) |
|
|
Anticancer drugs - Miscellaneous agents
a. Gefitinib and erlotinib - Group, mechanism b. Imatinib - Group, mechanism, use c. Bortezomib - Mechanism d. Transretinoic acid - Mechanism, use |
a. Gefitinib and erlotinib
I. Signal transduction inhibitors - Tyrosine kinase inhibitors II. They inhibit tyrosine kinase linked to epidermal growth factor receptor (The receptor tyrosine kinases are largely associated with growth factors. Gefitinib reversibly binds to the ATP site on the kinase and completely inhibits phosphorylation. This blocks all of the downstream signaling by the receptor) b. Imatinib I. Signal transduction inhibitors - Tyrosine kinase inhibitor II. Inhibits the BCR-Abl tyrosine kinase found in chronic myelogenous leukemia (CML) (In the patients which have the chromosomal translocation Philadelphia chromosome that results in the formation of a tyrosine kinase that the cell cannot regulate) c. Bortezomib I. Proteasome inhibitor (The proteasome is a large complex of proteins that si responsible for the regulation of protein expression and the degradation of damaged or used proteins in the cell. It regulates the expression of cell cycle proteins. Malignant cells are more sensitive to inhibition of proteasome function than normal cells) d. Transretinoic acid I. Stimulate growth of normal myeloid and erythroid progenitors and cause DIFFERENTIATION of myeloid leukemic cells -> Can induce remission in acute promyelocytic leukemia (Stringer) |

