![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
252 Cards in this Set
- Front
- Back
|
Chronic Anxiety Disorders
|
-Panic Disorder (4%) - recurrent episodes of intense anxiety
-Generalized Anxiety Disorder (5%) --> chronic persistent anxiety -Social Phobia (5-10%) --> intense fear of negative evaluation during social contact -Post-Traumatic Stress Disorder (8%) --> intense fear, helplessness, nightmares, flashbacks for prolonged period after exposure to a traumatic event Obsessive-Compulsive Disorder (6%) --> persistent, recurrent thoughts causing anxiety |
|
|
Drugs Used to Treat Anxiety
|
Benzodiazepines
5-HT/NE Uptake Inhibitors (drug of choice) Burpirone (5HT receptor partial agonist) |
|
|
Benzodiazepines for Anxiety
|
-safe, effective anxiolytics
-anxiolysis occurs within 1-2 hours -pharmacokinetics dictate use -dependence with chronic use (part physical & part psychological) -limited usefulness for chronic anxiety |
|
|
Benzodiazepines
(Length of Action) |
-Alprazolam (Xanax) - short acting
-Diazepam (Valium) - Intermediate acting -Chlordiazepoxide - Intermediate acting -Lorazepam (Ativan) - Long acting |
|
|
Benzodiazepines
(Pharmacokinetics) |
-Absorption is rapid for Alprazolam, Diazepam & Triazolam (1 hour to peak)
-Absorption is slower for Oxazepam, Lorazepam, Prazepam (2-3 hours to peak) - longer onset is better for helping pt sleep -Biotransformation (main determinant of half-life) takes place in the Liver: 1. Microsomal oxidation to produce hydroxylated & N-desalkylated metabolites that have biological activity 2. Glucuronidation of 3-OH groups -Pts with cirrhosis or the elderly have increased t1/2s & require decreased dose |
|
|
CNS
|
-a small perturbationat one point can cause major, unanticipated changes at a distant point
|
|
|
Cerebral cortex
|
-consists of frontal, temporal, parietal and occipital lobes.
-divided by information processed: -somatosensory, visual, auditory, and motor -sensory and motor integration, vision, hearing and memory -produces abstract thought, memory and consciousness -integrating role for autonomic nervous system as well as somatic and vegtative functions of the CV and GI systems |
|
|
Limbic System
|
-region of the brain composed of: hipposcampus, amygadaloid complex and septum
-regulation of emotion, the autonomic system and neuroendocrine function and short term memory |
|
|
Basal ganglia
|
-contains caudate nucleus, putamen, globus pallidus and lentiform nucleus
-regulates segments of extrapyramidal motor system needed for motor control |
|
|
Thalamus
|
-in the center of the brain below the cortex and basal ganglia and above hypothalamus.
-integrates sensory and motor functions |
|
|
hypothalus
|
-below the thalamus and is main integrating region for entire autonomic system
-regulates body temp, water balance, blood pressure, adenohypophysis secretions, sleep and emotions |
|
|
Midbrain and Brain stem
|
-connect the cerebral hemispheres and thalamus-hypothalamus to the spinal cord
-swallowing and vomiting CV and respiratory systems -receptors for visceral afferent sensory information are here -reticular activation system= sleep wakefulness, level of arousal and coordination of eye movements -nuclei of cranial nerves are here as well as the major monoamine-containing neurons of the brain |
|
|
Cerebellum
|
Standing upright and walking is controlled here. It also has a role in learning and memory
|
|
|
Spinal Cord
|
-spinal cord runs from the caudal end of the medulla oblongata to lower lumbar vertebrae
-dorsal horn, ventral horn and itermediolateral cell column -sensory info comes into the dorsal portion -motor stimuli and autonomic output exit via ventral horn |
|
|
Hierarchicial
|
-sesnory perception and with motor control
glutamate = main transmitter for projection neurons -long have a stimulatory effect |
|
|
Local circuit nuerons are shorter ones-transmitters are glycine and GABA
|
-make branches that are very close to the cell body which have synapses on the cell body and dendrites of both the projecting neurons and other local
-local project on the long projecting (or can also work on each other) -inhibitory effect on the long: can be either feedback or feed forwardI (will work ahead of the signal) |
|
|
Neuronal systems
|
1. heirarchical
2. non-specific or diffuse |
|
|
Heirarchical system
|
-characterized by long projection neurons- transmit over long distance
-local circuit neurons that are smaller whose axons branch immediate vicinity of cell body -inhibitory = release glycine (spinal cord) or GABA (gamma amino butyric acid) (supraspinally) |
|
|
Non-specific or diffuse neuronal systems
|
-uses monoamines, norepi, dopamine and 5-HT (serotonin, 5-hydroxytryptamine) as transmitter
-innervate several regions of brain don't work by specific synaptic connects work by diffuse release of transmitter so that response is governed more by receptor location -employ metabotropic (G protein mediated) receptors, have a long-lasting effect and are involved in global functions such as sleeping and waking, attention, appetite and emotions |
|
|
Ionic Control of Transmission
|
-presynaptically decreasing conductance or inward flow of Ca that is needed for fusion and release of neurotransmitter will decrease release of transmitter
-postsynaptically susceptibility to excitation may be reduced by causing hyperpolarization by either increasing outward flux of K or increasing inward flux of Cl -decreasing outward of K will result in hypopolarizaiton which will increase excitability -endogenous transmitters or drugs will increase this |
|
|
Neurotransmitter
|
-substance that is released from a presynaptic nerve terminal into the synaptic cleft that alters the excitablity of neurons
-cause excitation by opening ion channels to allow net influx of positively charged ions that leads to depolarization or cause inhibition by selective opening of ion channels = hyper polarization |
|
|
channels
|
a. ligand-gated channels = binding of neurotransmitter to the channel controls opening and closing of the channel
b. G protein-coupled receptors in which the G protein, activated by the neurotransmitter binding to the receptor, directly interacts with the ion channel c. G protein-coupled receptors in which the G protein activates an enzyme such as phospholipase C and produces a diffusible second messenger that interacts with the ion channel b and c are also called metabotropic receptors -voltage gated ion channels that are opened or closed by changes in membrane voltage |
|
|
Neutral amino acids
|
-glycne and GABA
-work thorugh cuasing hyperpolarization or inhibitory post synaptic potentials (IPSP) opening chloride channels |
|
|
GABA
|
-receptors grouped as GABAa or GABAb
-GABAa work thorugh chloride channels -GABAb coupled to G proteins that either inhibit calcium channels or activate potassium channels -calcium channesl are presynaptic and inhibit transmitter release while the potassium channels when activated = hyperpolarization thereby inhibiting impulse transmission |
|
|
Amino acid neurotransmitter
|
-glutamate and aspartate
-found in hih concentrations in many regions of the CNS -effect is excitatory -Glutamate receptors are either ligand-gated or G protein-coupled glutamate excitatory aspartate-inhibitory |
|
|
Acetylcholine
|
-acts thorugh both nicotinic and muscarinic receptors
-mediated through G protein coupled receptors, either inhibiton through M2 receptors that open potassium channels or excitation through M1 receptors that decreases membrane potassium permeability -more M1 than M2 -M1 will stimulate and cause decrese in K, M2 will be inhibitiory and cause increase in K |
|
|
Monoamines
|
-dopamine, norepi, serotonin (5-HT)
-epinephrine containing neurons are also present but little is known about their function |
|
|
dopamine
|
-dopamine containing neurons link substantia nigra to neostriatum and the ventral tegmetnal region to limbic structures (limbic cortex)
-regions involved in parkinson's diesease and psychosis -dopamine receptors: D1-like and D2-like -G-protein copuled receptors -D1-like couple thorugh Gs to increase adenyl cyclase - D2-like couple through Gi to inhibit adenyl cyclase |
|
|
locus ceruleus or lateral tegmental area of reticular formation
|
location of most noradrenergic neurons
|
|
|
noradrenergic receptors
|
-alpha-1, alpha-2 and beta are G-protein coupled receptors
-norepinephrine increases potassium conductance to hyperpolarize neurons via alpha 2 -norepi can indirectly cause excitation by inhibiting inhibitiory neurons |
|
|
alpha-1
|
- work thorugh Gq and stimulate phopholipase C
- cause deploarization by decreaseing potassium conductance |
|
|
beta
|
-via increasing adenyl cyclase
|
|
|
Serotonin-containing neurons
|
-originate in the raphe or midline regions of pons and upper brain stem
-diffusely innervate most regions of CNS -all except one of 5-HT receptors are G-protiein coupled -hyperpolarization due to increased potassium permeability or conductance -regulation of sleep, temp, apetite and neuroendocrine control, mood and anxiety -5-HT3 receptor when stimulated leads to emesis and antinociceptive actions so that antagonists are used in managing cancer chemotherapy-induced nausea |
|
|
Histamine
|
-in the ventral posterior hypothalamus project upwards and downwards to the whole CNS
-four receptor subtypes are thought to regulate arousal, body temp and vascular function throug hdecreased potassium conductance or increases in inositol tris-phosphate or diacylglycerol |
|
|
Peptides
|
-opiid peptides (enkephalins and endorphins), neurotensin, substance P, somatostain, cholecytokinin, vasoactive intestinal peptide, neuropeptide Y and throtropin-releasing hormone.
-G protein coupled -multiple subtypes -opioid peptide have cell bodies at all levels of CNS with long and short connections -involved in pain -work through mu and kappa and delta receptors |
|
|
Other possible transmitters
|
-purines, NO, and brain lipids act as endogenous cannabinoids such as anadamide and 2-arachidonyglycerol are possible neutransmiters through some such as purines and NO also are consdiered to be neuromodulators
-brain lipids listed interact with a cannabinoid receptor, CB1, -CB1 binds delta-9-tetrahydrocannabinol, the primary psychoactive ingredient in cannabis |
|
|
Neuromodulators
|
-originate from non-synpatic sites such as glia and influence the excitablity of nerve cells
-adenosine, and other purines, neurosteroids produced in the CNS, eicosanoids and NO may function as neuromodulators -circulating steroids may also have neuroodulatory activity |
|
|
Benzodiazepines
(Contraindicates) |
-Avoid if mental alertness is required for daily functions (e.g. driving or operating machinery)
-Avoid in individuals with propensity for drug dependence (e.g. drinkers, 20% of the population). Potentially fatal if used with alcohol or other CNS depressants -Avoid in pregnant women due to birth defects |
|
|
Benzodiazepines
(Other Therapeutic Actions) |
Sedation (higher doses)
Muscle relaxation Anti-spastic Anticonvulsant Amnesia (during surgery) Alcohol withdrawal Anxiolytic at low doses |
|
|
Benzodiazepines
(Mechanism of Action) |
BZs increase neuronal inhibition via interactions at GABA-A receptors (Chloride ion channels - increase frequency of opening)
-Causes hyperpolarization and inhibits the firing rate, which slows everything (reduced excitability throughout the nervous system). -Binding of Diazapam increases the affinity of GABA for its receptor |
|
|
Molecular Basis of Benzodiazepine Actions
|
Alpha-1 subunit receptors mediate hypnosis/sedation
Alpha-2 & Alpha-5 receptors mediate anxiolysis Alpha-3 receptors --> non-specific Alpha-4 receptors --> insensitive to benzodiazepines Alpha-6 receptors --> insensitive to benzodiazepines |
|
|
Benzodiazepine effect on GABA
|
-Benzodiazepine on GABA-A receptors increaeses the affinity of the receptor for GABA.
-Benzodiazepines have no direct effect on GABA receptors, but increase the frequency of chloride channel opening to enhance the response to GABA. -Benzodiazepines can't cause any more inhibition than the neurotransmitter itself. |
|
|
Benzodiazepines
(Adverse Effects) |
-very safe; overdose not fatal
-rebound anxiety, aggression, irritability -synergistic with alcohol (can be fatal) -memory/cognitive impairment -psychomotor impairment (sedation, ataxia) |
|
|
Benzodiazepines
(Tolerance & Dependence) |
Pharmacodynamic tolerance:
-Little tolerance to anxiolytic effects (sedative/hypnotic > anticonvulsant > antispastic >> anxiolytic) --> limited use to seizure disorders b/c it requires ongoing medication Physical dependence: -Withdrawal syndrome (mild to severe) -Most difficult with short-acting benzodiazepines -Taper dose gradually and/or substitute with a long-acting benzodiazepine |
|
|
Usefulness of benzodiazepines in anxiety disorders
|
Panic - alprazolam, excellent efficacy; high dependence risk
GAD - reasonably effective, chronic use troublesome--last choice Social Phobia - not useful Acute Stress Disorder - very effective for acute anxiety PTSD - not useful in chronic PTSD |
|
|
Use of 5-HT/NE Uptake Inhibitors in Anxiety Disorders
|
Panic
-SSRIs; Paroxetine, Sertraline -SNRIs; imipramine - 2nd choice GAD (Generalized Anxiety Disorder) -SNRIs; venlafaxine -SSRIs; Paroxetine Social phobia -SSRIs; Paroxetine, Sertraline -SNRIs; venlafaxine PTSD -SSRIs; Paroxetine, Sertraline |
|
|
5-HT/NE Uptake Inhibitors - SSRIs & SNRIs
(Adverse Effects) |
Temporary nervousness/activation
Insomnia Prolonged incidence of tremor GI disturbances Decreased libido Contraindicated in pregnancy |
|
|
Proposed cellular action of serotonergic drugs in the treatment of anxiety
|
-Anxiety is mediated by excessive neuronal activity in the hippocampus
-Can reduce activity through the GABA or the serotonin receptors -SSRIs increase serotonin, which in turns acts on 5-HT1A & inhibits activity -Must be taken for a couple of weeks before therapeutic levels are obtained (Benzodiazepines for immediate, SSRIs for long-term) |
|
|
Buspirone
|
-Full/Partial agonist at 5-HT1A receptors
-Anxiolytic efficacy questionable -Does not act via GABA-A receptors -No sedative, anticonvulsant or anti-spastic (muscle relaxing) activity -No tolerance, dependence or rebound anxiety BUT... -Clinical improvement occurs only after days to weeks of therapy -Useful only for chronic anxiety states, not for acute therapy |
|
|
Summary of Pharmacologic Treatment of Anxiety Disorders
|

|
|
|
Other therapeutic uses of SSRIs
|
-Depression (higher doses)
-Pain management -Premenstual dysphoric disorder -Sleep apnea - increases respiratory drive (paroxetine) |
|
|
Insomnia
|
Difficulty getting to sleep, awakening early or not being refreshed by sleep
Transient - very common, caused by stressful events Chronic - may arise from disease or correctable behavior |
|
|
Insomnia
(Sedative vs Hypnotic) |
Sedative - a drug that produces calmness & relaxation & reduces arousal
Hypnotic - a drug that promotes sleep; facilitates onset & maintenance All anxiolytic & sedative benzos have hyponotic properties if the dose is increased Not useful for chronic |
|
|
Insomnia
(Treatment) |
Selective Type 1 Benzodiazepine Receptor Agonists
Sedating SSRIs or SNRIs Non-selective Benzodiazepines Ideal Drug to Treat Insomnia: -Rapid onset, no residual effects (sedation, memory loss) -No rebound insomnia |
|
|
Pharmacologic Treatment of Transient Insomnia
|
Type 1 BZ receptor agonists:
-Zaleplon (Sonata; short-acting - 3 hours) -Zolpidem (Ambien; 6 hrs duration, no hangover & little rebound insomnia) -Eszopliclone (Lunesta; 8 hr duration, no hangover, no tolerance, little rebound insomnia) |
|
|
Pharmacologic Treatment of Chronic Insomnia
|
-Associated with depression, anxiety or sleep apnea (SSRIs or SNRIs)
-Long acting - anxious patient (Flurazepam, diazepam) -Intermediate acting - difficulty stay asleep (eszopiclone, temazepam) -Short acting - must be alert in the morning (zolpidem) |
|
|
Problems with Non-Selective Benzodiazepines for Treatment of Insomnia
|
-Next day sedation, memory loss, difficulty with concentration
-Development of tolerance -Rebound insomnia w/ abrupt discontinuation -Synergistic with alcohol |
|
|
Type 1 Benzo Receptor Agonists
(Ideal Hypnotics) |
-Rapid onset, short t1/2, minimal side effects
-Well tolerated by elderly -Similar efficacy to BZs -Little next day sedation -No significant drug interactions -Less rebound insomnia or withdrawal than BZs -Additive with EtOH, not synergistic |
|
|
Schizophrenia
(Clinical Features) |
Positive Symptoms (distortion or excess of normal function)
-Disturbance of perception (hallucinations) -Disturbance of thought content (delusions) -Disorganization of thought, speech, & behavior Negative Symptoms (a decrease or loss in normal function) -Decreased expression of feelings; diminished emotional range -Poverty of speech -Decreased interests & diminished sense of purpose & social drive Cognitive Domain -Difficult setting priorities, multi-tasking -Perseveration - failure to un-learn old patterns that don't work anymore |
|
|
Schizophrenia
(Course) |
-Typically begins in late adolescents & early adulthood, though symptoms may begin at any age
-Late onset form: affects post-menopausal women -Males have earlier age of onset than females (males are more vulnerable to schizophrenia & often have a worse course of disease) -Has genetic & environmental components (genetic component = 15-20% of the cause) Most important environmental factors = exposure to toxins during prenatal development that may later result in schizophrenia |
|
|
Schizophrenia is Heterogeneous
|
-Syndrome described by a variety of clinical symptoms
-Probably involves multiple etiologies that express themselves with a similar set of symptoms |
|
|
Schizophrenia
(Different Courses/Categories) |
-1/3 of pts respond well to antipsychotic drugs & can generally return to a fairly normal life style (relapses and then recovery)
-1/3 of pts do not respond as well, and show long-term decrements in function over many years (decline with each relapse of the disease) -1/3 of pts tend to be least responsive & often require long-term intensive or custodial care for decades (continues to decline until they no longer function well) |
|
|
Chlorpromazine & the pharmacological revolution
|
-Studied originally for usefulness as a SEDATIVE
-Delay and Deniker found in open trials that PSYCHOTIC SYMPTOMS WERE CONTROLLED & this effect was more prominent than sedation |
|
|
Clinical Efficacy of Phenothiazines
|
Very subjective
Based on a physicians subjective impression of whether the pt has improved or not These compounds are not a CURE for schizophrenia, but do have positive effects |
|
|
Mechanisms of Antipsychotic Action
|
-Classical drugs appeared to be functional dopamine receptor antagonists
-1972: Antipsychotic drugs competitively blocked the increase in cAMP induced by dopamine -1976: Haloperidol radiolabeled - receptors could be studied directly (excellent correlation found between haloperidol binding & antipsychotic efficacy; but not between DA-induced cAMP synthesis) -1978-9: Concept of 2 dopamine receptors: 1. D1: linked to cAMP synthesis but not important behaviorally 2. D2: not linked to cAMP, but more important behaviorally -1989-91: Cloning of dopamine receptors |
|
|
Dopamine & its Receptors
|
D1-like:
-Increases cAMP -Preferentially recognize 1-phenyl-tetrahydrobenzazepines -Linked to stimulatory types of G-proteins D2-like: -Inhibits cAMP -Inhibits GIRK (a potassium channel) -Preferentially recognize substituted benzamides (e.g. Sulpiride) or spiperone -Linked to inhibitory types of G-proteins |
|
|
Antipsychotic-Related Treatment Side Effects & the Role of Dopamine
|
"Neuroleptic" (acute)
-Parkinsonian-like --> motor: muscle rigidity, shuffling gait, decreased facial expression, drooling -Emotional: apathy, dysphoria -Cognitive: impaired thinking -Akathesia (involuntary hyperactivity): subjective & objective motor restlessness Tardive Dyskinesia: -Abnormal involuntary movements affecting major muscle groups, may be irreversible Endocrine -prolactin mediated (glactorrhea, sexual dysfunction, amenorrhea) |
|
|
Antipsychotic-Related Treatment Side Effects & the Role of Dopamine
(Mechanisms) |
"Neuroleptic" (acute)
-Motor side effects (dopamine receptors in basal ganglia) -Emotional & cognitive (dopamine receptors in limbic system & cortex) Tardive Dyskinesia: -Abnormal involuntary movements affecting major muscle groups, may be irreversible -Apparently involves decreased dopaminergic function - worsened by levodopa & reduced antipsychotic dose Endocrine -prolactin mediated - DA increases prolactin secretion (dopamine receptors in pituitary) |
|
|
Antimuscarinics work to treat APD-induced parkinsonism
|
-Typical antipsychotics block inhibitory D2 receptors.
-Antimuscarinics block effects of increased amounts of ACh resulting from increased firing -This increases firing of striatal cholinergic interneurons If we combat excess firing with antimuscarinic drugs, then we can block a lot of the motor problems |
|
|
Antipsychotics
(Side Effects) |
Anticholinergic (antimuscarinic)
-dry mouth, urinary retention, constipation, blurred vision, sinus tachycardia, confusion, memory impairment Antihistaminergic -sedation, weight gain, confusion, disturbed concentration Alpha1-Adrenergic antagonism: -orthostatic hypotension, reflex tachycardia, sexual dysfunction, priapism |
|
|
"Old" Atypical Antipsychotics
|
Risperidone (antagonist of seratonin receptors)
-Good with positive symptoms, but not as good for the negative symptoms as the newer atypicals. -High affinity for Dopamine D2 & serotonin 5HT2 receptors. -Lack of Parkinsonian side effects Clozapine (binds to many receptors) = best antipsychotic -Can cause agranulocytosis in 1-3% of population. -Causes major weight gain -Extremely toxic when first administered; most start slowly and gradually increase to therapeutic dose -Causes profound sedation & anticholinergic effects |
|
|
"Newer" Atypical Antipsychotics
(Clozapine-like) |
-Alanzapine
-Sertindole -Ziprasidone -Quetiapine -Aripiprazole (D2 - functionally selective or partial agonist) -Asenapine |
|
|
Typical Antipsychotics
|
-Chlorpromazine
-Haloperidol -Thiothixene -Provide better therapy for negative symptoms |
|
|
4 Most Commonly Used Antipsychotics
(Side Effects) |
Haloperidol --> weight gain & severe Extrapyrmideal side effects - EPS (Parkinsonian-like symptoms)
Risperidone - rare EPS, doesn't work well for negative symptoms Olanzapine - rare EPS, does NOT cause Agranulocytosis Clozapine - causes agranulocytosis, rare EPS, severe sedation, major weight gane |
|
|
Human Imaging Studies
(Antipsychotics) |
-D2 receptor occupancy predicts antipsychotic potency
-Only 60% receptor occupancy is required for therapeutic effects -Antipsychotic doses can be titrated to reduce side effects without loss of therapeutic efficacy -Dopamine D2 receptor hypothesis of the etiology of schizophrenia has MERIT. |
|
|
Ideal Antipsychotic Drug
("Atypical Antipsychotic Drug") |
-Decrease in positive & negative symptoms
-Improved cognitive performance -No extrapyramidal side effects -No tardive dyskinesia -No endocrine side effects |
|
|
Recent Strategies for the search for new "atypical" antipsychotics
|
Selectivity for single dopamine receptor isoform
-D4 selective antagonists (failed in clinical trials) -D1 selective antagonists (failed in clinical trials) Concomitant occupation of dopamine & other receptors -muscarinic -alpha-adrenergic -serotonergic Selective targeting of other receptors -Serotonergic (5-HT2A failed in clinical trials) -glutamatergic (NMDA?) |
|
|
Pharmacology of Antipsychotic Drugs
|
Interaction with receptors occurs rapidly as drug distributes
Direct receptor-mediated drug side-effects are seen immediately -Antimuscarinic effects -Autonomic effects (e.g. via alpha1 adrenoreceptor blockage) -Acute neurological effects (EPS) or typical APDs Maximal therapeutic benefit is temporally delayed (by several weeks) -Tardive dyskinesia (typical APDs) also temporally delayed (by years) |
|
|
New drugs target cognitive function in schizophrenia
|
Cognitive deficits --> including impairments in areas such as memory, attention, & executive function --> are a major determinant & predictor of long-term disability in schizophrenia. Unfortunately, available antipsychotic medications are relatively ineffective in improving cognition. Scientific discoveries during the past decade suggest that there may be opportunities for developing medications that will be effective for improving cognition in schizophrenia.
|
|
|
Alcohol
(Dosage - in a 70kg person) |
Beer: 3-6% by volume (12oz)
Wine: 5 oz Liquor: 45% by volume (1.5 oz) This will give you a BAC of 0.03% (loss of a little fine motor coordination) |
|
|
Alcohol
(Absorption & Distribution) |
-Absorbed by passive diffusion from the stomach (25%) & the small intestine (75%)
-More rapid absorption in the small intestine -Food in the stomach delays absorption because it delays gastric emptying into the small intestine -The rate of distribution to a particular tissue is proportional to the blood supply of the tissue (brain has large blood supply, so it will rapidly accumulate ethanol that is absorbed) |
|
|
Alcohol
(Elimination) |
Ethanol is eliminated from the blood at the rate of 8-10g/hour for a 70 kg person
1-3% of a dose is excreted via the lungs 2-4% excreted via the urine -Takes 60-90 minutes to get rid of 1 standard drink -Blood alcohol is decreased by about 0.015%/hour |
|
|
Disulfiram
(Effect when drinking Alcohol) |
-Causes flushing of the face, neck shoulders, & chest
-Get palpitations, difficulty breathing, become nauseous & eventualy BP goes down -This can occur with as little as 1/2 a drink |
|
|
Alcohol
(Effects on the Liver) |
Causes:
-Hepatitis (loss of appetite, fever, jaundice, pain) -Hypermetabolism (may be associated with thyroid hormones being produced more) -Fatty liver (fat from adipose tissue is mobilized and will collect in the liver; may be related to an effect of the adrenal cortex - cortisol mobilizes fats) -Cirrhosis that can be fatal (results in scarring of the liver; liver becomes harder & smaller, get varicose veins in the liver, eventually the veins may rupture & death will result) |
|
|
Alcohol
(Effects on the GI tract) |
Increases:
-Acid secretion (HCl production increases) -Irritation -Pancreatitis |
|
|
Alcohol
(Effects on the Kidneys) |
Diuresis
|
|
|
Alcohol
(Effects on the CV system) |
-Small increases in heart rate & cardiac output (heart myopathy)
-Increased incidence of Hypertension -Moderate consumption seems to be beneficial |
|
|
Alcohol
(Effects on the Endocrine system) |
-Glucocorticoids increase (may be associated with fatty liver)
-Inhibition of testosterone synthesis (feminization in males) -Altered estrogen metabolism -Decreased release of posterior pituitary hormones (ADH --> greater urine volume; Oxytocin --> may help relieve menstrual cramps) -Infertility |
|
|
Alcohol
(Effects on the Fetal Alcohol Syndrome) |
Congenital malformations & Retardation
|
|
|
Alcohol-Drug Interactions
(Drugs with Disulfiram-like Activity) |
-3rd generation Cephalosporins
-Metranidazole -oral sulfonylurea hypoglycemics -quinacrine If prescribed, must warn pt not to have ANY alcohol |
|
|
Alcohol-Drug Interactions
(Drugs with CNS Depressing Activity) |
-Barbiturates (interact synergistically with alcohol)
-1st generation Antihistamines -Phenothiazines (add to the alcohol effect) -Benzodiazepines (through the same receptors as alcohol) -Ethanol & these drugs further depress the CNS -Chronic ethanol may cause tolerance to general anesthetics |
|
|
Alcohol-Drug Interactions
(Other Drugs at Non-CNS sites) |
Aspirin --> can cause GI irritation (additive effect with alcohol)
Isoniazid --> used for treating TB; can cause liver damage; increased risk of hepatitis (additive effect with alcohol) Salicylates - increased GI irritation |
|
|
Alcohol-Drug Interactions
(Acute Ethanol & Metabolism) |
Ethanol competes with other drugs for metabolism by the P450 enzymes.
Thus, it PROLONGS the effects of the 2nd drug |
|
|
Alcohol-Drug Interactions
(Chronic Ethanol & Metabolism) |
-The P450 enzymes are increased which may lead to more rapid metabolism of the 2nd drug (concentration will decrease with the usual dose; decreased therapeutic ability)
-These drugs include: barbiturates, anticoagulants, oral hypoglycemics & ACETAMINOPHEN, which becomes possibly more TOXIC to the liver by the metabolic mechanism involving CYP2E1 and the toxic product N-acetyl-p-benzoquinone - imine & glutathione -Ethanol not only increases CYP2E1 to increase production of the toxic compound, but also reduces the amount of the protective reduced glutathione in the liver. |
|
|
Alcohol
(Lecture Pearls) |
-Liver cirrhosis resulting from alcohol is the 9th leading cause of death in the US
-Alcohol has a very low therapeutic index -If blood alcohol is between 0.03 & 0.08, then you are 2x more likely to be in a car accident -If BAC 0.1%, then you are 6x more likely to be in a car accident -If more than 0.4%, then you will be dead -If you have 3 standard drinks, then BAC will be about 0.067-0.092% on an empty stomach & 0.05% with a meal |
|
|
Alcohol
(Genetics) |
-If your parents were alcoholics, but you are raised by non-alcoholics, then you will STILL have a greater than average chance of alcoholism
-If you are a child of non-alcoholic parents, but are raised by people who are alcoholics, then the chance of being an alcoholic is no greater than the general population. |
|
|
Alcoholics
(2 Types) |
Type 1: seen with males & females (generally starts at >25 years old)
-Must have a genetic component & an environmental component Type 2: mainly seen with men (onset at ~18 years of age) -Associated with inappropriate aggressive behavior |
|
|
Alcohol
(Zero Order Kinetics) |
-The Km for alcohol dehydrogenase = 2mM
-When you have 0.08% BAC, it's equal to 16mM (well beyond the Km for the enzyme and it is working maximally) Supply of NAD: -6 strong drinks use up 2 moles of NAD (2 moles of NAD in the liver = 1.5kg) -Therefore, turnover is dependent upon continually generating NAD to maintain metabolism |
|
|
Alcohol
(CYP2E1) |
-CYP2E1 uses NADPH to produce caetaldehyde & NADP+ from alcohol
-It can metabolize up to 10% of a dose of alcohol -This is MOST important when talking about drug interactions -Can get rid of 10mL of 100% ethanol/hour -1 standard drink has 15-20 mL of alcohol |
|
|
Pain
(3 Categories) |
1. Pathological pain --> pain associated with inflammation or trauma
2. Acute pain --> results from brief/transient high intensity stimulation (i.e. cutting yourself). This activates the nociceptors, which results in perceiving pain (this can lead to tissue damage). 3. Neuropathic pain --> chronic and persistent pain resulting from either damage to peripheral nerves (nerve endings) or CNS lesions (i.e. stepping on a tack) Acute pain which is not promptly treated can lead to chronic pain that is more difficult to treat |
|
|
Opioid vs. Non-opioid analgesics
|
Opioid Analgesics --> act in the CNS to blunt the perception of pain
Non-opioid Analgesics --> act in the periphery where the pain signal originates (generally works by reducing the inflammation that causes the pain) Glucocorticoids have anti-inflammatory activity, and by this means will reduce pain |
|
|
Pain Fibers
|
Thinly myelinated A-delta fibers and unmyelinated C fibers (more common)
A nerve pain fiber with nociceptors has its cell bodies in the dorsal root or trigeminal ganglion of the face. A central axon synapses on a second order neuron in the dorsal root ganglion where the noxious stimulus is detected. The action potential is carried to the CNS where it is perceived as pain. -Nociceptors respond to chemical (acid), temperature (heat, and mechanical (sticking & cutting) stimulation -There are various ion channels, which are on the nerve terminal. Through these ion channels, the stimulus is perceived. |
|
|
Pain Ion Channels
|
TRPV1 (the non-selective Calcium & Sodium Ion Channel)
-Responds to temperature. Stimulus propagating the signal has a threshold of ~40 degree Celsius Peripheral Sensitization --> injury and/or inflammation result in the production of many substances that sensitize the nociceptor by changing its threshold for firing impulses. Sensitization allows low-intensity stimuli to cause pain through the nociceptors. |
|
|
Inflammatory "Soup" that alters Nociceptors
|
-Macrophages --> release Interleukins
-Mast cells --> release PGE2, bradykinin, & histamine -Damaged tissue --> releases protons, ATP & adenosine -Platelets --> release ATP -These all contribute to the inflammatory response, which goes into pain & sensitizes you to more pain -Decreased synthesis of PGE2 reduces inflammation & thus pain due to sensitization of nociceptors. Although this action is of therapeutic benefit, it may cause a possible problem relative to ulcers & GI bleeding (PGE2 has a protective action in the GI tract) |
|
|
NSAIDS
(2 Main Toxicities) |
1. GI irritation (to the point of ulceration)
2. CV problems (which cause clotting & infarcts) |
|
|
NSAIDS
(2 Groups) |
1. Non-selective --> Inhibits both COX1 & COX 2
2. Selective --> selectively inhibit COX 2 -Only 1 group of selective NSAIDS = Celecoxib - "coxibs" |
|
|
NSAIDS
(COX Inhibitors) |
-In the GI tract, the main COX enzyme is COX1, which produces PGE2 --> which has a protective effect against erosion, irritation, & ulceration
-Platelets only have COX1. The COX1 will produce Thromboxane A2, which leads to aggregation of the platelets & clotting. If you inhibit synthesis of thromboxane A2, you will decrease platelet aggregation & DECREASE clotting. -Endothelial cells in the blood vessels --> COX1, leads to synthesis of thromboxane A2, which promotes clotting -Selective inhibition of COX2 leads to less prostaglandin synthesis to balance against thromboxane A2 = anti-clotting -In inflamed tissues, we get the induction of COX2 |
|
|
Non-Opioid Analgesics
(Basis of Toxicity) |
-Non-selective COX2 inhibitors also inhibit COX1. This will decrease synthesis of PGE2, which helps protect the GI tract.
-Therefore, continued use of non-selective COX inhibitors will cause GI problems/irritation/ulceration. -Selectively inhibiting COX2 will not cause the same GI problems. However, COX inhibitors (like Celebrex) change the balance between thromboxane A2 and PGE2. TBX A2 favors clotting, while PGE2 opposes clotting. Selective COX2 inhibitors FAVOR CLOTTING, thus they favor formation of infarcts. -Non-selective COX inhibitors can cause CV clots, but the risk is decreased. Similarly, Celecoxibs can cause GI irritation, but risk is decreased. |
|
|
NSAIDS
(Aspirin) |
Aspirin (acetylsalicylic acid) is unique because its inhibition of COX is IRREVERSIBLE because its acetyl group is trnsferred to & irreversibly acetylates COX.
-In the body, the half-left for aspirin = ~15 minutes (very rapidly converted) |
|
|
Aspirin
(Uses) |
-Low dose aspirin prevents infarcts & clots
-Platelets, which make PGE2 from COX1, have the COX1 inhibited. Platelets do not have nuclei, so they do not have the capacity ot replace inactivated COX (permanent inhibition of COX) -The effect of aspirin on platelets lasts 8-10 days (life span of platelets = 8-10 days and then new platelets are made). -Aspirin has relatively little effect on the COX activity in other cells where new enzymes are synthesized in ~8 hours. |
|
|
Aspirin
(Toxicities) |
Too much aspirin, can cause salicylate intoxication (a possibly lethal effect)
Salicylism is characterized by: headaches, dizziness, mental confusion & tinnitus (ringing of the ears) -Aspirin & salicylic acid are eliminated through the kidneys. One way to increase elimination is to treat with sodium bicarbonate to alkalinize the urine, which keeps aspirin in the alkylated form so that more will be eliminated. |
|
|
Ibuprofen & Naproxen
(Non-Selective NSAIDs) |
-Ibuprofen has a t1/2 = 2 hours
-Naproxen has a t1/2 = 15 hours Both have similar toxicities characteristic for the NSAIDS (GI & CV problems) |
|
|
Major depression
|
-if you have five or more of symptoms of depression and have them for at least 2 weeks or more
-primary: arising from reasons we do not know; idiopathic -subsequent to med s -also part of bipolar disease |
|
|
Bipolar disease
|
-people swing from depression to mania
-2 categories: Bipolar I and Bipolar II |
|
|
Bipolar I
|
-depression plus mania
-behave in manic way: boundless energy, boundless ambition, try to do everything |
|
|
Bipolar II
|
-depression lesser amount of mania (hypomania)
-still have extra energy ambition etc -not quite as bad as mania |
|
|
Disthymic depression
|
-if you have 2 or more and you have it for lesser frequency (moderate-dysthymic)
|
|
|
Minor depression
|
-even fewer symptoms at a fewer times is minor
|
|
|
Monoamine Hypothesis
|
-earliest that is still around
-based on three observations 1. reserpine 2. isoniazid, iproniazid 3. drugs used today to treat depression increase monoamines |
|
|
Reserpine
|
-blocks uptake of NE (dopamine, serotoning)
-diminishes the monoamine that are in nerve terminals in the CNS + PNS -taking reserpine leads to depression |
|
|
Isoniazid, Iproniazid
|
-were experimental drugs which were being tested to treat people with TB (isoniazid used for this now)
-people treated with these drugs began to feel happiness -year later psychiatrist decided to try these on depressed patients and he found that their modd improved -we know that these are MAO inhibitors (inhibit monamine oxidase you increase amount of neurotransmitter in brain an dother parts of the body) -increase in monoamine |
|
|
Current drugs today to treat depression...
|
-increase monoamines
-monamines are related to depression and a decrease in monoamines makes you depressed and increasing them will treat depression -1-2 days will increase monoamines- can be a problem; it takes 2-3 or more weeks before people feel relief from depression |
|
|
Two hypothesis together
|
-if we give a drug---> monoamines increasing--> happens in a day or two--> this somehow leads to an increase in BDNF--> increases volume/size of particular regions of the brain
-this idea would explain why it takes 2-3 weeks to see an effect from increase in monoamines (you have to have new neurons made, new proteins, etc) |
|
|
Drugs to treat depression
|
-may be effective on treating depression (maybe emphasized)
-choice on which class to use is largely based on which type of drug will be effective with the fewest adverse/toxic effects |
|
|
Toxicities of SSRIs
|
-jittery, restless, agitated, headache, diarrhea, nausea
-some of these go away after about a week or so |
|
|
SNRIs
|
-next if SSRIs aren't working
-not only inhibit reuptake of serotonin, but also inhibit reuptake of NE -venotaxine, desnotaxine, duloxetine |
|
|
Toxicities of SNRIs
|
-similar to SSRIs
-sweating, dizziness, agitation -if people have liver disease, these can cause liver failure DO NOT USE WITH PEOPLE WITH LIVER DS -cause hypertension and cardiac prolems -more adverse effectsthan the SRRIs |
|
|
MAO inhibitors
|
-phenelzine (isoniazide and other above are not used)
-tranycipramine, selegeline -all work by mechanism of increasing monoamines in the terminal thus more can be released -one of the problems with inhibiting MAO is that you hav eto watch the foods that you eat that contain tyramine |
|
|
Bupropion
|
-reuptake inhibitor of NE and dopamine
-used in combination with SSRI |
|
|
Lithium therapeutic window
|
-.5-1.25 mM- Rx (therapeutic)
-1.25-2 mM you get nausea vominting, abdominal pain, tremor, CV problesm -exceed 2mM -cardiac arrhythmias, convulsions, coma and death |
|
|
all drugs for depression
|
-work for major but for mild or moderate it is arguable whether they have any better effect than placebo
-orginal studies done by drug companies get them approved but results of independent studies show poorer results -people get relief from taking placebo and then shortly after finding out it is placebo become depressed again -getting the right drug is trial and error -on average people try 2-3 different drugs until they hit the right one that helps them |
|
|
Talk therapy
|
-go for seven or more week s
-talking about depression with someone qualified -receive as much improvement as you will taking the drugs -do both (drugs and therapy) then have more improvement (combination better- studies shown) |
|
|
2 other drugs
|
1. Aripiprazole (abilify)
2. Quetiapine (seroquel) -these drugs cause tardive dyskinesia: irreversible effect wher eyou can't help making faces, you glucose goes up akathenia (felling of inner restlessness) -anripiprazole: show some improvement in 25% of people treated -placebo: 15% -incidence of akathenia in people taking placebo: 4% -"" for apipripazole: 25% (1 out of 4) -cost of drugs are 25 times price of other drugs |
|
|
Antimicrobials
|
Unique - curative rather than palliative
Relatively common Rx: 15-20% of prescriptions One of the most misused classes of drugs |
|
|
Bacteristatic & Bactericidal
(Definition) |
Bacteristatic antibiotics --> inhibits bacterial replication, but the inhibition is reversed upon drug removal
Bactericidal antibiotics --> the drug has an irreversible lethal effect on the bacteria |
|
|
Bacteristatic & Bactericidal
(Mechanism) |
-Bacteristatic antibiotics, if given long enough, will eventually result in the bacteria dying
-Bactericidal antibiotics, if not given in a large enough dose, then it may end up not killing the bacteria. -Most antibiotics that are bactericidal generally only affect growing microbes -Combining bactericidal & bacteristatic antibiotics may decrease the effect of the bactericidal antibiotic because you stop replication, which is required for bactericidal antibiotics (a bacteristatic antibiotic that blocks bacterial growth may prevent the action of a bactericidal drug) |
|
|
Antibiotics
(Immune System Considerations) |
-It's important for a person to have an intact immune system (phagocytes) because the immune system aids the effects of the antibiotic
Limitations: -If pt has HIV & is taking glucocorticoids, certain anti-cancer drugs or if cancer pt is being treated with radiation --> then it decreases the immune response. -If pt has Diabetes & glucose concentrations are up, then it inhibits macrophages & supplies energy for bacteria. |
|
|
Superinfection
|
A second infection on top of a preexisting infection. Can occur in 2 ways:
1. Get another infecting bacteria from outside 2. Outgrowth of normal bacteria that are normally kept in check due to an ecological balance between many types (>400 bacteria in person's GI tract) -When you overuse broader-spectrum antibiotics & kill multiple kinds of bacteria, you are more likely to get a superinfection |
|
|
Antibiotic vs Antimicrobial
(Definition) |
Antibiotic --> a substance made by a living organisms that kills or gets rid of another living organism
Antimicrobial --> often not made by a living organism (may be chemically made in the laboratory) |
|
|
Antibiotics
(2 Important Considerations) |
1. Selectivity --> selective to kill the bacteria & not the patient
2. Resistance --> bacteria are becoming resistant to antibiotics. The greater the selectivity of the antimicrobial, the more possible it will be to overcome resistance. If bacteria are resistance or have a very selective drug, then we can give a lot more (higher dose) of that antimicrobial & possibly overcome the resistance. |
|
|
Drug Resistance
|
-Generally genotypic changes in the infecting microorganism, which persist during further growth in the absence of the drug.
Drug resistance arises by: 1. Mutation & Selection 2. Plasmid Infection (more prevalent) |
|
|
Drug Resistance
(Mutation & Selection) |
If we have a bacterial infection & we have 10^6 population of bacteria, then 1 bacterium may be resistant by virtue of a mutation to the particular antibiotic that you are using. The antibiotic will eliminate 10^6 - 1. The 1 that is resistant is not selected for and that 1 bacteria will quickly become a million bacteria all of which are resistant to the antibiotic
|
|
|
Drug Resistance
(Plasmid Infection) |
Plasmids --> small circular pieces of DNA that can exist in bacteria & have multiple genes. It is possible for each of these genes to make a protein that encodes resistance to a particular kind of antibiotic (ex: penicillin resistance, sulfonamide resistance, tetracycline resistance, etc on different genes).
-If you treat someone wiht penicillin and those bacteria have penicillin resistance, then they will NOT be affected by penicillin & will grow. Additionally, you are generating a population of bacteria that can be resistant to tetracycline & sulfonamide, as well. Now you have multiple-resistance. You cannot treat this population with any of these drugs. |
|
|
Sulfonamide Structure
|
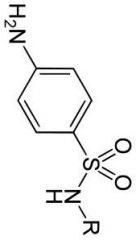
Para-amino (N4) benzene group
Sulfide Amide |
|
|
Sulfonamides
(History) |
-Synthesized in 1908
-A group of drugs that were tried & found to be successful, but were successful for the wrong (not initial) reason that they tried. -Used in the war industry as a dye for wool (have a great affinity for protein in the wool) -A drug, Prontosil, was synthesized & used. It turns out that Prontosil is broken down to make Sulfonamide |
|
|
PABA
(Structure) |
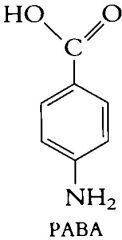
An endogenous substance in bacteria
The form of the molecule with O- resembles the naturally occurring PABA in the bacteria --> this accounts for the antibacterial activity of the sulfonamides because they all work in the same way. |
|
|
Sulfonamides
(Pharmacology) |
-Oral administration (can also be taken topically) --> short-acting (4-8 hours), intermediate-acting (10-25 hours), long-acting (35 hours)
-Sulfonamides & bilirubin are bound at the same sites on albumin (compete for binding; in newborn infants, bilirubin can pass the BBB & cause Kernicterus --> encephalopathy, binds to basal ganglia & subthalamic nuclei) -Can cross the BBB & placental barrier (do not give to pregnancy or nursing women) |
|
|
Sulfonamides
(Metabolism & Excretion) |
Metabolism:
-N4-acetylated in liver Excretion: -Eliminated via kidneys -Advantage --> in the tubules, the concentration of sulfonamides is higher than the blood (higher - more effective concentration to fight off bacteria) -Disadvantage --> many sulfonamides are NOT SOLUBLE. Therefore, there is risk of Crystaluria (formation of crystals in kidneys that can damage the tubules). Must use Sulfisoxazole (relatively soluble sulfonamide; short-acting - 4-8 hours) |
|
|
Sulfonamides
(Toxicity) |
-Hypersensitivity reactions (Stevens-Johnson syndrome --> a rare but serious condition where you have severe bleeding, lesions erupting all over the body, sloughing of the mucosal membranes & eventually death. It is reversible if you catch it early enough; if not caught early, then pt can die)
-GI disturbances -Blood dyscrasias -Hemolytic anemias -Deficiency in Glucose-6-phosphate dehydrogenase |
|
|
Sulfonamides
(Uses) |
-Acute urinary tract infections caused by Gram- bacteria (E. coli, proteus, etc)
-Nocardiosis (Cerebral & pulmonary abscesses caused by bacteria) Alternative Treatment: -Trachoma (cause of blindness) -Infections (has been superseded by a combination of 2 drugs --> Sulfamethoxazole-trimethoprim) |
|
|
Sulfonamides
(Combination of 2 drugs) |
1. Trimethoprim --> dihydrofolate reductase (FH2 --> FH4)
-This reaction occurs in bacteria AND humans -Selectivity? Trimethoprim is needed in much higher doses (5 order of magnitude more) to inhibit the Human FH2 reductase by 50% than to inhibit the Bacterial FH2 reductase by 50% (concentrations do not effect human enzymes) 2. Sulfamethoxazole (aka Bactrim & Septra) -Trimethoprim & Sulfamethoxazole act synergistically for killing bacteria. -Combination is used for the same things that you use sulfonamides for, but is also good for CHRONIC urinary tract infections. |
|
|
Sites of Inhibition
(Nucleic Acid Synthesis) |
Sulfonamides
Trimethoprim Rifampin Fluoroquinolones |
|
|
Sites of Inhibition
(Cell Wall Synthesis) |
Penicillin
Cephalosporins Vancomycin Isoiazid Bacitracin |
|
|
Sites of Inhibition
(Protein Synthesis) |
Aminoglycosides
Erythromycin Tetracyclines Clindamycin Chloramphenicol |
|
|
Sites of Inhibition
(Cell Membrane) |
Amphotericin B
Miconazole Fluconazole |
|
|
Penicillin
(Basics) |
-Route of elimination = Kidneys
-Penicillin is NOT metabolized -Drug is dosed in units (1 unit = 0.6 micrograms) -Have beta lactam ring |
|
|
Bacterial Cell Wall Structure
|
-Bacteria are HYPERtonic relative to the environment, so the cell wall prevents water from coming end (prevents lysis)
-Beta lactams prevent the proper synthesis of bacterial cell walls & cause lysis -Cell wall = peptidoglycan (longitudinal strands with vertical strands connecting them) -->made up of peptides & sugar molecules (N-acetylmuramic acid & N-acetylglucosamine) -->alternating MurNAc & GlcNAc strands --> attached to N-AcMur = peptide chain with 4 AAs (L-Ala, D-Glu, L-Lys, D-Ala); Attached to L-Lys is 5 Glycines, which form a peptide bond (glycine bridge) with D-Ala on the other vertical strand |
|
|
Bacterial Cell Wall Synthesis
(Stage 1 Cytoplasm) |
-Begin with N-Acetylglucosamine-1-P, which reacts with UTP to release Pyrophosphate to make --> UDP-GlcNAc
-UDP-GlcNAc reacts with Phosphoenolpyruvate, which adds to the GluNAc to make --> UDP-MurNAc -UDP-MurNAc undergoes 3 separate reactions to add L-Ala, D-Glu, & L-Lys -At the same time, L-Ala is acted on by Alanine Racemase, which changes L-Ala to D-Ala. D-alanyl synthetase links 2 D-Ala together to yield a dipeptide -Finally, D-Ala-D-Ala is added to the end of the tripeptide chain to make --> UDP-MurNAc-pentapeptide |
|
|
Bacterial Cell Wall Synthesis
(Stage 1 Cytoplasm - INHIBITION) |
The 2 steps (below) are inhibited by Cycloserine.
Cycloserine looks like D-Ala & thus, inhibits these reactions (2nd line treatment for TB) (UDP-MurNAc undergoes 3 separate reactions to add L-Ala, D-Glu, & L-Lys At the same time, L-Ala is acted on by Alanine Racemase, which changes L-Ala to D-Ala. D-alanyl synthetase links 2 D-Ala together to yield a dipeptide) |
|
|
Penicillin G
(Salt Toxicities) |
Depends on the salt that is given
-If pt is taking a digitalis glycoside or a drug for congestive heart failure, then a lot of K+ will counter the effects of the glycoside & will DECREASE effectiveness -If pt has renal problems & you give a lot of penicillin G as a K+ salt, then you will get HYPERKALEMIA -If using the sodium salt & the pt has hypertension, then you will increase the pt's sodium levels. |
|
|
Penicillin G
(Elimination) |
-90% of Penicillin that is injected IM will be excreted through the kidneys unchanged. Of the 90% that goes out, only ~10% is by filtration & the other 90% is by tubular secretion (Elimination is almost completely tubular)
Probenecid inhibits tubular secretion & increases the concentration & length of time that Penicillin is in the circulation (2nd line for treating gout - prevents reabsorption of uric acid) |
|
|
Penicillin G
(Distribution) |
Penicillin G is widely distributed.
It enters interstitial fluid, gets into bone, synovial fluid & will cross the placenta. Normally, it will NOT get into CSF (does not readily cross the BBB). If the meninges are inflamed, then they will allow the passage of Penicillin G |
|
|
Penicillin G
(Uses) |
Aerobes:
-Streptococcus infections (A, B, C & G strains) -Strep. pneumoniae (resistant) --> accounts for 35% of middle ear infections (otitis media) -Neisseria gonorrhea & Neisseria meningitidis (resistant) -Treponema pallidum (syphilis) --> spirochete; VERY sensitive to Penicillin G -Borrelia burgdorferi (Lyme Disease) --> spirochete -Bacillus anthracas (antrax) Anaerobes: -Gram+ Glostridium perfringens & tetani (CANNOT be used for Clostridium difficile) -Gram- Bacteroides - Oropharyngeal infections (NOT used for Bacteroides fragilis) |
|
|
Penicillin G & Staph infections
|
Most Staph infections are resistant to Penicillin (hospital acquired infections = Nosocomial infections)
|
|
|
Problem with Pts taking antibiotics
|
-many antibiotics are given for 7-10 days, after a few days patients begin to feel better and stop taking them, this allows for the infection to come back and cause resistance
|
|
|
Macrolide Types
|
-Erythromycin
-Clarithromycin -Azithromycin -Telithromycin |
|
|
Macrolides
|
-own their antibiotic activity bc they selectivity inhibit protein synthesis since it is so different from the protein synthesis in humans and doesn't interfere
|
|
|
Erythromycin
|
-half life is 1.5 hours
-macrolide, inhibits protein synthesis |
|
|
Clarithromycin
|
-half life of 6 hours
-macrolide, inhibits protein synthesis |
|
|
Azithromycin
|
-half life 3 days
-part of the benefit is that it is highly concentrated in the tissues and then it slowly released -used differently bc of its long half life, usually given a loading dose and then once a day for 7-10 days |
|
|
Telithromycin
|
-liver toxicity
-doesnt work on pts with myasthenia gravis -was used a lot but bc it is believed to work better than other drugs |
|
|
Uses of Macrolides
|
-mycoplasma pneumonia
-strep infections in which the person is hypersensitive to penicillin and its derivatives -treponema -legionella (legionares disease) -helicobacter pylori- usually causes ulcers, antibiotic treatment is now given to treat ulcers -used for mycoplasmid avium complex (found in pts with AIDS) -chlamydia |
|
|
Erythromycin Problems
|
-along with clarithromycin can inhibit P450 metabolism of certain drugs
-Increases theophylline (too much causes convulsions)and increased warfarin (causes bleeding disorders) and carbamazepine (antiepileptic that now in higher concentration causes seizures) -GI distress (cramping, diarrhea -causes Torsades de pointe |
|
|
Torsades de pointe
|
- twisting points
-with standard EKG you have the standard points QRS and T, -the macrolides have the ability to block the efflux of potassium in the ventricles and make the Q-->T interval longer, causing sudden cardiac death, -2 fold higher in people taking erythromycin (all macrolides but mainly erythromycin, but also clarithromycin) . - If you combine another drug with erythromycin that inhibits the metabolism of erythromycin there is a 5 fold increase in sudden cardiac death. |
|
|
Modes of Resistance of Gram Positive Bacteria
|
1. Can make beta-lactamases (or penicillinase)
2. Mutated form of the PBPs (no longer inhibited by penicillin G) 3. Inability to activate Autolysins (enzymes that break down the cell wall) |
|
|
Modes of Resistance of Gram Negative Bacteria
|
1. Mutations in Porins (prevents beta lactams from getting across the outer membrane to inhibit PBPs)
2. Can make beta-lactamases (or penicillinase) 3. Some bacteria (ex: Neisseria) have a pump that pumps the penicillin & other beta lactams out of the bacteria |
|
|
Broader Spectrum Antibiotics
(Uses) |
Will treat all the same things as Penicillin G, but only 1/2 as effective
Treats a range of Gram Negative bacteria that Penicillin G will not treat: -E. coli, Proteus, Shigella, & Typhus Will work aginst Klebsiella & Pseudomonas |
|
|
Carbapenems
|
Imipenem (combined with Cilastatin)
-Has a 2nd beta lactam ring -Renal dipeptidase will break down imipenem, so Cilastatin inhibits the renal dipeptidase & allows imipenem to last longer. Meropenem --> resistant to dipeptidase Reserved for Serious Multi-Drug Resistant bacteria (DO NOT work against MRSA) |
|
|
Cephalosporins
|
Can confer resistance
Divided into generations: 1st generation: -Similar in activity to Penicillin G -Mainly treats Gram+ infections 2nd generation: -More like Amoxicillin & Ampicillin -Can treat Gram+ & Gram- infections 3rd generation: -Mainly treat Gram- infections 4th generation: -Cefepime --> more Beta lactamase resistant 2nd & 3rd generations have a Disulfiram-like effect (must warn pt NOT to drink ALCOHOL) |
|
|
Clindamycin
|
-can be given orally and inhibits protein synthesis, eliminated by the liver through the bile and through the feces
|
|
|
Clindamycin uses
|
-bacteroides (gram - infections)
-clostridium perfringes (anaerobe gram +) -alternative strep infections |
|
|
Pseudomembranous Colitis
|
-sloughing of the intestinal mucous membrane
-clindamycin allows the outgrowth of clostridium difficile which secretes a toxin that attacks the mucous membrane of the intestinal tract causing the sloughing, now you have to get rid of the C. difficile -this is done by vancomycin |
|
|
Vancomycin toxicity
|
-ototox (damaging ear)
-nephrotox (kidney damage) -elimination of vancomycin is through the kidney and normal half life is 6 hours, but with reduced kidney function it is 250 hours. -must be careful about pairing drugs with similar toxicities |
|
|
Vancomycin Uses
|
-C. difficile (must be given orally bc this is an intestinal infection)
-MRSA, (first choice, however sometimes it will not be responsive) -Alternative Strep infections due to penicillin allergy -Enterococcus faecium -Enterococcus facaelis |
|
|
Dolfupristin/Quinupristin
|
-together are called Synercid
-only good for enterococcus faecium and used if linezolid fails -administered by IV |
|
|
Linezolid
|
-if pt is alreads taking an SSRI or an SNRI, Linezolid is an MAO inhibitor (MAO-A metabolizes serotonin) causing a serotonin syndrome which involves possible convulsions , dizziness and confusion, this should only be used if it is a life threatening infection if pt is on SSRI SNRI.
|
|
|
Linezolid uses
|
-MRSA
-Enterococcus faecium -Enterococcus facelis |
|
|
Aminoglycosides
|
-streptomycin
-gentamycin -tobramycin -amikacin -neomycin |
|
|
Streptomycin
|
-first used of the aminoglycosides
|
|
|
Gentamycin
|
-most frequently used,
-resistance can develop |
|
|
Tobramycin
|
-most frequently used
- resistance can develop |
|
|
Amikacin
|
-developed to get around resistance to gentamycin and tobamycin, bc they metabolize the antibiotic, these sites were modified so that the sites with inactivation cannot be inactivate
|
|
|
Aminoglycosides
|
For severe infections aminoglycosides may be combined with one of the penicillins, these are given IV, a caution when this is done you can mix both in the same syringe or back because they react with one another and precipitate out and must be injected separately
-work at the site of the 30s ribosome |
|
|
Gram negative bacteria
|
-have and outer membrane and cytoplasmic membrane and have a two step process for the amninoglycoside to get into the bacteria
-antibiotic must go through porins -once in periplasmic space must be transported by and O2 dependent transporter, consequence is that aminoglycosidesare used only for Aerobic gram - bacteria |
|
|
Ways bacteria create resistance
|
-mutation of porins
-metabolize antibiotic, modify and inactivate -Mutation at 30s ribosome -transporter actively pumps out antibiotic |
|
|
Aminoglycoside toxicity
|
-ototoxicty
-nephrotoxicity -reserved for moderate to severe gram - infections |
|
|
Metronidazole
|
-believed to work by inhibits anaerobic energy production or inhibit DNA synthesis. Used only for ANAEROBES, gram + or gram -
|
|
|
Metronidazole uses
|
-Bacteroides
-Clostridium difficile - Helicobacter - Vincent's ginvivostomatitis - ANUG |
|
|
Metronidazole toxicities
|
- Nausea
- Heart burn - Can produce a disulfur like effect, have to caution them to not have alcohol - Carcinogenic and mutagenic and shouldn’t be used in pregnant women |
|
|
Tetracycline
|
- inhibit protein synthesis, mainly eliminated through the bile via the feces,
-varying half life (6-15hrs) can use them orally and IV, |
|
|
Tetracycline cautions
|
-cautions include: they are chelators (compund that is a claw like molecule that can bond to di or trivalent cations such as calcium magnesium and aluminum,
-when it does this they become insoluble and are not absobed well. For this reason you have to tell them to be careful to not drink milk or dairy products and cant be taking antacids because these all prevent the absorption of tetracyclines) |
|
|
Tetracycline Toxicities
|
-GI irritation (more frequent)
-photo sensitivity -overdose can cause liver toxicity -can get into bone and teeth due to chelating agent -should not be prescribed to children who are under 8 years old, pr pregnant women -can be toxic to kidneys but not frequent -taking outdated tetracycline causes fanconi-like syndrome (kidney damage) |
|
|
Tetracycline Types
|
-tetracycline
-doxycycline -demecloncycline -minocycline |
|
|
Minocycline
|
-has a derivative called tigecycline
-accumulates in gingival fluid to a higher concentration than the plasma -useful in dental infections -used in microspheres to be used in perio disease |
|
|
Tetracycline Uses
|
-mycoplasma pneumonia
-rocky mountain spotted fever -chlamydia -helicobacter -anthrax -can be combined with metronidazole and bismuth subsalycilate (pepto bismol) |
|
|
Choramphenicol
|
-infrequently used
-effective for serious gram + and gram - and for Rocky mountain spotted fever - Not used much anymore because if caused complete shut down of bone marrow |
|
|
Fluorquinolones
|
-metabolized and eliminated through the kidneys.
-Acts on DNA gyrase |
|
|
Fluorquinolone Problems
|
-cause arthropathy (condition of the joints where there is an erosion of cartilage, not used in children or pregnant women)
-can effect tendons in geriatric pts and they can snap, -nausea and vomting. (most common problem) |
|
|
Fluorquinolone Types
|
-Ciprofloxacin
-Norfloxacin |
|
|
DNA gyrase activity
|
-prevents DNA from tangling up when it is being uncoiled during synthesis
|
|
|
Fluroquinolone uses
|
-aerobic gram + and - infections, particularly for recurrent urinary infections and Rocky Mountain Spotted fever
-used for serious infections with pts with cystic fibrosis. |
|
|
Topical Antibiotics
|
-frequently used in combo
-bacitracin (gram +) -Polymixin B (gram -, detergent breaks up bacteria) -Neomycin (aminoglycoside, gram + and - infections) -Neosprorin: (gram -, too toxic to take internally |
|
|
Antibiotics that inhibit protein synthesis
|
-macrolides
-tetracycline -clindamycin -chroamphenachol -Linezolid -quinupristin/dolfupristin |
|
|
Antifungal Drug Types
|
-Amphotericin B
-Azole -Echinocandins -Caspofungin |
|
|
Amphotericin B
|
-toxic to kidneys and heart on occasion, comes in many diff forms , least toxic is most expensive and vice versa, reserved for serious system fungal infections,
-selectivity is not great - Forms channels in the membrane and allows contents of the cells to leak out and the fungus dies, creates channels next to ergosterol (equivalent in human cells is cholesterol, ergosterol is in fungal membranes and binds better to this than cholesterol) used for pts with HIV candidiasis infections |
|
|
Azole
|
-used for oropharyngeal candidiasis , interferes with the synthesis of ergosterol via a 14 alpha demethylase
|
|
|
Azole types
|
-Fluconazole
-Itraconizole -Clotrimazole : -Miconazole: |
|
|
Miconazole
|
used for vaginal yeast infections
|
|
|
Clotrimazole
|
used for athletes foot
|
|
|
Echinocandins
|
- used for various fungal infections, thought to replace amphotericin B.
-Caspofungin |
|
|
Pre-Med patients for dental work
|
-Pts with artificial heart valves
-Previous endocarditis - Enlarged heart - Transplant heart pts -For treatment that will involve bleeding (does not include injection of local anesthetic |
|
|
Oral Amoxicillin
|
- drug of choice 1 hour before the procedure, give IM then 30 min before
|
|
|
Pt cannot take oral medication
|
nject amoxicillin or cephalosporin
|
|
|
Pt has allergy to penicillins
|
-give clindamycin or azithromycin, can give a cephalosporin if not rxn to penicillin
|
|
|
Pt cannot take oral meds and allergy to penicillin
|
-give clindamycin parenterally or cephalosporin
|
|
|
Tuberculosis
|
-About 3 million ppl in the world die from TB and about a billion ppl are infected with it . In non HIV pts it is the most frequent cause of infectious disease death in the world.
-If you test and find that the mycobacterium tuberculosis (infecting organism) is sensitive to Isoniazid & Rifampin, then you can use them BOTH in combination. If you use both, then you will treat for at least 4 months |
|
|
First Line TB drugs
|
-Isoniazid
-Rafampin -ethambutol -Pyrizinamide |
|
|
3 categories of Depression
|
1. Major depression
2. disthymic depression 3. Minor depression |
|
|
TB difficult to treat
|
-HIV pts are immunocompromised & are more likely to keep having the infection
-Generally starting out with 10^8 to 10^9 bacteria (very large number to treat) -Mycobacteria grow intracellularly, as well as extracellularly (drug must penetrate cells) -TB goes to the brain, so it must cross the BBB -Mycobacteria form CASEOUS lesions (cheesy) |
|
|
Lithium
|
(used to treat bipolar ds)
-given as lithium bicarbonate Li2CO3 -various effects not sure which one effects bipolar disease -while drug is effective must be carefully MONITORED -lithium likes sodium: lithium will go into sodium channels as well as sodium can, but lithium, unlike sodium, is not as efficiently pumped out (accumulates in cell), concentration of intracellular potassium goes down and leads to hyperpolarization and is perhaps part of the problems or problems that are seen if you get to high a concentration of lithium in the blood -the only way to treat the toxicities (after stopping lithium) is dialysis to try and get rid of the lithium |
|
|
SSRIs
|
-Fluoxetine, Citalopram, Escitalopram (s-isomer of citalopram, unlike Prilosec (omeprazole), isomers work better because one isomer counters the effect of the s-isomer), Paroxitene, Sertraline
-Selectively inhibit reuptake of serotonin from serotoneric neurons in the brain -all bind to the transporter for serotonin reuptake and allostericly change the transporter so that it no longer takes up serotonin as well -drugs are usually taken long term -people taking this long term stop taking it on their won because these cause sexual problems; in women: anorgasmia (not being able to achieve orgasm); in men- increase latency to ejaculation; 5-10% of peple on one of these drugs there is significant weight gain |
|
|
Symptoms of Depression
|
1. Emotions -general feeling of depression, use interest and lack of pleasure
2. Ideation- feeling of worthlessness, guilt, thoughts about death or attempting suicide 3. Somatic Symptoms -insomnia, hypersomnia (all you want to do is sleep), lose apetite, eat all the time, don't want to move around, sense of agitation, having trouble concentrating |
|
|
Alcohol
(Metabolism) |
-90-95% of ethanol is oxidized to acetaldehyde (CH3CHO) in the liver by alcohol dehydrogenase (CH3CH2OH + NAD+ --> CH3CHO + CADH + H+)
-Metabolism follows Zero Order Kinetics (a constant amount per unit time) -Alcohol dehydrogenase is also in the gastric mucosa, but women have ~1/2 that of men -A small percentage of ethanol is metabolized by P450 (Ethanol may increase P450 enzymes that also metabolize other drugs or it may compete with other drugs for metabolism by P450). -Acetaldehyde is TOXIC and is converted to Acetate by aldehyde dehydrogenase (CH3CHO + NAD+ --> CH3COO- + NADH + H+) -Disulfiram inhibits aldehyde dehydrogenase. Thus, it has been employed to discourage drinking in alcoholics by resulting in unpleasant effects. Disulfiram also affects P450 metabolism of some drugs: (i.e. phenytoin) |
|
|
Penicillin G
(Toxicities) |
Main toxicity = Hypersensitivity (1-10%)
2 Types: 1. Immediate (within 1 hour) -Involve Anaphylaxis & IgE -0.002% chance of an immediate hypersensitivity reaction -Characterized by: swelling, rashes, flushing, itching, drop in BP, difficulty breathing & can be fatal if not treated rapidly -Treatment = Epinephrine 2. Non-Immediate (>>1 hour) -Characterized by: rashes & itching -NOT treated with epi (local corticosteroid or an antihistamine) -Do not try to substitute another beta-lactam drug if pt is allergic to Penicillin G Other toxicity: Neurotoxicity -Occurs when given HIGH doses of Penicillin G (>20-60 million units) -More likely in neonates, elderly, & pts with preexisting brain injuries |
|
|
Penicillin G
(Carbonyl Group) |
The carbonyl group can be matched to a K+ or Na+ salt.
-It can make a salt with Procaine or Benzathine (positively charged). -Procaine & Benzathine are NOT SOLUBLE because they are given IM. When you inject them IM, you form a depot, which allows the Penicillin to be slowly released into the general circulation. Procaine --> Inject 1x/day Benzocaine --> Inject 1x/week -Cannot achieve high plasma concentrations of Penicillin G intramuscularly, so the bacteria must be very sensitive to it (can't be used for bacteria requiring high concentration of Penicillin G) -IM procaine stays for 1-2 days & benzathine stays for 1+ weeks, so if you run into a toxic effect, then you are SOL |
|
|
Typical Antipsychotic Drugs
|
Chlorpromazine, Haloperidol, Thiothixene
• Resolution of hallucinations, delusions, & disorganized thought processes (positive effects) • Loss of initiative, loss of interest in the environment & more limited range of affect (little display of emotion) --> poor compliance because it makes them feel bad; more likely to relapse • Drowsiness & slowness to respond to external stimuli (zombie phenomenon - not totally there; lack of interest), although subjects are easily aroused with intact intellectual functions (no ataxia or incoordination) • Antiemetic actions against non-GI-induced emesis (given to prevent emesis caused by general anesthesia) • Maximal therapeutic benefit is delayed (Takes a few weeks to get full antipsychotic effects) |
|
|
Broader Spectrum Antibiotics
|
Broader spectrum penicillin derivatives are can treat more types of bacteria. Involves changes to Penicillin G:
-Ampicillin (add an amino group --> benzene-CH(NH2)C(=O)... -Amoxicillin (add hydroxyl group to benzene) These derivatives are more HYDROPHILIC than Penicillin G & are able to go into porins; thus, effective on Gram Negative bacteria These derivations are NOT penicillinase resistant & are often combined with another drug (sole purpose of the 2nd drug is to inhibit penicillinase): •Ampicillin + Sulbactam = Unasyn •Amoxicillin + Clavulanic acid = Augmentin (only ORAL) •Ticarcillin + Calvulanic acid = Timentin •Piperacillin + Tazobactam = Zosyn •All are acid Stable --> (Amoxicillin>Ampicillin) •Associated with GI problems: -->Ampicillin = 8-10% -->Amoxicillin = 3% |
|
|
Dopamine Pathways & Their Interactions
|
-Neurons located in the substantia nigra project to different parts of the brain. Axons terminate & impulses synapse on cholinergic neurons in the striatum (caudate-putamen), which functions in fine motor control.
-The D2 receptors on the cholinergic target neurons are inhibitory; when occupied by D2 dopamine antagonists, the inhibitory effects of dopamine are blocked & the cholinergic neurons become disinhibited. Results in increased firing & release of more ACh, which activates muscarinic receptors = antipsychotic-induced parkinsonism. These side effects can be treated by coadmin. of appropriate antimuscarinic drugs. •Pre-frontal cortex (imp. for cognitive impairing effects) •Nucleus Accumbens - thought to be important for drug abuse/addition mechanisms that involve dopamine (many schizophrenics have problems with abuse of alcohol, nicotine, etc) •Dopamine released from the pituitary into the hypothalamic-pituitary portal system regulates (inhibits) release of prolactin. |
|
|
Mechanism of Pain Reduction by Non-Opioid Analgesics
|
-Membrane of cells have Phosphatidylcholine (carbond backbone with a phosphate & choline, fatty acid, & arachidonic acid-AA)
-AA is cleaved from the phosphatidylcholine by Phospholipase A2. -This initial step is inhibited by glucocorticoids that induce the synthesis of lipocortin that binds to & inhibits the phospholipase A2, thus inhibiting formation of AA. -AA can be worked on by lipooxygenases to make leukotrienes or can be metabolized by COX 1 or 2. COX 2 is induced by a painful stimulus & inflammation. COX enzyme converts AA to PGH1. PGH1 can form Thromboxanes and/or Prostaglandins. -Non-opioid analgesics (aka NSAIDS) work by inhibiting COX 1 & 2. They block conversion of AA to form PGE2 and thromboxanes. |
|
|
Acetaminophen
(Non-Selective NSAIDs) |
-Acetaminophen DOES HAVE anti-inflammatory properties AND is a Selective COX Inhibitor (as previously thought)
-Does not appear to have GI disturbances, but has the potential to cause serious liver toxicity that can be irreversible & lethal (especially with alcohol consumption) -It is metabolized in the liver where it produces benign metabolites & a non-benign metabolite: N-acetyl benzoquinone via the P450 enzyme (normally not a problem) -A small amount of N-acteyl benzoquinone can react with Glutathione to produce a glutathione N-acetyl benzoquinone, which can be eliminated. -If combined with alcohol, it can be deadly because: 1. Alcohol induces p450 enzyme that makes N-acetyl benzoquinone. So N-acteyl benzoquinone is produced. 2. Alcohol consumption (damaging to the liver), will decrease the amount of glutathione that is available to inactivate the toxic metabolite (N-acetyl benzoquinone builds up & reacts with various molecules/proteins/DNA & lead to lethal liver damage) |
|
|
Neurotrophic Hypothesis
|
-Based upon substance brain derived neurotrophic factor (BDNF)
-made in brain -plays role in plasticity (ability of brain) to change the number of neurons; it affects neurogenesis (making of more nerves) -BDNF can lead to increase in volume/size in certain parts of the brain -people who are depressed have a decreased volume/size of particular areas of the brain -In animal studies: if BDNF infused into animal brains ---> if they are depressed, they seem to be less depressed -in humans who are depressed: BDNF are decreased -drugs that are used--->lead to increase in BDNF--> treats depression |
|
|
Tricyclic antidepressants
|
-Core of the molecules are 3 ring structures
-Inhibit reuptake of serotonin & NE -Imipramine, Desmipramine (metabolite of imipramine), Amytryptiline, Noramytryptiline(metabolite of amytryptiline) -Parent drug & metabolite have activity: part of the longer activity is the formation of the metabolite which also has activity (so parent drugs should last longer) -Only used if no results from SSRis or SNRI -Not 1st choice because they effect other receptors -Block muscarinic, H1 receptors & alpha 1 receptors -Muscarinic receptors blocked --> dry mouth & constipation -H1: result = sleepiness, sedation -alpha 1: cause orthostatic hypotension |
|
|
Antibiotics
(Suppurative Abscess) |
Abscess --> a suppurative (pus forming) infection that derives from dead & dying cells in the area of infection, which will lead to poor circulation. This has a consequences:
-Effective antibiotic concentration is difficult to achieve at the site of infection -Supply of antibodies, phagocytes, oxygen and nutrients decrease & waste products (including CO2) increase: -->This causes bacteria to stop replicating & sometimes lyse (thus, bactericidal drugs will not work) -Leukocytes, which can fight infections, will go down - Lysis of host cells may release previously unavailable intracellular metabolites that the bacteria may now utilize to bypass the block created by the antibiotic -When you come across an abscess in a pt: --> Drain the pus for optimal antibiotic action |
|
|
Staph Treatment
(Penicillinase-Resistant Penicillins) |
-Some beta-lactamases are more selective for penicillin molecules (penicillinases) & others are more selective for cephalosporin molecules (cephalosporinases)
-Staph is resistant because they produce a penicillinase that inactivates the penicillin. Thus we must modify Penicillin G: -->Methicillin -->Nafcillin (most used - unique b/c it's eliminated through the liver by bile) -->Oxacillin (most used) -->Cloxacillin -->Dicloxacillin -These are ONLY used when bacteria are penicillin-resistant (If not resistant, then Penicillin G is more effective) -Some staph are resistant to all alternative --> Methicillin Resistant Staph aureus (MRSA) |
|
|
Monobactams
|
Aztreonam (only available drug)
ONLY has the beta lactam ring (no 2nd ring) -->Anaphylactic reactions occur by a reaction to the 2nd ring...so Monobactams do NOT have anaphylactic reactions Uses: SERIOUS AEROBIC infections -Septicemia -Serious pneumonia -Osteomyelitis -UTIs -Pseudomonas aerogenosa For serious Gram Negative infections there is an alternative: -Combination of a broader spectrum penicillin + an Aminoglycoside |
|
|
Alcohol
(Effects on the CNS) |
Acute Effects:
-Behavioral depression -Depression of respiration (main cause of death) -Altered temperature regulation with heat loss (actually getting colder due to vasodilation) -Arteriole & capillary dilation in the skin & GI tract Chronic Effects: -Tolerance --> decreased sensitivity to intoxication, reduced CNS depression (brain learns to counter the effects of alcohol with a stimulatory effect) by induction of metabolizing enzymes & learned behavioral compensation - when you feel terrible in the morning, it's because the depressive effects have left with the alcohol and the overcompensated stimulatory effect is still there. -Physical Dependence: withdrawal reaction when consumption is stopped --> characterized by tremors (delerium tremens), sweating, nausea, hallucinations, delirium, convulsions. Withdrawal can be life-threatening. Often treated with Benzodiazepines. |
|
|
Antipyresis
(NSAIDs) |
NSAIDs can reduce Fever (different from Hyperthermia)
-Hyperthermia is an increase in body temperature (occurs when the body produces more heat than it can eliminate; i.e. vigorous exercise) -Fever occurs due to a change in the "setpoint" for body temperature (like a thermostat); the body has decided to maintain the temperature higher. •Fever is produced by exogenous or endogenous pyrogens: -Some cytokines produce endotoxin (i.e. Gram- bacteria). These substances stimulate endothelial cells in the hypothalamus, ventricles & brain to get an increase in COX2. PGE2 synthesis increases & PGE2 is released & interacts with the PGE2 receptor EP-3 on glial cells to release cAMP, which causes the elevation of the thermoregulatory setpoint (through the action of the hypothalamus). •Anti-pyretics (acetominophen, ibufrofen, aspirin, etc) inhibit COX2 & prevent the increase of PGE2, thus allowing the temperature set point to remain at "normal" |
|
|
Bacterial Cell Wall Synthesis
(Stage 2 Cell Membrane) |
-UDP-MurNAc-pentapeptide reacts with Lipid-P to make --> Lipid-P-P-MurNAc-UDP-pentapeptide
-This product reacts with UDP-GlcNAc to yield --> Lipid-P-P-MurNAc-pentapeptide-GlcNAc -Glycyl-tRNA is then used to add the 5 Glycines onto L-Lys on Lipid-P-P-MurNAc-pentapeptide-GlcNAc [NONE of the above steps are inhibited by Penicillins] -The Lipid-P-P-MurNAc-peptapeptide(5 glycine)-GlcNAc is added onto the growing end of the cell wall (Peptidoglycan) and Lipid-P-P remains. --> This step is inhibited by Vancomycin (binds to D-Ala-D-Ala) --> Bacteria resistant to Vancomycin change D-Ala-D-Ala to D-Ala-D-Lactate (binding affinity is reduced 1000 fold, so the usual dose of Vancomycin will not inhibit this step) -Lipid-P-P reacts with phosphatase to give Lipid-P, which goes through the cycle again --> This step is inhibited by Bacitracin (a topical antibiotic) |
|
|
Bacterial Cell Wall Synthesis
(Stage 3 Cell Wall) |
The 5 glycines on Peptidoglycan-MurNAc-pentapeptide(L-Lys)-GlcNAc form a bond with the next to last D-Ala & the last D-Ala is removed.
--> This reaction is catalyzed by a Transpeptidase, which yield MurNAc-GlcNAc-4AAs + 5 glycines --> This step is inhibited by ALL Beta-Lactams Penicillin resembles the last 2 D-Alanines. Penicillin- Binding-Proteins (PBPs), such as transpeptidases, react with penicillin. So the transpeptidase reacts with penicillin & opens the beta-lactam ring = IRREVERSIBLE & the transpeptidase can no longer function to link the 2 strands •Penicillin Resistance --> Bacteria may produce a Beta-lactamASE that reacts with the beta lactam bond instead of transpeptidase. It binds reversibly & causes a hydrolysis reaction. The beta-lactamase comes off active & inactivates penicillin •Beta lactamases are made from a genome of a bacterium & are frequently carried on plasmids |
|
|
Penicillin G
(Administration & Absorption) |
•Orally, IM, or IV administration
-Orally, only 1/5-1/3 of what is given is absorbed since Penicillin G is acid-labile (beta-lactam bond cleaved by HCl) -->Orally, take at least 1 hour before eating or at least 2 hours after eating (will minimize the problems of absorption & given better adsorption into systemic circulation) --> Takes 30-60 minutes to be in plasma -IM, 1/2 of penicillin gets absorbed --> Takes 30 minutes to be in plasma To combat acid-lability, you can give a variant of Penicillin G --> Penicillin V (has an oxygen in the structure that makes it acid stable). Unlike Penicillin G, Penicillin V will NOT absorb the food in the GI tract •Generally dose Penicillin G ~every 4 hours (we do not see a plateau concentration, but it's ok b/c it binds irreversibly so the effect stays the same) •Penicillin G is very selective because Cell wall synthesis does not exist in human cells |
|
|
Sulfonamides
(Mechanism) |
-Bacteria make 6-pyrophosphoryl-methyl-7,8-dihydropteroin, which reacts with PABA & is catalyzed by dihydropteroate synthetase to make dihydropteroate. Dihydropteroate has a glutamate molecule added onto the end, which forms dihydrofolate (FH2). FH2 is then converted to tetrahydrofolate (FH4) by dihydrofolate reductase.
-TH4 (important in bacterial & human cells) transfers carbons to molecules, such as dUMP (deoxyuridylic acid). dUMP is catalyzed by N-5,10-methylene FH4 to dTMP (deoxythymidylic acid). The methylene group is put onto dUMP to convert it to dTMP. -Folate (taken up in the diet) can be converted to dihydrofolate (FH2). Since sulfonamides look like PABA, they will block formation of folate compounds (FH2 & FH4) competitively inhibiting dihydropteroate synthetase. It also inhibits making dTMP (thymidine), purines & methionine. |
|
|
Sulfonamides
(Mechanism - DNA synthesis inhibition) |
-It is possible for humans & bacteria to get preformed purines & methionine. However, there is not enough thymidine that can be converted to dTMP. If purines, methionine, & thymidine are very low, then we inhibit DNA, RNA, and protein synthesis = bacteristatic
-If enough purines & methionine are present, but low levels of thymidine, then only dTMP synthesis is inhibited = bactericidal -The block in folate synthesis leads to inhibition of DNA, RNA, & protein synthesis & the sulfonamides are bacteristatis. Under conditions where only DNA synthesis is inhibited "thymineless death" occurs (i.e. the sulfonamides are bactericidal) -The selectively of sulfonamides for bacterial synthesis is due to the fact that humans can make FH2 & FH4 from Folate in the diet, whereas bacteria cannot & rely on the synthesis using dihydropteroate synthase (humans do not have or use this enzyme) |

