![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
|
Definition of Pharmacology? |
The study of medicine
|
|
|
Pharmacotherapy/therapeutics? |
Therapeutics is concerned with Prevention of disease Treatment of suffering
Pharmacotherapy is the application of drugs for Disease prevention Treatment of suffering |
|
|
Know role FDA (responsibilities, stages) |
Four stages: Preclinical investigation Clinical investigation Review of new drug application (NDA) Postmarketing surveillance
They issue blackbox warning
Controls and approves ALL drugs OTC/RX
Another branch of the FDA, the Center for Biologics Evaluation and Research (CBER), regulates the use of bio- logics including serums, vaccines, and blood products.
The FDA oversees administration of herbal products and dietary supplements through the Center for Food Safety and Applied Nutrition (CFSAN)
Herbal and dietary supplements can be marketed without prior approval from the FDA; |
|
|
Differences between Trade/Generic |
A chemical name is assigned using standard nomencla- ture established by the International Union of Pure and Ap- plied Chemistry
The generic name of a drug is assigned by the U.S. Adopted Name Council. With few exceptions, generic names are less complicated and easier to remember than chemical names.
A drug’s trade name is assigned by the company market- ing the drug. The name is usually selected to be short and easy to remember. The trade name is sometimes called the proprietary or product or brand name.
The rule of thumb is that the active ingredients in a drug are described by their generic name. The generic name of a drug is usually lowercased, whereas the trade name is capitalized. |
|
|
Schedules (drugs in each) |
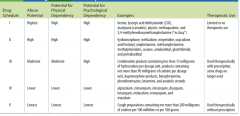
cheduled drugs are classified according to their potential for abuse: Schedule I drugs have the highest potential for abuse, and Schedule V drugs have the lowest potential for abuse.
Not all drugs with an abuse potential are regulated or placed into schedules. Tobacco, alcohol, and caffeine are significant examples.
Schedule V is the only category in which some drugs may be dispensed without a prescription because the quantities of the controlled drug are so low that the possibility of causing dependence is extremely remote. |
|
|
Controlled Substance |
In the United States, a controlled substance is a drug whose use is restricted by the Controlled Substances Act of 1970 and later revisions.
Hospitals and pharmacies must register with the Drug Enforcement Administration (DEA) and then use their assigned registration numbers to purchase scheduled drugs.
Hospitals and pharmacies must maintain complete records of all quantities purchased and sold.
a special order form must be used to obtain Sched- ule II drugs, and orders must be written and signed by the health care provider.
Telephone orders to a pharmacy are not permitted. Refills for Schedule II drugs are not per- mitted; patients must visit their health care provider first. |
|
|
Drug Classes |
Therapeutic Classification Based on what the drug does clinically Examples Anticoagulants Antidepressants Antineoplastics
Pharmacologic Classification Based on the drug's mechanism of action, or how the drug produces its effect At molecular, tissue, or body-system level More specific than therapeutic classification |
|
|
Prototype drug |
“Prototype” Drug—Serves as Model for a Drug Class
Is well understood Has known action and adverse effects Is used to compare other drugs in the same pharmacologic class May not be the most widely used drug in its class Disagreements may exist over which drug should serve as prototype drug. |
|
|
Know all the routes (pros/cons) |
The enteral route includes drugs given orally and those administered through nasogastric or gastrostomy tubes. Oral drug administration is the most common, most con- venient, and usually the least costly of all routes. It is also considered the safest route because the skin barrier is not compromised.Medications absorbed from the stomach and small intestine first travel to the liver, where they may be inactivated before they ever reach their target organs. This process, called first-pass metabolism,
Topical drugs are those applied locally to the skin or the membranous linings of the eye, ear, nose, respiratory tract, urinary tract, vagina, and rectum.
The parenteral route delivers drugs via a needle into the skin layers, subcu- taneous tissue, muscles, or veins.
The IV route is used when a very rapid onset of action is desired. As with other parenteral routes, IV medications bypass the enzymatic process of the diges- tive system and the first-pass effect of the liver. Although the IV route offers the fastest onset of drug action, it is also the most dangerous. Once injected, the medication cannot be retrieved. If the drug solution or the needle is contaminated, pathogens have a direct route to the bloodstream and body tissues. |
|
|
Know definition and meaning of pharmacokinetics |
The term pharmacokinetics is derived from the root words pharmaco, which means “medicine,” and kinetics, which means “movement or motion.” Pharmacokinetics is thus the study of drug movement throughout the body. In prac- tical terms, it describes how the body deals with medica- tions |
|
|
Know the FOUR components |
Absorption is a process involving the movement of a substance from its site of administration, across body mem- branes, to circulating fluids. Drugs may be absorbed across the skin and associated mucous membranes, or they may move across membranes that line the gastrointestinal (GI) or respiratory tract. Most drugs must be absorbed to produce an effect.
Distribution involves the transport of drugs throughout the body. The simplest factor determining distribution is the amount of blood flow to body tissues. The heart, liver, kid- neys, and brain receive the most blood supply. Skin, bone, and adipose tissue receive a lower blood supply; therefore, it is more difficult to deliver high concentrations of drugs to these areas.
Metabolism, also called biotransformation, is the process of chemically converting a drug to a form that is usually more easily removed from the body. Metabolism involves com- plex biochemical pathways and reactions that alter drugs, nutrients, vitamins, and minerals.
Drugs are removed from the body by the process of excretion. The rate at which medications are excreted determines the concentration of the drugs in the bloodstream and tissues. This is important because the concentration of drugs in the bloodstream determines their duration of action. Pathologic states, such as liver disease or renal failure, often increase the duration of drug action in the body because they interfere with natural excretion mechanisms. |
|
|
Which organ is primary metabolizer? |
The liver is the primary site of drug metabolism, although the kidneys and cells of the intestinal tract also have high metabolic rates. |
|
|
First past effect? |
drugs absorbed after oral administration cross directly into the hepatic portal circulation, which carries blood to the liver before it is distributed to other body tissues. Thus, as blood passes through the liver circulation, some drugs can be completely metabolized to an inactive form before they ever reach the general circulation. This first-pass effect is an important mechanism, since a large number of oral drugs are ren- dered inactive by hepatic metabolic reactions. Alternative routes of delivery that bypass the first-pass effect (e.g., sub- lingual, rectal, or parenteral routes) may need considera- tion for these drugs. |
|
|
Loading dose? maintenance doses? |
Few drugs are administered as a single dose. Repeated doses result in an accumulation of drug in the bloodstream, as shown in. Eventually, a plateau will be reached where the level of drug in the plasma is maintained continu- ously within the therapeutic range. At this level, the amount administered has reached equilibrium with the amount of drug being eliminated, resulting in the distribution of a continuous therapeutic level of drug to body tissues.
The plateau may be reached faster by administration of loading doses followed by regular maintenance doses. A loading dose is a higher amount of drug, often given only once or twice to “prime” the bloodstream with a sufficient level of drug. Before plasma levels can drop back toward zero, intermittent maintenance doses are given to keep the plasma drug concentration in the therapeutic range. |
|
|
Pharmacodynamics |
The term pharmacodynamics comes from the root words pharmaco, which means “medicine,” and dynamics, which means “change.” In simplest terms, pharmacodynamics re- fers to how a medicine changes the body. A more complete definition explains pharmacodynamics as the branch of pharmacology concerned with the mechanisms of drug ac- tion and the relationships between drug concentration and responses in the body. |
|
|
Potency? Efficacy? |
First is the concept of potency. A drug that is more potent will produce a therapeutic effect at a lower dose, compared with another drug in the same class.
efficacy, which is the magnitude of maximal response that can be produced from a particular drug.
From a pharmacotherapeutic perspective, efficacy is almost always more important than potency. In the previous ex- ample, the average dose is unimportant to the patient, but headache relief is essential.
|
|
|
median lethal dose? |
For example, the median lethal dose (LD50) the dose of drug that will be lethal in 50% of a group of animals. |
|
|
Median effective dose? |
drug’s median effective dose (ED) is the dose required to produce a specific therapeutic response in 50% of a group of patients. Drug guides sometimes report the ED50 as the average or standard dose. |
|
|
Therapeutic index |
The ED50 and LD50 are used to calculate an important value in pharmacology, a drug’s therapeutic index, the ratio of a drug’s LD50 to its ED50.
The larger the difference between the two doses, the greater the therapeutic index.
In Figure 5.2a, the therapeu- tic index is 4 (40 mg ÷ 10 mg). Essentially, this means that it would take an error in magnitude of approximately 4 times the average dose to be lethal to a patient. Thus, the thera- peutic index is a measure of a drug’s safety margin: The higher the value, the safer the medication. |
|
|
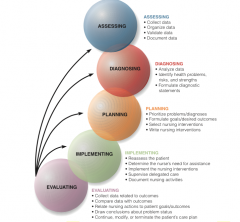
Know the phases |
|
|
|
Basic labs to assess for med admin. |
wevwev |
|
|
Definition of med error |
medication error is “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care pro- fessional, patient, or consumer.” |
|
|
Know the steps for reporting |
dwv |

