![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
216 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Haemostasis (natural process) |
-the arrest of bleeding from a broken or ruptured vessel (prevents significant blood loss after vascular injury) -formation of a localized plug in injured vessels and blood is kept fluid and clot free -depends on the blood vessel wall, platelets & other blood cells, and coagulation proteins |
|
|
|
4 step process |
1) Local Vasoconstriction: reduces blood flow to injury site
-injured blood vessel constricts (initiated by neurogenic mechanisms/pain receptors and released vasoconstricters due to pain receptors such as endothelin) - lasts for upto 30 minutes (briefly reduces flow)
2) Primary Haemostasis (platelet plug formation): platelet aggregation which acts as a plug
-intact endothelium acts as a physical barrier -separates circulating platelets from thrombogenic (clot forming) substances in extravascular space -vasuclar injury exposed floating platelets to procoagulant endothelium matrix to initiate 4 events:
2.1 Platelet adhesion: upon endothelial injury, platelets bind to exposed subendothelial matrix proteins (collagen)
*platelets bind adhesive proteins via transmembrane glycoprotein receptors (GP1b-IX-V binds von willebrand factor, GPVI and a2B1 binds directly to collagen, and GP1c binds fibronectin)
2.2 Platelet activation: adherent platelets undergo activation (shape becomes elongated with cytoplasmic extensions, granule release (release of contents of preformed cytoplasmic granules)):
2.2.1 a granules: contain vWF, P-selectin, FV & FXIII 2.2.2 dense granules: contain ADP, serotonin and Calcium
-increased throboxin A2 production -activation and expression of GPIIb
2.3 Platelet agression & Platelet plug formation: -agonist activated platelet GPIIb/,IIIa receptors bind fibrinogen, cross bridge with adjacent platelets and form a primary haemostatic plug-effective primary haemostatic plug-effective
-effective in small blood vessels but not in larger vessels or severely injured ones -active and aggregated platelets form phospholipid membrane surface for the clotting process
3) Secondary Haemostasis (blood clotting and coagulation): blood clotting strengthens and reinforces platelet plug -main goal is to form stable fibrin clot at site of injury -serial activation of coagulation factors, formation of insoluble, cross linked fibrin, stability in of the primary platelet plug and a compact irreversible clot formed
4) Fibrinolysis: dissolves the clot once the vessel integrity has been restored
- the steps run simultaneously without the completion of the other steps -process that dissolves and removes the fibrin clot -ensures localization of fibrin clot -removes clot after healing -meditated by plasmin, a proteolytic enzyme that degrades fibrin mesh: release of plasminogen activators, plasmin production from inactive precursors (plasminogen), and clot lysis plus release of degraded products |
|
|

Secondary Haemostasis (the coagulation cascade) |
-3 key features:
1) sequence of enzyme reactions involving blood borne coagulation factors
2) major activation requires - negative charge phospholipid rich membrane surface on platelets and endothelial cells -an enzyme (activated coagulation factor), a substrate (pro enzyme form of downstream coagulation factor), a cofactor& Ca
3) divided into 3 main pathways: intrinsic, extrinsic and common pathways
-involves serial activation of inactive plasma proteins ( a and B globulins) to zvrive enzymes and cofactors - each activated enzyme reacts with the next inactive plasma protein to activate it -proceeds until fibrinogen, the final substrate us converted by fibrin to active enzyme thrombin -all enzymes in the cascade are serine proteases -all enzymes and cofactors in the cascade are referred to as coagulation or clotting factors ( 12 coagulation/clotting factors, from Roman numerals I to XII, "a" beside activated form of coagulation factor, most factors synthesized in the liver) -no factor 6 as the same as factor 5 |
|
|
|
Biochemical and Physiological classification of coagulation factors |
1) contact group: XII (12), XI (11), prekallikrein, HMW, kinninogen (require contact with negatively charged surface for activation) 2) prothrombin groups: II (12), VII (7), IX (9), X (10) (require vitamin K for synthesis) 3) fibrinogen group: I (1), V (5), VIII (8), XII (12) (large molecules absent from serum) |
|
|

Intrinsic, Extrinsic and Common Pathway |
1) Intrinsic: all components occur in the bloodstream (activates 8, 9, 11, 12, HMW kininogend, and prekallikrein)
-slow responding and begins when blood makes contact with collagen in damaged blood vessel wall - factor 12 forms a primary complex with high molecular weight HMWK and prekallikrein on collagen to activate the factor -FXIIa activates factor 11 which activates factor 9 -FIXa forms a complex with FVIIIa, Ca, and phosphadatidylserine (intrinsic tenase) which activates FX for thrombin generation
2) Extrinsic: requires a factor not normally present in blood (ex. Tissue factor in wall of blood vessel) (tissue factor and factor 7)
-quick responding, begins when damage occurs to tissues surrounding the injury
-involves interaction between factor 7 calcium, and tissue factor (factor 3, tissue bound thromboplastin: cell bound glycoprotein largely expressed in extravascular tissue, exposed to blood when endothelial barrier is breached)
-upon contact with blood, the damaged cells release tissue factor (forms complex with calcium called extrinsic tenase)
- the TF- factor 7a in turn activate FX of the common pathway (thrombin generation) or FIX of the intrinsic pathway -factor X binds to Xa and activates prothringin to thrombin
3) both intrinsic and extrinsic eventually activate the common pathway ( factor 1 (fibrinogen to fibrin), 2 (prothrombin), 5, 10) -both intrinsic and extrinsic lead to the common pathway where fibrin is produces to seal off breach in blood vessel wall
3.1 Activation of FX: by intrinsic and extrinsic tenase complexes -FXa rhen forms a complex with FVa, Ca+++ and phosphatidylserine (prothrombinase complex)
3.2 Conversion of prothrombin (FII) to thrombin (FIIa): prothrombinase complex complex above converts to FIIa
3.3 Cleavage of fibrinogen (FI) to fibrin (FIa): thrombin cleaves fibrinogen to fibrin monomers
3.4 polymerization of fibrin: fibrin monomers undergo spontaneous polymerization
3.5 Stabilisatuon of fibrin polymers by FXIII: FXIIa induces cross linkage and stabilization of fibrin monomers |
|
|
|
Receptor families |
1) G protein coupled receptors (largest family of cell surface receptors)
*found by Alfred Gliman and Martin Rodbell *updated form by Lefkowit, and Kobilika in 2012 -meditate the cellular responses to an enormous diversity of signaling molecules (hormones such as glucagon, proteins, and neurotransmitters such as Ach, DA, NA) -most regulate cAMP and calcium as second messengers
2) ligand gated ion channels 3) tyrosine kinase linked receptors 4) steroid receptors (nuclear receptors) |
|
|
|
Muscarinic Receptors (G protein coupled receptors)
|
1) M1: nerves: increases IP3 plus DAG cascade and thus Calcium production
2) M2: heart and nerves: decreases CAMP production, activates potassium channels and inhibits calcium channels *basal forebrain, act as auto receptors to control acetylcholine synthesis and release*
3) M3: glands, smooth muscle and endothelium: increases IP3 and DAG cascade
4) M4: CNS: decreases CAMP production and activates potassium channels
5) M5: CNS: increases IP3 and DAG cascade
(hydolyze PIP2)
-M2 and M4 coupled to Gi (inhibit and negatively regulate adenylyl syclase and decrease CAMP production)
-M1,M3 and M5 coupled to the Gq (hydolyze PIP2)-M2 and M4 coupled to Gi (inhibit and negatively regulate adenylyl syclase and decrease CAMP production)-M1, M3 and M4 located in cerebral cortex and hippocampus meditate effects of Ach on learning and memory-M1 and M4 occur in striatum, and meditate cholinergic signaling in extrapyramidal motor circuits ,M3 and M5 coupled to the Gq (hydolyze PIP2)-M2 and M4 coupled to Gi (inhibit and negatively regulate adenylyl syclase and decrease CAMP production)-M1, M3 and M4 located in cerebral cortex and hippocampus meditate effects of Ach on learning and memory-M1 and M4 occur in striatum, and meditate cholinergic signaling in extrapyramidal motor circuits (hydolyze PIP2)-M2 and M4 coupled to Gi (inhibit and negatively regulate adenylyl syclase and decrease CAMP production)-M1, M3 and M4 located in cerebral cortex and hippocampus meditate effects of Ach on learning and memory-M1 and M4 occur in striatum, and meditate cholinergic signaling in extrapyramidal motor circuits -M1, M3 and M4 located in cerebral cortex and hippocampus meditate effects of Ach on learning and memory
-M1 and M4 occur in striatum, and meditate cholinergic signaling in extrapyramidal motor circuits
|
|
|
|
Nicotinic Receptors (ligand gated) (positive charge and excitatory) |
- activation leads to the inward flow of sodium ions - neuronal nAChR composed of 5 subunits: most have 2 alpha and 3 beta subunits - 12 identified (8a (a2 - a9) and 3b ( b2-b4)) with a1 and b1 in skeletal muscle -homomeric receptors a7 can form a channel (5 form an active channel) 1) NM: skeletal neuromuscular junction: pentameric structure with (2alpha, beta,lambda and sigma): sodium depolarizing ion channel 2) NN: post synaptic cell bodies, dendrites: a and b subunits only with 2 alpha and 3 beta: sodium depolarizing ion channel |
|
|
|
A-adrenoreceptors (targets of drugs and carecholamines such as epinephrine and norepinephrine) |
1) a1 (a1a, a1b, a1d): -Na > Ad -found in post junctional effector organs such as vascular muscle, arterioles -causes smooth muscle contraction -increases IP3 and DAG - Phenylephrine is agonists -prazozin is antagonists 2) a2 (a2a, a2b, a2c) - Ad> NA -found in presynaptic nerve endings and post synaptic brain and some blood vessels -inhibits transmitter release and decreases sympathetic outflow - decreases cAMP and calcium - clonidine is agonist -yohimbine is antagonist *2 different g proteins for each of the receptors |
|
|
|
B-adrenoreceptors (aCtivated by norepinephrine and epinephrine) (GPCR) |
1) B1: - Iso > NA > Ad - found in heart, adipose tissue and JG kidney cells - increases CAMP-an agonist is dobutamine and an antagonist is atenelol -an agonist is dobutamine and an antagonist is atenelol 2) B2: -Iso > Ad > NA - located in the bronchi, blood vessels and uterus - increases CAMP -an agonist is Salbutamol 3) B3 - NA> Ad, found in adipose tissue and not blocked by propranolol *1 g protein for beta 1 and beta 2 |
|
|
|
Dopamine receptors (GPCR) |
1) D1 type: have D1 and D5 receptors which increase CAMP and cause post synaptic inhibition 2) D2 type: have D2, D3 and D5 type which decrease CAMP and cause pre/post synaptic inhibition/ stimulate hormone release |
|
|
|
Subtype of Histamine receptors (GPCR) (bind to histamine as primary ligand: produce allergic response) |
1) H1: -wide spread in vascular airways involved in inflammation (allergies) and bronchial inflammation -G protein 2nd messenger: Gq (increases IP3 and DAG) -an agonist us histaprodigen and an antagonist is mepyramine 2) H2: - located in the stomach - stimulates acid secretion -G protein 2nd messenger: Gs (increases cAMP) -an agonist is histaprodifen and an antagonist is mepyramine 3) H3: -located in the CNS -inhibits neurotransmitter release -G protein 2nd messenger: Gi (decreases cAMP) - an agonist is methimmepip and clobenpopit 4) H4: - found in bone marrow and blood cells -involved in inflammation -G protein 2nd messenger: Gi (decreases cAMP) - an agonist is 4 methyl histamine and an antagonist is JNJ7777120 |
|
|
|
20 receptors of 5HT/ serotonin |
-antidepressants modify 5HT and not classically agonists -simatriptan is used to treat migraines: caused by too much blood flow into the brain causing pain - agonists if 5HT 1B and 1D receptors constrict blood vessels |
|
|
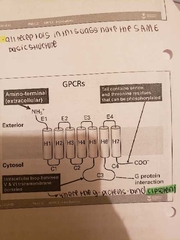
All of the protein linked receptors have s similar structure |
-occur in the cell membrane -respond in seconds -have a single polypeptide chain that has 7 transmembrane helices -have an extracellular amino terminal -tail contains serine and the nine residues that can be phosphorylated -intracellular loop between H4 and H5 domains where the g proteins bind |
|
|
|
Herterotrimeric G proteins (active when GTP is bound and inactive when GDP is bound) (GTPases) |
-composed of 3 different polypeptide chains (alpha, beta and gamma) - both alpha and gamma subunits have lipid molecules that help them bind to the plasma membrane - the alpha subunit has GDP bound (inactive state) - beta and gamma form a dimer that is tightly bound |
|
|
|
Heterotimeric proteins on and off switching |
-extracellular ligand binds to a protein linked receptor causing a change in confirmation - releases GDP and replaces it with GTP - the switch is turned off when the G-protein hydrolyzed its own bound GTP and converts it into GDP (occurs when tk much GTP in the cell) -befife this occurs the active protein diffuses away from the receptor and delivers its message for a prolonged period of time |
|
|
Dissociation of a heterotrimeric g protein activates it's two components |
-GTP binding causes conformations change in the surface if the alpha subunit - causes release of the beta gamma dimer -allows alpha subunit to interact with target proteins -the alpha subunit us a GTPase and hydrolysis GTP to GDP - the associated by beta gamma complex
(SWITCH OFF SIGNALLING SEQUENCE)
- an isolated alpha subunit is an inefficient GTPase and takes minutes if alone -activity enhanced by binding of a second protein called a regulator G-protein signalling (RGS: plays crucial role in shutting off the Gprotein) |
|
|
|
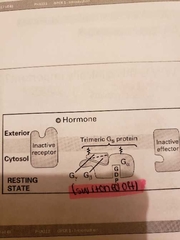
Cycle of events for G-protein coupled receptors |
1) binding of hormone induced a conformational change in receptor 2)activated receptor binds to Ga subunit 3) binding induces conformational changes in Ga: bound GSP dissociates and is replaced by GTP: Ga dissociates from the beta gamma dimer 4) hormone dissociated from receptor; Ga binds to effector activating it (Receptor is switched off but signaling continues) 5) hydrolysis of GTP to GDP causes Ga to dissociate from effector and to associate with Gbeta and lambda (Inactive G protein) |
|
|
|
G protein effects |
1) Gs: the as subunit; activates adenylyl cyclase and increases cAMP plus activates calcium channels 2) Gi: the ai subunit: inhibits adenylyl cyclase and thus decreases cAMP plus activates potassium channels 3) Gq: the aq subunit: activates PLC-B turn over which generates IP3 which is a second messenger for calcium and increases its amount |
|
|
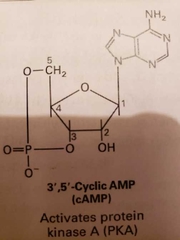
cAMP (activates protein kinase A) |
-synthesized from ATP by plasma membrane bound enzyme called adenylyl cyclase -rapidly and continuously destroyed by phosphodiesterase enzymes -hydrolyses cAMP to AMP -has 2 catalytic domains -pKA sticks phosphates onto compounds to alter activity -inactive pKA has 2 catalytic subunits and 2 regulatory subunits that bind cAMP (binding causes disassociating of regulatory subunits from the complex) |
|
|
|
Phosphodiesterase enzymes |
-atleast 10 subtypes exist (PDE 3 and 4 selective for cAMP and 5 selective for cGMP) -inactivated weakly by drugs (methylaxanathines (theophylline & caffiene), rolipram (PDE 4 selective), slidenafil (viagra: PDE 5), milrinone (treat a heart failure) -PDE inhibitors used to treat cardiovascular and respiratory disease -theophylline inactivates phosphodieasterase and is used in the treatment of asthma |
|
|
|
B-adrenoreceptor |
-b1 and b2 are both coupled to cAMP - function of b1 and b2 depend on location and what is inside the cell - cAMP in skeletal muscle breaks down glycogen and stores it as carbs (also inhibits synthesize in liver and skeletal muscle) -also relaxation of smooth muscle (increased cAMP) |
|
|
|
Switch on and off |
1) ON: -when adrenaline binds to the B adrenoteceptor, adenylyl cyclase is active through Gs
-cAMP is formed as a result by the as subunit exchanging GDP for GTP
-as dissociates from Bgamma diner and activates adenylyl cyclase
-life of active as is short as GTPase activity of as is stimulated when as binds to adenylyl cyclase
2) OFF:
-bound GTP is hydrolyzed by GDP as soon as it is bound to adenylyl cyclase, inactivating aS and adenylyl cyclase
-the as then reassociated with beta gamma dimer to reform inactive Gs molecule |
|
|
|
PKA |
-2 regulatory and 2 catalytic subunits: 2 catalytic subunits released -cAMP activates A-kinase, which phosphorylates/activates phosphorylase kinase which phosphorylates/activates glucose kinase that releases glucose and 1 phosphate used for fight for flight response -phosphorylates 2 other enzymes:
1) phosphorylates phosphorylase kinase which phosphorylates the glycogen phosphorylase enzyme -glycogen phosphorylase releases glucose residues from glycogen
2) glycogen synthase (final step in glycogen synthesis): active all the time, but its phosphorylation inhibits glucose synthesis |
|
|
|
Phosphatase (1, 2, 3 and 4) |
-dephosphorylate proteins phosphorylated by PKA -phosphotase 1 plays an important role in response to cAMP (counteracts phosphorylation by adrenaline stimulated muscle cells) - Fischer and Krebs award in 1992 for reversible protein phosphorylation as a biological regulatory mechanism -the purpose of these cascade reactions are for amplification (1 molecule or adrenaline binds to receptor and activates 100s of g proteins) |
|
|
|
Functions of cAMP |
-enzymes involved in energy metabolism -cell division and differentiation -ion transport -ion channels -conteactile proteins in smooth muscle |
|
|
|
CREB (transcription factor activated by neuronal activity) (key pathway for producing changes in synaptic transmission) |
-involved in the formation of long term memory -overactivation of CREB enhances long term memory formation -inhibitors of phosphodiesterase 4 enhbce memory |
|
|
|
Muscarinic M2 receptors in the heart |
-acetylxholine decreases force and rate of heart beats -coupled to Gi protein, causes decrease in cAMP production decreasing force of contraction - opens potassium channels by beta-gamma subunit and causes hyperpolarization of SA node cells (decreasing rate of firing and thus HR) |
|
|
|
Actions of cAMP in heart cells |
-interacts directly with pacemaker current channels (if) allow sodium to move in faster -activates PKA which phosphorylates calcium channels to increase open state and plateau current (ica: more calcium in heart cells) -PKA phosphorylates phospholamban, a protein associated with Calcium ATPase pump (allowing it to work faster, pump calcium in and out of heart faster increasing heart rate) |
|
|
|
G proteins and diseases |
1) Cholera caused by bacteria which secretes cholera toxin -causes persistent activation of adenylyl cyclase -massive amounts of cAMP produced -causing excess secretion of fluid from GI epithelium = chronic diarrhea due to NaCl buildup 2) Pertussis toxin (Ptx) secreted by bacterium causing whooping cough -inactivates a-subunit of Gi (prevents deactivation of adenylyl cyclase) -massive amounts of cAMP produced -increases mucus secretion and interferes with cellular functions |
|
|
|
Calcium as a second messenger (discovered in 1947) (intracellular injection of calcium caused muscles to contract) |
-to use calcium as an intracellular signal the resting systolic calcium levels are kept low and achieved in several ways
-a large gradient drives calcium from the ER (10^-3 M) to the cytosol (10-7 M)
|
|
|
|
Keeping Calcium out of the cell |
1) energy dependent calcium atpase pump: in the plasma.membrane uses energy of ATP to pump calcium out of the cell 2) anti porters of Na and Ca: nerve and muscle cells have anti porters that pump Na in and Ca out 3) calcium pump in the ER membrane keeps the cutosolic calcium level low 4) a low affinity pump high capacity calcium pump (when cell is damaged and Ca rises to a high level): in the inner mitochondrial membrane takes up Ca from the cytosol 4)numerous proteins in the cytosol bind to the calcium to inactivate it such as calmodulin |
|
|
|
2 pathways of calcium signaling |
1) nerve cells: depolarization of the plasma membrane causes influx of Ca through voltage gated calcium channels
2) other cells: binding of extracellular ligand to the cell surface receptors, IP3 is then released and binds to the binding sites on the gate and causes the release of Ca from the ER *most important are PIP and PIP2
-skeletal and cardiac muscle must have extracellular calcium to contract -smooth muscle has free calcium |
|
|
|
Hydrolysis of PIP2 |
-breakdown begins with a log and binding to a gprotein linked receptor (more than 25 cell surface receptors use this pathway)
- an activated receptor stimulates the trimeric g-protein gq which activates phospholipase (PLC-B)
-Phospholipase cleaves PIP2 to generate 2 products: IP3 and DAG which have roles in cell signaling |
|
|
|
IP3 and DAG |
-IP3 diffuses through the cytosol and releases calcium from the ER by binding to IP3 gated CA release channels in the ER (positive feedback) *the Ca released is fed back to release more calcium* -DAG remains in the membrane and activates protein kinase C (3 types: beta, gamma and sigma) the beta class is activated by G protein receptors
|
|
|
|
Smooth muscles |
-store calcium in the ER and SR -released when IP3 receptor is activated (IP3 generated by Gq linked receptor activation) -do not require electrical stimulation for the release of calcium |
|
|
|
Myosin light chain (contraction of smooth muscle activated by myosin light chain phosphorylation) |
-catalysded by MLCK -activated when bound to Ca calmodulin -moyosin phosphatase reverse phosphorylation and cause relaxation |
|
|
|
Role of Calcium in cells |
1) activate membrane ion channels 2) regulate enzyme activity 3) modulate protein function 4) regulate gene expression 5) kill cells (necrotic and apoptic) 6) life and death messenger
7) causes smooth muscle to contract (liberation from the SR triggers muscle contraction of smooth or cardiac muscle)
*the chemical signal carried out by calcium is more important than the charge*
-calcium influx through ligand gated channels involve LTP -calcium influx through voltage gated channels in the pre synaptic terminal evoke transmitter release |
|
|
|
Sources of Calcium to the cells |
1) extracellular fluid (2 mM) 2) plasma membrane channels 3) voltage gated channels (N,P, Q and L type) 4) ligand gated channels (glutamate and other neurotransmitters are linked to calcium channels) 5) store operated channels: open in response to depletion of ER calcium stores |
|
|
|
2 mechanisms that terminate the calcium response |
1) IP3 is radpidle dephosphorylated by specific enzymes 2) the calcium that enters the cytosol is rapidly pumped out of the cell 3) not all IP3 is phosphorylated as some is phosphorylated to IP4 4) meditates slower, more prolonged responses in the cell and refilling of intracellular Ca stores 5)the enzyme that catalyses IP4 is activated by the increase in cytosolic Ca induced by IP3 (IP4 meditates opening of store channels and increased level of calcium) |
|
|
|
Regulation |
-resynthesis of the phospholipid is or else the cell would run out of substate -13 reactions -2 cycles: ILC (in the membrane) and IPC (in the cytosol) |
|
|
|
DAG activates protein kinase C (PKC) |
-PKC is a calcium dependent enzyme (highest concentration in brain where it phosphorylates other ion channels) -DAG increases affinity of PKC for Ca, activating the enzyme -involved in the secretion, modulation of ion conductance and control of growth plus differentiation |
|
|
|
G protein linked receptor desensitization (how to get smooth muscle to contract and relax) |
1) receptor inactivation: receptors become altered so they no longer interact with the G proteins
2) receptor sequestration: receptors moved temporarily inside the cell so they no longer have access to the ligand
3) receptor down regulation: receptors destroyed by lysosomal after internalization
-desensitizatuin depends on phosphorylation of the receptor by PKA, PKC or a member of the Gprotein linked receptor kinase
-GRKs phosphorylate multiple serine and threonines (released after prolonged contract of ligand and receptor) on the receptor after ligand binding
-once a receptor has been phosphorylated it binds to a member of the arrestin family of proteins
-homologous or heterologous desensitization
|
|
|
|
Receptor phosphorylated by 3 enzymes |
1) BARK (B-adrenergic receptor kinase) -most important in homologous desensitization as it is only capable of phosphorylation the active agonist bound form of receptor -activate GRK, bind to arrestin and loss of gprotein 2) PKC (protein kinase C) 3) cAMP dependent protein kinase |
|
|
|
Receptor up and down regulation |
Examples include: -cholesterol LDL receptors up regulation by statins -opiate receptors up regulation by antagonist naloxone -beta adrenoreceptors up regulation bh antagonists -agonists tend to down regulate receptors |
|
|
|
Ligand gated ion channels |
-best studied example is the nicotinic acetylcholine receptor -5-HT3 -GABAa and GABAc -Glycine -Glutamate *voltage gated channels are very selective* *ligand gated channels are non specific* (Na, K and little Ca are the same size and pass through the pore) -opening of the chunnel causes a large net influx of Na (30,000 ions/channels/ms) and a small influx of K plus Ca -causing membrane depolarization and skeletal muscle contraction -prolonged contact of the receptor with elevated concentrations of Ach results in desensitization of the receptor |
|
|
|
Nuclear receptors |
-not embedded in the membrane but present in the soluble phase of the
-receptors become mobile on the presence of their ligand and can translate from the cytoplasm into the nucleus to cause a reaction -many act as lipid sensors involved in the regulation of lipid metabolism within the cell 1) Class 1: consist largely of receptors for steroid hormones, including glucocorticoid and mineralocorticoid receptors (GR and MR) -also oestrogen, progesterone and androgen (ER, PR and AR) -in abscence of their ligand, located in the cytoplasm completed with heat shock proteins or other proteins -form homodiners -in the nucleus they can transactivate (switch on gene transcription) or transgress genes (switch of gene transcription) -the ligand must be lipid soluble to cross the plasma membrane |
|
|
|
Structure of Nuclear receptors |

1) N terminal: displays the most heterogeneity; harbours AF1 which bind to other cell transcription factors 2) Core domain: highly conserved and consists if the structure responsible for DNA recognition and binding (2 zinc fingers/ cysteine or histidine loops in the amino acid chain held in a conformation by zinc ions) 3) Hinge region: a highly flexible region allows the molecule to dimeriee with other nuclear receptors and bind in a variety of configurations 4) C terminal domain: contains the ligand binding module specific to each class of receptor (a highly conserved AF2 region is important in ligand-dependent activation) |
|
|
|
Effects of steroids |
1) Anabolic and androgenic steroids: -mimic the effect if testosterone and hihydrotestosterone -increase protein synthesis resulting in build up of tissue (anabolism) -maintain masculine characteristics (androgenic) 2) Catabolic steroids: -break down muscle -used to treat swelling (anti-inflammatory) -cortisone and prednisone 3) Thyroid hormones: -T3 and T4 act on nearly every cell of the body -increase basal metabolic rate, affect protein synthesis, regulate bone growth, neuronal maturation, and increase the body's sensitivity to catecholamines |
|
|
|
Voltage gated ion channels |
-membrane proteins that conduct ions at high rates regulated by the voltage across the membrane -role in propagation of the nerve impulse and cell homeostasis -voltage sensor: region of protein which has charged amino acids that relocate when there is a change in the membrane's electric field (movement of sensor causes conformational change in the gate of the conducting pathway controlling ion movement) |
|
|
|
Types of Voltage gated channels |
1) Voltage gated sodium channels:
-2 voltage sensitive gated -at resting membrane potential (-75 mV) the mgate is closed and the hgate is open -when the membrane becomes depolarized (due to sodium entering) the mgate opens -after a short time the h-gate closed (-50 mV) -anaesthetics such as lidocaine block the channel and reduce pain -drugs also block Na channels in arrhythmias -tetrodotoxin blocks action potential by binding to Na+ channels (puffer fish, and blue ringed octopus)
(causes paralysis of skeletal muscle, numbness of lips and tongue, loss of sensation, stopped breathing: state of near death and unable to communicate for days)
2) Voltage gated potassium channels:
-only 1 gate activated by depolarization (much slower to respond) -n gate is closed, when Na enters and causes depolarization, the channel opens -contributes to membrane depolarization in the heart (hERG channel) if unable to allow K+ influx then can cause long QT syndrome -dendrotoin (57-60 amino acid protein) blocks K channels and prevents depolarization of nerve plus muscle cells
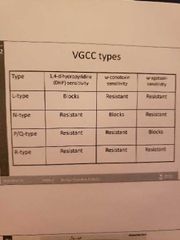
3) Voltage gated calcium channels:
-complex of several subunits: a1, a2s, b1-4 and y -the a1 subunit forms the ion conducting pore -subunits can be targets of drugs (pregabalin binds to a2s subunit and treats neuropathic pain)
-N-type (neural) -L-type (excitation and contraction of muscles plus hormone secretion in endocrine cells) -P/Q type -R type (resistant to blockers and toxins involved in poorly defined processes in the brain) -clinically useful drugs that block calcium channels include dihydropyridines (amlodipine, felodipine, nifedipine: block L type channels on vascular smooth muscle) -verapamil also blocks cardiac calcium channels treating angina and arrhythmias |
|
|
|
Tyrosine kinase linked receptors |
-superfamily of 1-TM linked structural and functional classification -have an enzyme called tyrosine kinase built into the receptor; when active these receptors recruit the cytoplasmic tyrosine kinase -Binding of these receptors results in the dimerisation of these receptors -PDGF is a diemeric ligand and can cross link two receptors (1 molecule) -EGF is a monomeric ligand that brings 2 molecules and 2 receptors together -an active protein is able to phosphorylate ge neighbouring receptor (interphosphorylation) -the dimeric complex is the active EGF receptor: at this stage it is able to phosphorylate and activate enzymes plus interact with adapter proteins |
|
|
|
EGF |
-receptor which initiates a series of signal transduction cascades through association with other proteins 1) activation of Ras 2) altered gene expression via MAP kinase 3)signaling via PI3 kinase 4) activation of PLC and PLA |
|
|
|
MAP Kinase (activated by EGF) |
-results in activation of an adapter protein called Ras GTPase -MAPK activation results in translocation into the nucleus and phosphorylation of proteins that regulate gene expression |
|
|
|
Ras (activated by EGF) |
-GTP binding protein and exists as a monomer -on and off state when GTP is bound and u bound -Ras does not directly bind to the receptor |
|
|
|
Ras activation |
1) accelerated by GEF, binds to RAS GDP complex to dissociate GDP 2) GTP binds to Ras and dissociation of GDP occurs as more GTP in cell (average lifetime of GTP-Ras complex is 1 minute) 3)deactivation of Ras requires another protein 4) GAP binds to the complex increasing its activity 100x -ras proteins such as GAP are associated with cancer |
|
|
|
Activation of MAPK alters gene expression |
-MAPK activates the kinase p90RSK then they both translocate to the nucleus -alter gene expression |
|
|
|
Why focus on EGF? |
-human carcinomas express high level of EGF receptors -monoclonal antibodies which block the activation if EGFR and ErbB2 have bern developed -Herceptin is a monoclonal antibody which blocks the ErbB2 receptor (look for receptor in breast cancer if expressed than use the drug) -Iressa is an inhibitor of the EGF receptor (non small lung cancer that has returned and unresponsive to chemotherapy) |
|
|
|
Insulin |
-polypeptide hormone made from 2 chains a and b -produces in the pancreas by b cells of the islets of langerhans 1) mRNA is translated into an inactive protein called preproinsulin which contains an amino terminal signal allowing passage through the ER membrane
2) post translational mechanisms remove this sequence to produce pro insulin 3) after forming 3 disulphide bonds, peptidase cleaves prounsulin to insulin 4) insulin is packaged and stored in secretory granules |
|
|
|
Insulin release |
-released in response to elevated blood glucose concentration into B cells 1) glucose is metabolized by glucokinase and converted to ATP 2) ATP/ADP ratio increases -GLUT2 meditate entry of glucose into B cells 3) ATP gated K channels in the membrane close preventing K leak causing depolarization 4) Ca channels are activated which transport Ca into the cell causing exocytosis of insulin
|
|
|
|
Insulin receptor substrate (IRS) proteins |
-phosphorylate several substrates including:
1) IRS protein 1-4 2) Shc 3) Gab 1 4) Cbl 5) APS 6) P60dol
-adapter proteins which affect the number of signaling cascades -each provides a docking site for other signaling proteins via SH2 domains -immediate substrates for insulin (tissue specific differences in tole of IRS proteins to meditate insulin action) |
|
|
|
Nicotinic acetylcholine receptor (nAChR) |
-located in the neuromuscular junction and the CNS plus spinal cord
-composed of 5 transmembrane segments (2 a, b, gamma and sigma) encoded by 4 separate genes
-the a polypeptide chains have binding sites for acetylcholine
- when 2 Ach molecules bind they open the channel for 1 Marc and the close -clusters of negatively charged amino acids are either enf of pore EXCLUDE -ve charged ions and ions more than 0.65 mm |
|
|
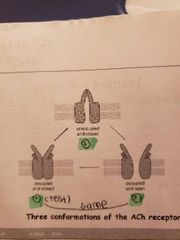
3 conformations of Ach receptor |
1) occupied and closed: at rest when no agonist is bound to it 2) unoccupied and closed: before acetylcholine has a chance to diffuse away from binding sites 3) occupied and open: acetylcholine is bound to receptor sites on the a subunit |
|
|
|
Nicotinic receptors |
-heteromeric (2a4 and 3b2 subunits most commonly studied) and homomeric (5a7 subunits) |
|
|
|
GABA receptor subtypes (LGIC) |
1) GABAa: ligand gated ion channel 2)GABAb: G-protein coupled receptor (a dimer made from 2 subunits,l 3)GABAc: ligand gated ion channel
-GABAa and c are linked to an chloride channel causing hyperpolarization (always inhibitory in nature) (l -GABAa (6a, 3b, 3y subunits) -various binding sites on the GABA receptor for clinical drugs (allosteric binding) -convulsuants: too much excitation on the brain causes epilepsy, and increased activity of GABA causes more Cl, hyperpolarization and lessens excitation |
|
|
|
GABAa |
-anticonvulsants and consultants act on the same site to produce opposite effects -BDZ needs GABA at its receptor sites in vitro to function, invivo GABA is always in the body and opens Cl channel longer |
|
|
|
GABA agonists |
-causes excitation by blocking effects of GABA by binding to its sites |
|
|
|
GABAa antagonists |
-bicuculline: competes with GABA for the binding site -picrotoxinin and TBPS are both non competitive antagonists at the GABAa receptor and bind away from the site |
|
|
|
GABA reuptake inhibitors |
-used in the treatment of epilepsy/anticonvulsants -Tigabine: is a derivative if nipecotic acid |
|
|
|
Barbituric acid: inactive parent compound modified for results to barbiturates |
-used for hypnotics (for severe insomnia amylobarbitone), sedatives, anticonvulsants (phenobarbitone), anesthetics (thiopentone: IV) -thiobarbituates: contain a sulfur molecule in place of an oxygen molecule (patients develop tolerance and a narrow therapeutic index and interact with alcohol) |
|
|
|
Benzodiazepines |
-the y subunit is essential for its activity at the receptor (increase affinity for GABA and open the Cl channel) -Flumazenil is an antagonist for the BDZ binding site used to treat BDZ overdose; has no effect on GABA binding -used as hypnotics (nitrazepan, flunitrazepan, and flurazepam which have prolonged action whilst loprazolam and temazepam act for a shorter amount of time) -anxiolytics used for short term relief of anxiety -main advantage over barbiturates is that - they do not directly open the Cl channel and lower chances of overdose |
|
|
|
Glutamate |
-L glutamate is major excitatory neurotransmitter in the CNS -if too much released then lethal ex. during CNS injuries -glutamate receptors play a vital role in synaptic plasticity and higher cognitive function -drugs that target glutamate include those for Alzheimer's and stroke |
|
|
|
Ionotripic receptors (glutamate activates all of these; hence look for a selective agonist) |
1) NMDA: involved in long term potentiation, learning, memory and synaptic plasticity (Calcium channel)
-coexist with AMPA and kainate -have a high Ca permeability -glycine is an agonist and must bind with glutamate to activate the receptor -tetrameric: GLUN1, GLUN2A-D And GLUN3A
-implicated in diseases such as stroke with too much Ca and excessive glutamate stimulation -parkinson's where pathways are overactive with glutamate -Huntington's where disrupted Ca homeostasis causes excitotoxicity
-parkinson's where pathways are overactive with glutamate -Huntington's where disrupted Ca homeostasis causes excitotoxicity -Huntington's where disrupted Ca homeostasis causes excitotoxicity
2) AMPA: widely expressed in the CNS jn rapid excitatory neurotransmission
-tetramers of 4 subunits GluA1-4 -AMPAkines used in treating neurological and psychiatric diseases -linked to formation of LTP and memory enhancing -upregulate neurotrophins such as BDNF
3) Kainate receptors:
-more restricted distribution -tetrameric combinations of 5 subunits GluK1-K5 -involved in oresynaptic regulation of neurotransmitter release -involved in excitatory imbalances linked to epilepsy
|
|
|
|
PI3 kinase and Akt pathways |
-PI3K plays a vital role in metabolic and cell division activities of insulin (heterodimeric enzyme of p85 subunit and p110 catalytic subunit subunit subunit |
|
|
|
PI3K secondary messengers |
-phospholipids recruit PI3-K dependent serine/the nine kinase (PDK 1 and PDK 2) and Akt by binding to PH domain -Akt is phosphorylated by THR308 by PDK1 and Ser473 by PDK2 leading to full activation of Akt -activated Akt phosphorylates and regulates the activity if many downstream proteins involved in cellular physiology |
|
|
|
Akt (PKB) |
- exist in several isoforms: a, b1, b2, and gamma -differ in phosphorylation sites -phosphorylates GLUT 4, PKC, GSK4 -all are critical in insulin meditated metabolic effects -inhibition of PI3K blocks insulin stimulated translocation of GLUT4 to cell surface and glucose uptake into the cells |
|
|
|
Classification of joints |
1) Functional: 1.1 Synarthroses: immovable joints 1.2 Amphiathroses: slightly moveable 1.3 Diarthroses: freely moveable joints 2) structural: 2.1 Fibrous: bones are joint by fibrous tissue, no joint present -suture: dense fibrous connective tissue ,(between the bones of the skull and develop into adulthood) -syndesmoses: a cord or band of connective tissue (long and fibrous tissue permits joint flex) -gomphoses: periodontal ligament that hold teeth in place (peg in socket) 2.2 Cartilageneous: articulating bones united by cartilage, not that moveable and lack a joint cavity -synchondroses (synarthroses): immovable, headline cartilage units bones (ex. epiphyseal plate,) -symphyses (amphiarthroses): fibrocartilage unite bones and absorb shock plus allow flexibility (ex. intervertebral discs) 2.3 Synovial: most of body's joints and are diarthroses, all have a fluid filled joint cavity -plane: allow gliding movements and no rotation (ex. Intercarpals, intertarsals and vertebrae) -hinge: elbow and knee joints; movement in one plane, cylindrical projection bone fits into trough shapes surface of another bone
-pivot: only movement allowed is rotation of the bone in long axis (shoulder: cork in screw) -condyloid: metocarphalangeal joints in first knuckles; oval fits into complementary concavity -saddle: more free movement than condyloid; each surface has concave and convex structure that fits together (ex. Carpometacarpal joints of the thumb) -ball and socket: most free moving synovial joint, movement in all planes (ex. Shoulder and hip) |
|
|
|
Joint movement |
1) Hyperextension/Extension/ Flexion: moving back, moving slightly forward, bending too forward -bring leg back, extend knee, bent knee 2) Gliding: the wrist from left to right (Mr. Miyagi movement) 3) Abduction/ adduction: open fingers and close fingers 4) Pronation/ supination: back of hand and front of hand 5) Dorsiflexion/plantar flexion: toes pointing up and toes pointing down 6) eversion and inversion: move foot away and move foot towards inside 7) protraction/ retraction: chin forward and chin back 8) elevation and depression: lift jaw up and bring jaw down 9) circumduction: circular motion |
|
|
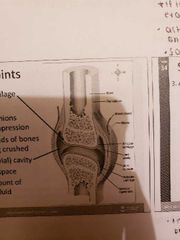
Synovial Joints (EXAM DRAW DIAGRAM) |
1) Articular cartilage: spongy hyaline cushions which absorb compression and protect ends of bones from being crushed 2) Joint cavity: small amount of synovial fluid with potential space 3) Articular capsule: 2 layered, outer (fibrous capsule of dense irregular connective tissue) and inner (loose connective tissue which makes synovial fluid) *lines all internal joints 4) Synovial fluid: filtrate of blood, has glycoproteins, nourishes cartilage and acts as a lubricant 5) Reinforcing ligament: thickened part of capsule (extracapsular and intracapsular ligaments) 6) nerves: detect pain and monitor stretch 7) Blood vessels: rich blood supply and extensive capillary beds in synovial membrane produce blood filtrate |
|
|
|
Reinforcing ligaments |
-ring of cartilage which help bones fit better -provide strength and support |
|
|
|
Bursae and tendon sheaths |
-contain synovial fluid -not joints but act like them -prevent friction (ball and socket in shoulder) -bursa is a flattened sac lined by the synovial membrane: ligaments, muscles and bones over lie each other and rub together -tendon sheath is only on tendons and subjected to friction |
|
|
|
Meniscus in some joints (crescent shaped) |
-bone ends of different shapes and poor fit -allow 2 kinds of movement (ex. Jaw) - ex. Knee and TMJ |
|
|
|
Parts of the skeletal system |
1) Bones: support the body and protect soft organs plus allow for movement, storage of minerals 2) Joints 3) Cartilages 4) Ligaments |
|
|
|
2 divisions of the skeletal system |
1) Axial skeleton: skull, ribs and vertebrae 2) Appendicular skeleton: pelvic and extremities that hang off the skeleton |
|
|
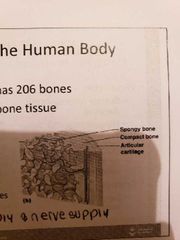
Types of bone tissue |
1) Compact bone: homogeneous 2) Spongy bone: many open spaces and small needle like pieces of bine |
|
|
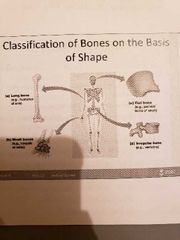
Classification of Bones |
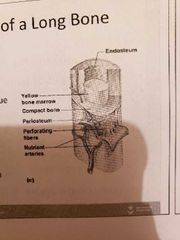
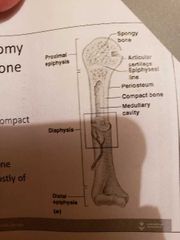
1) Long bone (humerus of arm and femur): contain mostly compact bone 1.1 Diaphysis: composed of compact bone and shaft Epiphysis: composed of mainly spongy bone and the ends of the bone 1.3 Periosteum: outside covering of the diaphysis 1.5 Arteries: supply bone cells with nutrients 1.2 Epiphysis: composed of mainly spongy bone and the ends of the bone1.3 Periosteum: outside covering of the diaphysis 1.4 Sharpey's fibre: secures periosteum to bone1.5 Arteries: supply bone cells with nutrients1.6 Articular cartilage: covers the external surface of the epiphyses (made of hyaline cartilage): shock absorber and decreases friction 1.7 Medullary Cavity: cavity of the shaft contains yellow marrow (fat) in adults and red marrow in children for blood cell formation 1.6 Articular cartilage: covers the external surface of the epiphyses (made of hyaline cartilage): shock absorber and decreases friction 1.4 Sharpey's fibre: secures periosteum to bone1.5 Arteries: supply bone cells with nutrients1.6 Articular cartilage: covers the external surface of the epiphyses (made of hyaline cartilage): shock absorber and decreases friction 1.7 Medullary Cavity: cavity of the shaft contains yellow marrow (fat) in adults and red marrow in children for blood cell formation 1.7 Medullary Cavity: cavity of the shaft contains yellow marrow (fat) in adults and red marrow in children for blood cell formation : cavity of the shaft contains yellow marrow (fat) in adults and red marrow in children for blood cell formation 2) short bones (carpals and tarsals) : cube shaped, mostly spongy bone 3) Flat bones (skills, ribs and sternum): thin layers of compact bone around a layer of spongy bone 4) Irregular bones (hip and vertebrae): irregular in shape |
|
|
|
Classification of joints |
1) Functional classification (focuses on movement):
-syanthroses: immovable -amphiathroses: slightly moveable joints -diathroses: freely moveable joints
2) Structural classification:
-fibrous: suture, syndesmoses, gomphoses -cartilageneous: synvhondroses, symphyses -synovial: gliding, hinge, pivot, condyloid, saddle, ball and socket
|
|
|
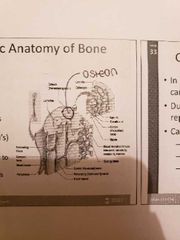
Miscroscopic Anatomy of Bone |
1) Osteon: a unit of bone 2) Central Haversian canal: carried blood vessels and nerves 3) Volkman's canal: perpendicular to the central canal which carries blood vessels and nerves |
|
|
|
Bone and skeleton growth |
-in embryos, skeleton is primarily hyaline cartilage -during development it is replaced by bones (cartilage remains in joints, bridge of the nose) -epiphyseal plates allow for growth of long bone during childhood -new cartilage is continuously formed and old cartilage is converted into bone -bknes are remodelled and lengthened until growth stops |
|
|
|
Types of bone cells |
1) Osteocytes: mature bone cells 2) Osteoblasts: bone forming cells 3) Osteoclasts: bone destroying cells (increased mitochondria in these cells pumps more protons releasing acid and dissolving the bone; under control of the PTH) -calcitoin controls action of osteoblasts and causes bone deposits -bone remodeling occurs by both osteoblasts and osteoclasts - bone remodeling continues throughout life -more osteoclasts activity as we grow older - |
|
|
|
Factors affecting bone development, growth and repair |
1) Vitamin A deficiency: retards bone development 2) Vitamin C deficiency: results in fragile bines 3) Vitamin D deficiency: rickets, osteomalacia 4) Growth hormone deficiency: dwarfism 5) Growth hormone excessive: gigantism and acromegaly 6) insufficient thyroid hormone: delays bone growth 7) insufficient sex hormones: pro MIT bone formation, stimulate ossification of epiphyseal plates 8) Physical stress: stimulates bone growth -glucorticoids: activate osteoclasts and resorption of bone |
|
|
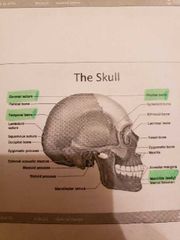
The axial skeleton and the skull |
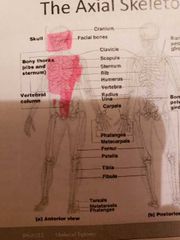
- axial skeleton firms the longitudinal part of the body -skull, vertebral column and bony thorax -the skull has 2 set of bones: cranium, and facial bones joined by sutures except mandible |
|
|
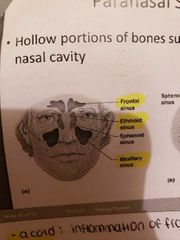
Paranasal sinuses |
-hollow portions of bones surrounding the nasal cavity -lighten the skill, give resonance and amplification to voice |
|
|
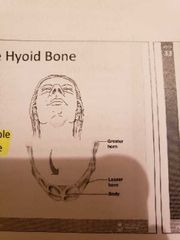
The Hyoid bone |
The only bone that does not articulate with another bone and serves as a moveable base for the tongue |
|
|
|
The fetal skull |
-large compared to the infants total body length -sutures are formed over the years -fontanelles are fibrous membranes connecting the bones which ultimately form sutures (within 24 months) |
|
|
|
The vertebral column |
-seperated by intervertebral discs (with cartilage) -normal curvature to the spine 1) Cervical curvature: C1 - C7 (smallest and lightest vertebrae) -bifid or forked from C2 to C6 -provides passage and protection for vertebral arteries 2) Thoracic curvature: T1 - T12 (12 pairs of ribs attached to them) 3) Lumbar curvature: L1 - L5 (thick, stout body, resist twisting movement: bad back complaint from this section) 4) Sacrum: 5 fuses vertebrae 5) Coccyx: 4 fuses vertabrae |
|
|
|
Abnormal spinal curvature |
1) Scoliosis: most common abnormality 2) hunchback: exaggerated curvature, usually in older females due to osteoporosis, spinal tuberculosis or osteomalacia 3) lordosis: sway back in pregnant women or obese women |
|
|
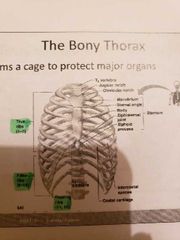
The bony thorax |
-to protect major organs -made up of the sternum, ribs and thoracic vertabrae |
|
|
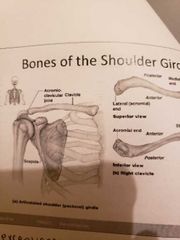
The pectoral shoulder girdle |
-composed of 2 bones: 1) clavicle: collarbone/ ball in socket 2) scapula: with shoulder blade -allow exceptional free movement |
|
|
|
The upper limb |
- divided into 4 regions with 30 bones per limb 1) Brachium (arm proper): extends from shoulder to elbow with 1 bone humerus 2) Antebrachium (forearm): extends from elbow to wrist (2 bones: radius and ulna) 3) Carpus (wrist): contains 8 small bones arranged in 2 rows 4) Manus (hand): 19 bones in 2 groups (5 meracarpals in palm, 14 phalanges in fingers) |
|
|
|
Bones of the pelvis girdle |
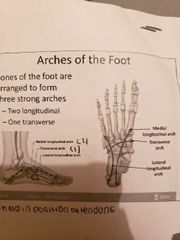
1) hip bones: ilium, ischium, pubic bone -female pelvis: broader hips, pubic arch length is greater, lighter bones, iliac bones more flared -the thigh has one bone called the femur -the leg has 2 bones: tibia and fibia -the foot: tarsus (ankle), metarsals (sole), phalanges (toes) -the bones of the foot are arranged to form 3 strong arches: 2 longitudinal and 1 transverse |
|
|
|
Functions of the respiratory system |
1) Gas exchange,: grab O2 and eject CO2 2) regulate blood pH: alters CO2 level with respiration 3) voice production: air movement past vocal cords 4) olfaction: in the nasal cavity (sense of smell) 5) innate immunity: physical protection of blood |
|
|
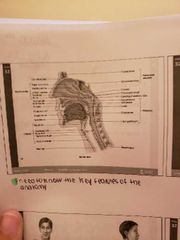
Anatomy of respiratory system |

1) Upper respiratory system:
1.1nose: cleans air, smell, humidifies air, paranasal sinus resonating chamber for speech
1.2nasal cavity 1.3pharynx: passageway for respiratory and digestive tract (nasopharynx is where auditory tubes empty: soft palate is on floor of nasopharynx (elevated when we swallow and held open when we sneeze)
1.3.1 oropharynx: extends from the uvula to epiglottis (oral cavity opens into oro pharynx where food passes through)
1.3.2 laryngopharynx: from epiglottis to oesophagus for food and drink
2) Lower respiratory system: 2.1 larynx: assortment of cartilages that are involved in voice production, allow us to hold our breath, close air tube to prevent food from entering trachea 2.2 trachea: the windpipe with connective tissue and smooth muscle supported by cartilage (smooth muscle constricts airway and cartilage maintains an open airway)
2.3 bronchi: symmetrical branches from trachea 2.4 lungs
-gaseous exchange takes place in the lungs -less and less cartilage in smaller areas |
|
|
|
Lung structure |
-divided into lobes (R=3 and L=2.to provide space for the heart) divided by septa -bronchi subdivided further into bronchi, then bronchioles then alveolar ducts and alveoli -cartilage amount decreases and smooth muscle increases -during exercise the alveolar duct diameter increases and during asthma attack it decreases |
|
|
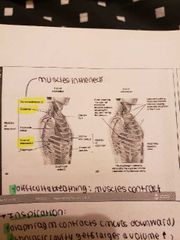
Respiratory membrane and ventilation |
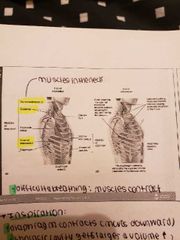
-gaseous exchange takes place in the alveoli and require a diffusion gradient -capillaries provide a large blood supply -air we breathe in is rich in oxygen 1) Respiratory membrane: extremely thin, fluid layer lines alveolus -alveolar epithelium squashed, thin interstitial space 2) Ventilation (inspiration and expiration) - caused by changes in thoracic volume which produce changes in air pressure and lungs -muscles of expiration: abdominals and internal intercostal -muscles of inspiration: diaphragm, external intercostal (scalene, pectoralis minor, sternocleidmastoid *accessory muscles only play a role when difficulty breathing* |
|
|
Respiratory membrane and ventilation |
-gaseous exchange takes place in the alveoli and require a diffusion gradient -capillaries provide a large blood supply -air we breathe in is rich in oxygen
1) Respiratory membrane: extremely thin, fluid layer lines alveolus -alveolar epithelium squashed, thin interstitial space
2) Ventilation (inspiration and expiration)
- caused by changes in thoracic volume which produce changes in air pressure and lungs -muscles of expiration: abdominals and internal intercostal -muscles of inspiration: diaphragm, external intercostal (scalene, pectoralis minor, sternocleidmastoid) (diaphragm contracts, thoracic cavity gets larger and increases in volume, internal muscles lift up the ribcage)
*accessory muscles only play a role when difficulty breathing* |
|
|
|
Pressure changes and airflow |
1) at the end of expiration: Patm=Palv with no air flow 2) during inspiration: increased thoracic volume decreases Palv (Patm > Palv) with air flow into the alveoli 3) end of inspiration: Patm = Palv with no air flow 4) during expiration: decreased thoracic volume = increased pressure inside of the alveoli -Patm < Palv and air flows out of the lungs 5) Compliance: the ability of lungs and thorax to expand (during expiration squash alveoli to hey air out of them) -premature children lack surfactant which decreases surface tension putting them at risk of respiratory distress syndrome (surfactant increases compliance making it easier for lungs yo inflate) |
|
|
|
Passive expiration |
-occurs due to lung recoil 1) caused by elastic fibres in connective tissue of lung 2) surface tension of wafer molecules on alveolar walls -surfactant opposed lung recoil (secreted by cells of alveolar epithelium) (lipoproteins interfere wirh surface tension produced by water) |
|
|

Pulmonary volumes and capacities |
1) Tidal volume: volume of air inspired or expired during normal inspiration or expiration
2) Inspiration reserve volume: amount of air inspired forcefully after inspiration of normal tidal volume
3) Expiration reserve volume: amount of air forcefully expired after expiration of normal tidal volume
4) Residual volume: volume of air remaining in respiratory passages and lungs after most forceful expiration
5) Inspiratory capacity: TV + IRV
6) Functional residual capacity: ERV + RV
7)Vital vapacity: IRV + TV +ERV (hoe much air can be removed from the lungs in one go) -influences by age, gender, body, size -trained athlete may have 40% more VC than usual -ultimate limit in athletes is efficiency with which oxygen reaches the tissues -assesses resistance to airflow
8) Total lung capacity: TV, RV, IRV, ERV (larger than vital capacity) |
|
|
|
Dead space |
-some inspired air never contributes to gas exchange -anatomical dead soace: volume of conducting zone conduits (150 mL) -alveolar dead space: alveoli that cease to act in gas exchange due to collapse or obstruction |
|
|
|
Pulmonary function tests |
1) spirometer: measures respiratory volumes and capacities (distinguishes between COPD (increased airway resistance such as bronchitis) (restrictive disorders tbat reduce total lung capacity due to structural changes (TB)) 2)minute ventilation: total amount of gas into or out if the respiratory tract in one minute 3) Forced vital capacity: gas forcibly expelled after taking a deep breath 4) forced expiratory volume: amount of gas exported during specific time intervals of the FVC (in one second is used clinically) *increases in TLC, FRC, and RV may occur as a result of obstructive disease *reduction in VC, TLC, FRC and RV result from restrictive disease 5) Alveolar ventilation rate: flow of gases into and out of the alveoli during a particular time (AVR= frequency (bpm) x (TV - deals space)) |
|
|
|
Dalton's Law of partial pressure |
-the air we breathe in is made of 4 main gases: N2, O2, H2O, and CO2. -the sum of the partial pressures of each individual gas is equal to the total pressure of air -more O2 in the inspired air than the alveolar air -more CO2 in the alveolar air than in the inspired air |
|
|
|
Partial pressure gradients during internal and external respiration |
1) Internal respiration: PCO2 in peripheral tissues is higher in arteries and PO2 is lower in tissues than in blood -CO2 from blood to tissues -O2 from tissues to blood 2) External respiration: -CO2 and O2 move from high to low -PO2 = lungs to blood -PCO2 = blood to lungs -diffusion occurs until equilibrium is met -blood PO2 and PCO2 equals the alveolus partial oteddure |
|
|
|
O2 transport |
-1.5 % dissolved in plasma -98.5 loosely bound to iron of hemoglobin in RBC -Hb is composed if 4 glovin chains surrounding a central haem group (2 aloha and 2 beta chains) -4 O2 per Hb -saturated hemoglobin is when all 4 harms of the molecule are bound to oxygen and partially is one of 3 are bounded (cooperativity of binding leads to saturation as binding of one encourages binding of another) -ths rate that Hb binds js regulated by oxygen pressure, CO2 pressure, temperature, blood pH and concentration of BPG |
|
|
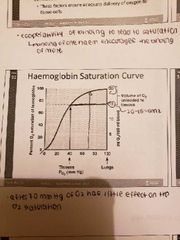
Haemoglobin saturation curve |

-98% saturated arterial blood has 20 mL of oxygen per 100 mL of blood (20 vol %) -as arterial blood flows through capillaries 5 mL of oxygen is released leaving 15 mL left -hemoglobin is almost completely saturated after 70 mmHg (further increase causes minimal binding) -only 20-25% of bound oxygen is unloaded during one systemic circulation -if oxygen levels in tissue drop then more oxygen dissociates from HB and is used by cells |
|
|
|
Carbon dioxide transport (transported in blood in 3 forms) |
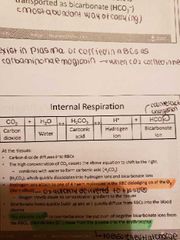
1) dissolved in plasma (7 to 10%) 2) chemically bound to Hn (20% carried in RBC as carbaminoharmoglobin) 3) bicarbonate ion in plasma: 70% transported this way 4) Internal respiration -carbon dioxide diffuses into the RBC -equation shifts to the right -Bohr effect: hydrogen ions formed attach to one of 4 haem molecules in RBC dislodging O2 to tissues -chloride shift: to balance rush of negative bicarbonate ions from RBCm chloride move from plasma to erythrocytes to ensure neutral charge 5) External respiration -when blood gets to lungs the process is reversed -bicarbonate ions move to RBC and bind with hydrogen to form carbonic acid split by carbonic anhydrase -CO2 levels rise in the cell and diffuses from blood into alveoli |
|
|
|
Factors that Ffect Hb saturation curve |
1) Temperature: increase in temperature increases O2 shifting curve to the right -H, PCO2 qnd BPG increase O2 unloading from hemoglobin 2) decrease in temperature: shifts curve to the left 3) greater need for O2 when cells are metbaolically active |
|
|
|
Control of respiration |
IX (excites the inspirstory muscle and causes eupnoea and becomes dormant during expiration) -DRG (dorsal respiratory group) is located near the root of nerve IX (excites the inspirstory muscle and causes eupnoea and becomes dormant during expiration)- Ventral respiratory group os involved in forced inspiration and expiration ) - Ventral respiratory group os involved in forced inspiration and expiration |
|
|
|
Pontine respiratory centers |
-influence and modify activity if ventral respiratory group -smooth out transition between inspiration and expiration |
|
|
|
Depth and rate of breathing & reflexes plus higher brain centers |
-depth is how actively the respiratory center stimulates the respiratory muscles -rate is how long the respiratory system is active 1) Pulmonary irritant reflexes: irritants promote constriction of air passages
2) inflation reflex: stretch receptors in the long are stimulated by lung inflation -upon inflation, inhibitory signals are sent to medullary inspiration centre to end inhalation and allow expiration 3) hypothalamic controls act through the limbic system go modify rate and depth of respiration (ex. Breath holding during anger) 4) rise in body temperature increases respiratory rate 5) cortical controls are direct signals from the cerebral motor cortex that bypass medullary controls (holding breath) |
|
|
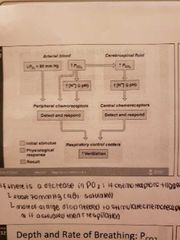
Central chemoreceptors |
-changing PCO2 levels are monitored by central chemoreceptors of the brain stem
-CO2 does not affect respiration but H ions do, the blood brain barrier is impermeable to H+ in medulla and thus stimulate the receptors -stimulaye DRG to increase depth and rate of breathing -hypercapnia: allows body to blow off more cow reestablishing homeostasis |
|
|

Peripheral chemoreceptors |
-located in the arch of the aorta, the carotid sinus -decrease in PO2 or increase in arterial PCO2 will stimulate Dorsal respiratory group to increase respiratory rate -central chemoreceptors are more effective |
|
|
|
Respiratory conditions |
1) Hypoventilation: when PCO2 levels are low and body slows its respiratory rate -holding breath or breathing slow increases CO2 in the blood which triggers chemoreceptors to signal DRG to increase ventilation which triggers chemoreceptors to signal DRG to increase ventilation 2) Hyoerventilation: increased depth and rate of breathing which quickly flushes CO2 from the blood due to hypercapnia (high CO2) 3) Respiratory acidosis: pH of CSF is the most powerful stimulus -pH greater than 7.35 caused by decline in pulmonary ventilation -not getting rid of CO2 when breathing *pH imblanaces caused by type 2 diabetes: fat oxidation causes ketoacidosis may be compensated for by deep rapid breathing as cannot use glucose 4) respiratory alkalosis: pH > 7.35 -caused by hypocaonia where pCO2 is less than 35 mmHg -hypoventilation pushes reaction to the right by increasing CO2 which lowers pH to notmL -lower breathing to allow CO2 buildup and increase the pH |
|
|
|
Depth and rate of breathing: PO2 |
-arterial oxygen is monitored by the aortic and carotid bodies -substntial drops in O2 to 6p mmHg sre mended for oxygen to become a stimulus for increased ventilation -if CO2 is not removed then the chemoreceptors become unresponsive to pCO2 stimuli (in chronic bronchitis and emphysema) -in these cases, PO2 becomes the principal respiratory stimulus (hypoxic drive) (do not supplement oxygen) |
|
|
|
Hypoxia |
-reduces novels of oxygen in blood (4 types) 1) Hypoaxemic hypoxia: high altitudes, drowning, aspiration, respiratory arrest, , CO poisoning, lung diseases 2) Ischaemic hypoxia: inadequate circulation due to diabetes type 2 or atherosclerosis 3) Anemic hypoxia: blood loss decreases RBC and O2 or diet 4) Histoxic hypoxia: metabolic poisoning due to cyanide or other chemicals -signs of cyanosis include bluntness of skin, lips, finger and nail clubbing -travel above 8000 feet induces symptoms of acute mountain sickness -acclimatization: respiratory and haematopoeitic adjustments to altitude (Chemorecetpors more responsive to PCO2 when PO2 declines) (breathe faster) |
|
|
|
Muscular system functions |
1) Body movement 2) Maintenance of posture (sitting and standing) 3) Respiration (diaphragm and intercostal contractions) 4) Communication (verbal and facial) 5) Constriction of organs and vessels 6) Heart beat 7) Production of body heat |
|
|
|
Properties of muscles |
1) Excitability: respond to stimulus 2) Contractility: ability of a muscle to shorten and generate pulling force 3) Extensibility: muscle can be stretched back to it's original length 4) Elasticity: ability of muscle to recoil to original resting length after stretched |
|
|
|
Types of muscle |
1) Skeletal: attached to bones and make up 40% of body weight, voluntary and control locomotion/facial expression/posture 2) Smooth 3) Cardiac: major source of movement of blood, controlled involuntarily by endocrine and ANS |
|
|
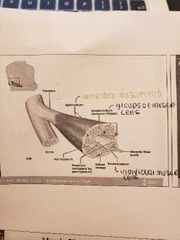
Connective tissue sheaths |
1) Epimysium (outermost layer): dense regular connective tissue surrounding entire muscle -seperates muscle from surrounding organs and tissues 2) Perimysium: collagen and elastic fibres surrounding a group of muscle fibres called a fascicle - contain blood vessels and nerves 3) Endomysium: loose connective tissue that surrounds individual muscle fibres -contain blood vessels, nerves, satellite cells *collagen fibres of all 3 layers come together at each end of muscle to form a tendon (muscle to bone) |
|
|
|
Nerve and blood vessel supply |
1) Motor neurons -stimulate muscle fibres to contract -neuron axons branch so that each muscle fiber (muscle cell) is innervated -form a neuromuscular junction (myoneural junction) 2) Capillary bed surrounds muscle fibres -muscles require large amounts of energy -extensive vascular network delivers necessary O2 and nutrients plus Carrie's away metabolic waste produced by muscle fibres |
|
|
|
Muscle fibre anatomy |
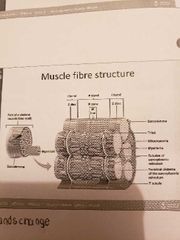
1) Sarcolemma (cell membrane) -surrounds the sarcoplasm (cytoplasm of fibre) -abundance of oxygen binding protein names myoglobin -openings called T-tubules (narrow tubes that extend into sarcoplasm at right angles to the surface: filled with extracellular fluid) 2) Myofibrils (cylindrical structures within muscle fibre) -bundles of protein filaments (myofilaments) -2 types of myofilaments: 2.1 Actin filaments: thin filaments 2.2 Myosin filaments: thick filaments -at each end of the fibre, myofibrils are anchored to the inner surface of the sarcolemma -when myofibril shortens then the muscle shortens (contracts) |
|
|
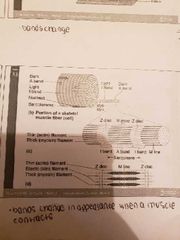
Skeletal muscle structure |
1) Sarcomere: the region between 2 Z-lines
2) I-band: the zone of thin filaments not superimposed by thick filaments (upon contraction the I band becomes smaller)
3) A-band: the entire length of a single thick filament
4) H-zone: the zone of thick filaments not superimposed by thin filaments (upon contraction the H zone becomes narrower)
5) M-line: the middle of the sarcomere |
|
|
|
Sarcoplasmic reticulum |
-elaborate smooth endoplasmic reticulum -runs longitudinally and surrounds each myofibril -forms chambers called terminal cisternae on either side if the T-tubules -one T tubule and the 2 terminal cisternae form a triad -SR stores Ca when muscle not contracting -when stimulated the calcium us released into the sarcoplasm causing contraction |
|
|
|
Actin filaments |
-2 strands if fibrous actin form a double helix extending the length of the myofilament attached at either end at sarcomere -composed of G actin monomers each of which had an active site -actin site can bind myosin during muscle contraction 1) Tropomyosin: an elongated protein which winds along the groove of the F actin double helical 2) Troponin: is composed of 3 subunits 2.1 Tn I site: binds to actin 2.2 Tn T site: binds to tropomyosin 2.3 Tn C site: binds to calcium ions -in smooth muscle, we have actin and.muosin but not troponin and tropomyosin -the troponin and tropomyosin complex regulate the interaction between active sites on G actin and myosin |
|
|

Thick myosin filaments |
-shaped like gold clubs -bind to active sites on actin molecules to form cross bridges -attach to the rod portion by a hinge region that can bend and straighten during contraction -ATp breakdown releases energy which is used to bend the hinge region of the myosin molecule during contraction |
|
|
|
Neuromuscular Junction |
-functional connection between nerve fibre and muscle cell -Acetylchokine released from nerve fibre stimulates muscle fibre -Na excited the postjunctional cells which trigger depolarization and voltage gated ca channels to open causing contraction |
|
|
|
Muscle contraction and relaxation |
1) Excitation: nerve action potentials lead to action potentials in muscle fibre 2) Excitation-contraction coupling: action potentials on sarcolemma activate myofilaments 3) Contraction: shortening of muscle fibre 4) Relaxation: return to resting length |
|
|
|
Sliding filament theory |
-explains relationship between thick and thin filaments as contraction proceeds -cyclic process begins with calcium release from the SR 1) calcium binds to troponin 2) troponin moves, moving tropomyosin and exposing actin active site 3) Myosin head forms cross bridge and bends toward H zone 4) ATO allows release of cross bridge |
|
|
|
Muscle contraction summary |
1) Nerve impulses reach NMJ 2) Acetylcholine is released from motor neuron 3) Ach binds with nicotinic receptors in the muscle membrane allowing Na ions to enter 4) Na influx generates an action potential into the sarcolemma 5) Action potential travels down T tubule 6) Sarcoplasmic reticulum releases calcium 7) Calcium binds with Troponin C to move the troponin/tropomyosin complex 8) binding sites in the actin filament are exposed 9) myosin head attach to binding sites and create a power stroke 10) ATP detached myosin heads and energizes them for another contraction 11) when action potentials cease the muscle stops contracting |
|
|
|
Functions of ATP in skeletal muscle contraction |
1) hydrolysis of ATP by myosin -energizes the cross bridges providing energy for force generation 2) binding of ATP to myosin - dissociates cross bridge bound to actin 3) Energizes the Ca pump to transport CA back into the SR -lowers cystolic calcium levels causing relaxation 4) Runs the Na/K pump in the sarcolemma -maintains the resting membrane potential of the sarcolemma -ATP provides immediate energy for muscle contraction produced from 3 sources 5.1 Creatine phosphate: most rapid method of generation, 1 ATP per CP used 5.2 Aerobic respiration: most efficient as generates 36 ATP per glucose molecule (requires oxygen break down to glucose) 5.3 Anaerobic respiration: occurs in abscence of oxygen and breaks down glucose to yield ATP and lactic acid |
|
|
|
Types of muscle fibres |
-skeletal muscle cells are specialized into 2 main types in humans and primates -specialization allows either (high work rates, long duration contractions -2 cell types ate differentiates kn whether gene for a slow or fast myosins isoemzyme is expressed on the cell 1) Slow twitch/high oxidative: -contract more slowly -moderate power output, consume ATP at moderate rates -rich blood supply due to high capillary density -more mirochonfira -smaller in diameter*long distance runners, aerobic* *long distance runners, aerobic* 2) Fast twitch/ low oxidative: -respond rapidly to nervous stimulation -maximal ATP consumption met only by glycolysis -contain myosin that breaks down ATP more radpidly -fewer and smaller mitochondria -less blood supply *short distance runners are anaerobic* |
|
|
|
Force of Muscle contraction |
-the larger the muscle the more muscle fibres -larger fibres can generate more force than smaller fibres -more muscle fibres generate more force -the more the motor units the greater the force -muscles contract strongest when the muscle fibres are 80 to 120% of their normal resting length |
|
|
|
Smooth muscle |
-longitudinal layer: muscle fitness run parallel to organ's long axis -circular layed: muscle fibres run around circumference of the organ and both layers participate in peristalsis -innervated by the ANS -final trigger git contraction is a rise in intracellular Ca -Ca is released from SR, interacts with calmodulin and MLCK enzyme to activate myosin -activated kinase transfers phosphates from ATP to myosin cross bridges which interact with actin to cause contraction -relaxation when intracellular Ca level drops |
|
|
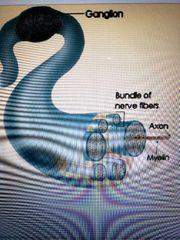
Neurons |
-a bunch of axons bundles together make up a nerve -some axons are covered with a myelin sheath made of of glial cells -the myelin sheath increases neuron efficiency and provided insulation -multiple sclerosis is the most common demyelenaging disease of the CNS |
|
|
The spinal cord and the brain |
1) Spinal cord -white matter: bundles of axons coated with a sheath of myelin -gray matter: masses of he cemm bodies and dendrites covered with synapses 2) Tract: collection if actions in the CNS 3) Nucleus: collection is neuron cell bodies in the CNS 4) Ganglion: collection of neuron cell bodies in the PNS however some in the brain such as basal ganglia |
|
|
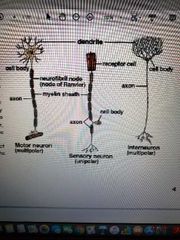
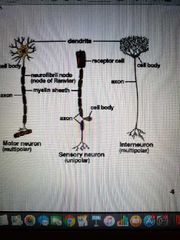
3 types of neurons |
1) Sensory neurons: carry signals from periphery into the CNS 2) Motor neurons: carry signals from the CNS to the outer parts (muscles, skin and glands) of the body 3) Interneurons: connect various neurons within the brain and spinal cord |
|
|
|
Affetent nerve fibres |
1) Afferent nerve fibres: axonal projections that arrive at a particular region 2) Efferent nerve fibres: exit the region *in the CNS: the afferent and efferent projections from the perspective if any brain *in the PNS: from the perspective if the spinal cord 3) Ascending: information travelling up the spinal cord such as sensory information 4) Descending: informaruint travelling down the spinal cord such as motor commands |
|
|
|
Depolarization and Hyperpolarization |
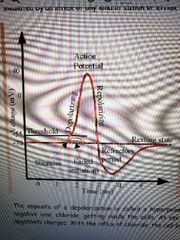
1) Depolarization
-at resting state a neuron is at -70 mV and if excited it will increase to-55 mV -when the threshold is reached an action potential is generated along the axon
2) Hyperpolarization -after reaching this maximum it must return to the resting state -must return below -70 mV to -80 mV before returning to -70 mV called the refractory period |
|
|
|
Sarcoplasmic Reticulum |
-elaborate smooth endoplasmic reticulum -runs longitudinally and surrounds each myofibril -forms chambers called terminal cisterns on either side of the T-tubules -a single T-tubule and 2 terminal cisternae form a triad -stores Ca when muscle not contracting -when stimulates then calcium is released into sarcoplasm -Ca out if the sarcoplasm back into the SR after contraction |
|
|

Actin filaments |
-2 strands of fibrous actin form a double helix then length if the myofilament -composed of G actin monomers with active site that bind to myosin during muscle contraction
1) Tropomyosin: elongated protein that winds along groove of the F actin double helix
2) Troponin: composed of 3 subunits 2.1 Tn I site: binds to actin 2.2 Tn T site: binds to tropomyosin 2.3 Tn C site: binds to calcium ions
- tropin/tropomyosin complex regulates interaction between myosin and G actin sites -elevated amounts show cardiac damage and muscle damage -in smooth muscle we have actin and myosin but not tropomyosin and troponin |
|
|
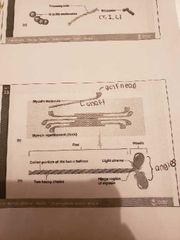
Thick myosin filaments |
-many elongated myosin molecules shaped like gold clubs -myosin heads: bind to active sites on the actin molecule to form cross bridges -attached to rod portion by hinge region that bends and straightens during contraction -energy released upon ATP breakdown bends hinge region of myosin during contraction |
|
|
|
Neuromuscular junction |

-functional connection between nerve fibre and muscle cell -Ach released from erve fibre stimulates muscle fibre -Na excites post-junctional cells and triggers depolarization which caused voltage gated Ca channels to open and cause contraction -extensive folding stimulated muscle fibres to contract (nicotinic Ach receptors) |
|
|
|
Muscle contraction and relaxation |
1) Excitation: nerve action potentials lead to action potentials in muscle fibres 2) Excitation-contraction coupling: action potentials on the sarcolemma activate myofilaments 3) Contraction: shortening of muscle fibre 4) Relaxation: return to resting length |
|
|

Sliding filament theory |
-relationship between thick and thin filaments as contraction proceeds -cyclic processes begin with calcium release from SR (impulse triggers excitation of muscle cells) 1) Calcium binds to troponin 2) Troponin moves, moving tropomyosin and expose actin active site 3) Myosin head forms a cross bridge and bends toward H zone 4) ATP allows release of cross bridge |
|
|
|
Heye |
Hh |
|
|
|
Cytoplasm of the axons |
-contains neurotubules which are the transport system along which packages containing essential materials are transported through the axoplasm |
|
|
|
Cytoplasm of the axons |
-contains neurotubules which are the transport system along which packages containing essential materials are transported through the axoplasm |
|
|
|
2 classes of proteins |
1) Kinesins: transport substances in an anterograde direction from cell body to axon terminal 2) Dyneins: responsible for retrograde transport from axon terminal to the cell body -act as molecular motors that carry organelles and other materials along neurotubukes |
|
|
|
Microtubules |
-guide the movement of vesicles down the axoplasm (cytoplasm within an axon) -stabilized by the presence of a protein called tau -in alzheimer's there are a large amount if molecules attached to tau and allow neurotubules to disintegrate -also, hyperphosphorylated tay joins other filaments to form a mesh that interferes with the transport of materials |
|
|
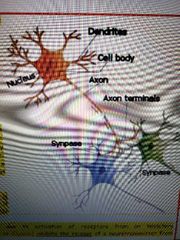
Neuron communication with other neurons |
-the axon if one neuron (presynaptic) transmits a signal to the next neuron by releasing a neurotransmitter which will attach to the receoties kn the dendrites or cell body of the next neuron (post synaptic) 1) Depolarization: -due to activation of receptors (ex. NMDA) from an EXCITATORY neurotransmitter (ex. glutmate) leads to the release of a neurotransmitter from that depolarized neuron 2) Hyperpolarization -due to activation if receptors from an inhibitory neurotransmitter such as GABA or glycine inhibits the release of a neurotransmitter from that neuron *the released neurotransmitter is specific to the depolarized neurin and can be excitatory or inhibitory |
|
|
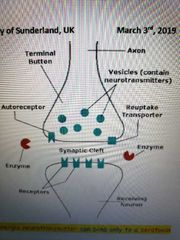
Neuron communication part 2 |
1) when activated and depolarized, the presynaptic neuron releases a neurotransmitter which attaches to its cognate receptor on the post synaptic neuron which will either depolarize (excite) or hyperpolarize (inhibit) the neuron 2) a neurotransmitter attaches to it's own receptors for a short time: broken down by enzymes and captured plus taken back or located in presynaptic vesicles 3) a neurotransmitter attaches to its ken receptor 4) a neuron can have various ore and post synaptic receptors |
|
|
|
Types of drugs |
1) Agonist: a drug that mimics a neurotransmitter action: binds to its cognate receptor activating it for a biological response 2) Antagonist: a drug that prevents a neurotransmitter from binding to it's own receptors - can also prevent an agonist drug to bind to it's own cognate receptor 3) Antagonist vs. Inhibitor -an inhibitor has affinity for it's own cognate receptor and produces a change to the receptor and elicits a response -GABA and diazepam 4) Partial agonist: an agonist ligand that produces a lower response than a full agonist after binding to the same njnved of receptors -can act as a competitive antagonist shein in presence of a full agonist 5) Inverse agonist: a ligand that produces an opposite action to that of an agonist |
|
|
|
Disinhibition |
-in the periaquedectal gray area kn the brain involved in pain, there are GABA neurons - these neurons control the descending neurons leaving the PAG and maintain them under tonic inhibition -GABA neurons receive inputs from opioid neurons; depolarization of opioid neurons would release enkephalins which will bind to opioid receptors on the GABA neuron causing hyperpolarization which remove inhibition that exerted in descending neurons leaving the PAG ; depolarization of opioid neurons would release enkephalins which will bind to opioid receptors on the GABA neuron causing hyperpolarization which remove inhibition that exerted in descending neurons leaving the PAG |
|
|
|
Blood brain barrier and cerebral blood |
1) the blood brain barrier: maintains the neural microenvironment by regulating the passive of molecules in and out of the brain (protecting the brain against toxins and microorganisms) 2) Cereberal blood volume: refers to the volume of blood present at a given moment within the neurocranium in order to perfuse the brain and meninges *the brain relies in blood flow and oxygen: must be restored as soon as possible after an accident. *without oxygen and nutrients, th brajn cells are damaged or die within a fee minutes |
|
|
|
The hindbrain |
-made up of the cerebellum, pons and medulla 1) Medulal: connected by the pons to the mid brain -contains cardiac, respiratory and vasomotor centers -reponsible for functions such as breathing, swallowing, urination/defecation, coordinating life saving reflexes, inciting vomiting, maintaining a steady heart rate and blood pressure |
|
|
|
The midbrain |
-the darkly pigmented Substantia Nigra contains a large number of dopamine producing neurons -the degeneration of pigmented neurons in SN is the principal pathology in Parkinson's disease |
|
|
|
The forebrain |
-split into 2 sections 1) Telencephalon: cereveral hemispheres of cerebrum and connections 2) Diencephalon: thalamus, and the hypothalamus -the cerebral cortex is the gray matter of the brain -most information processing occurs in the cerebral cortex -the telencephalon has the corpus callosum which connect the left and right sides of the cereberal cortex -the white matter in the brain, an enormous bundle of axons, connect the left and right cereberal hemisphere (comissure) |
|
|
|
The hippocampus (Dienceohalon of the forebrain) |
-the hippocampus is one of the first regions affected by Alzheimer's disease: memory loss and disorientation are among the early symptoms -responsible for short and long term memory |
|
|
|
The amygdala (telencephalon of the midbrain) |
-responsible for emotional processing, associated with conditioned learning especially fear/anger/rage |
|
|
|
The thalamus (in the diencephalon of the forebreain) |
-acts as a relay station for incoming sensory nerve impulses -axons from every sensory system except olfaction synapse here as the last relay site before information reaches the cereberal cortex -main sensory information relay hub if the brain |
|
|
|
The hypothalamus (diencephalon of the forebrain) |
-involved in the regulation of homeostatic control -maintains homeostasis by exerting control on the pituitary gland -regulates various sensations such as hunger, thirst, temperature, libido -4 F's: fight, flight, feed, **** |
|
|
|
The pituitary gland |
-the hypothalamus CONTROLS motivated behavior by regulating the release of hormones from the OG -small lea sized gland below the hypothalamus -in response to hormonal messages from the hypothalamus, the pituitary gland releases 1) Growth hormone 2) thyroid stimulating hormone 3) follicle stimulating hormone 4) leuteinizing jormone 5) prolactin 6) adrenocorticotropic hormone -in response to electrical messages from the hypothalamus, the pituitary gland releases ADH and oxytocin |
|
|
|
The pineal gland |
-small endocrine gland in the vertebrate -located in the diencephalon -releases melatonin a hormone which modulates sleeping patterns, seasonal function and sexual development |
|
|
|
Noradrenaline |
-produced in the noradrenergic neurons -process starts with the amino acid precursor of NA called tyrosine 1) Tyrosine js transported into the nervous system from the blood 2) once tyrosine is inside the neuron, it undergoes transformation by the action of 3 enzymes in sequence 2.1 Tyrosine hydroxylase: converts tyrosine into DOPA 2.2 DOPA decarboxylase: converts DOPA into dopamine 2.3 Dopamine beta hydroxylase: converts dopamine into noradrenaline -NA kind is taken back up and stored in vesicles or metabolized by monoamine oxidase -the form of uptake of NA is blocked by amphetamines, cocaine and tricycling antidepressants -NA that diffuses away from the receptors is subject to extra neuronal uptake into the surrounding tissues and metabolized by COMT |
|
|
|
Noradrenergic neurons |
-regulated by several receptors: mainly a1, a2, b1, b2, b3 -g-protein coupled receptors 1) a1 is coupled to Gq which increases intracellular calcium and causes smooth muscle contraction 2) a2 is coupled to Gi which decreases cAMP activity causing smooth muscle contraction 3) B is couples to Gs whuch increases intracellular cAMP activity causing heart muscle contraction, smooth muscle relaxation, and glycogenolysis 4)autoreceptor: receptor located on the presynaptic cell membrane and serves as a part of a feedback loop in signal transduction -turn off further release of NA and act like a brake for NA neurons -provide negative feedback signal tk noradrenergic neurons -majority of the NA containing neurons in the CNS are located in the locus coerulus |
|
|
|
Noradrenergic functions |
1) Noradrenaline: plays an important role in arousal, reward system, and pain 2) Norepineohrine: increases arousal, alertness, promoted vigilance, enhanced formation and retrieval of memory, focuses attention and increased anxiety -norepineohrine increases heart rate, blood pressure, triggers release of glucose from energy stores, increased blood flow to skeletal muscle, reduces blood flow to the GI tract and inhibits emptying of the bladder |
|
|
|
SNRI |
-inhibit reuptake and prevent serotonin and norepinephrine transporters from taking their respective transmitters back to their storage vesicles for later use -tricyclic antidepressants: increase norepinephrine activity -ADHD: reduces NA neurotransmission is associated with decreased alertness, low energy and attention problems plus cognitive disabilities -NA cannot cross the blood brain barrier |
|
|
|
Dopamine |
-dopamine starts with the precursor tyrosine -once tyrosine js inside the neuron it undergoes transformation by the action if 2 enzymes in sequence 1) Tyrosine hydroxylase: converts amino acid tyrosine into DOPA 2) DOPA decarvoxylase: converts DOPA into dopamine -the 3rd enzyme called dopamine beta hydroxylase which converts DA to NA is absent in dopaminergic neurons -DA is largely recaptured following its release by its own presynaptic transporter (re-uptake pump) -DA is metabolized by MAO and COMPT same enzymes as NA -DA receptors are a class of G protein coupled receptors: comprise of 2 family groups 3) D1 includes D1 and D5 (stimulation) -coupled to Gs and meditate excitatory neurotransmission 4) D2 has D2-D4 (inhibition)) -inhibuts adenylyl cyclase activity: coupled to Gi/Go and meditate inhibitory neurotransmission *loss of dopamine neurons in substantia nigra is main pathological features of Parkinson's disease leading to a marked reduction in DA function in this pathway *the mesolimbic system is also known as the reward pathway *the release of DA from the mesolimbic pathway into nucleus accumbens regulates incentive salience (motivation and desire for rewarding stimuli) *the dysregulatuin of mesolimbic pathway and its output neurons in nucleus accumbens play a significant role in the development and maintenance of addiction *a normally high DA transmission has been linked to psychosis and schizophrenia *DA meditates pleasure in the brain, produces nausea and vomiting by stimulating DA receptors in the CTZ in the medulla oblongat *Parkinson's disease: in the basal ganglia, DA neurons exert an inhibitory effect on cholinergic neurons; in nigrodtruatal dopaminergic neurons are degenerated while cholinergic neurons are intact resulting in a marked decrease in dopamine content (deficiency syndrome) and a relative predominance of cholinergic activity ; in nigrodtruatal dopaminergic neurons are degenerated while cholinergic neurons are intact resulting in a marked decrease in dopamine content (deficiency syndrome) and a relative predominance of cholinergic activity |
|
|
|
5-HT-Serotonin |

-the precursor is tryptophan 1) 5-HT is formed from dietary tryptophan which is converted to 5-hydroxytryptophan (in chromaffin cells, and neurons but not platelets) by tryptophan hydroxylase 2) 5-hydroxytryptophan can cross the blood brain barrier to increase central levels of 5-HT 3) after release 5-HT is recovered by neuronal uptake -tricyclic antidepressants block reuptake -SSRIs constitute an important group of anti depressants and anxiolytics 4) degradation by MAO -5-HT occurs in several large nucleinsuch as the pons and upper medulla often called the rapha nuclei (main source of serotonin for the brain) - with the exception of 5HY3 receptor which is ligand gated all other 5HT are G protein coupled 5) Numerous drugs target 5HT: such as antipsychotics, antidepressants, anxiolytics, anti emetics, and antimigrane drugs and psychedelic -SSRI: most commonly prescribed antidepressants in the treatment of major depressive disorder and anxiety disorder |
|
|
|
Suoerchiasmatic nuclei (SCN) of hypothalamus |
- contains neurons that exhibit a circadian pattern of activity and regulate melatonin secretion by the pineal gland in response light and dark cycle |
|
|
|
Acetylcholine |
-formed from 2 precursors 1) choline: derived from dietary and intraneuronal sources 2) acetylcoenzyme: which is made from glucose in the mitochondria of neurons 3) Ach is synthesized from choline and acetyl-coA by the enzyme choline acetyl transferable (ChAT) to form ACh 4) once released Ach must be removed rapidly in order to allow depolarization to take place 5) the hydrolysis of Ach is carried out by the enzyme acetylcholinesterase -the septum provides cholinergic fibres to the septal hippocampus tract -the primary cholinergic input to the cereberal cortex comes from the basal nucleus of Meynert |
|
|
|
The nucleus basal is |
- the nucleus basal is of Meynert is a source of Ach -projections provide primary source of Ach -cholinergic projections from the adjacent medical septum and diagonal band of broca to the hippocampus -cholinergic projection make jo a wide source of Ach in the brain |
|
|
|
Cholinergic receptors |
1) muscarinic receptors: g protein receptors -found at the neuromuscular junctions of cardiac and smooth muscle as well as on the glands and on the post ganglion neurons of the sympathetic nervous system
1.1 M2 and M4 couple with Gi/Go proteins and inhibit adenylyl cyclase reducing cAMP 1.3 M1, M3, M5 couple with Gq/G11 and increase Ca levels cis activation of phospholipase C 2) nicotinic: ligand gated channels -found at NMJ of skeletal muscles only on the post ganglionic neurons of the parasympathetic nervous system and many neurons in the brain -ligand gated Jon channels which meditate a rapid and depolarizing respons to acetylcholine |
|
|
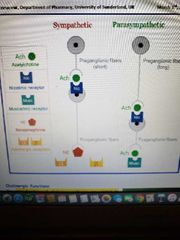
Acetylcholine in the ANS |
-Ach binds nicotinic receptors fi autonomic ganglia to trigger the release of norepinephrine if a sympathetic synapse is stimulated OR -Ach binds to tissue muscarinic feceotirs which will produce a parasympathetic or cholinergic response |
|
|
|
Cholinergic function |
-main functions if cholinergic pathways are related to arousal, learning, memory, and motor control -a large reduction in cholinergic activity in the brain of Alzheimer's patients: less Acg less CAT -reduced cholinergic activity results from degeneration of neurons in the basal forebrain (nucleus basalis of Meynert, diagonal band of broca, medial septal nucleus) |
|
|
|
Acetylcholinesterase |
-involved in the impulse transmissionbu rapid hydrolysis of neurotransmitter Ach in numerous cholinergic pathways -AchE inhibitors or anti-cholinesterar inhibit the cholinesterase enzyme from breaking down AcH increasing both the level and duration of neurotransmitter action |
|
|
|
Glutamate |
-most abundant amino acid in the CNS -functions such as an intermediate in neuronal metabolism ex. as a precursor for GABA -acts as a major excitatory neurotransmitter in the brain 1) Glu is synthesized in the nerve from 2 sources: 1.1 glucose: via the Krebs cycle 1.2 glutamine: by the enzyme glutaminase present in the mitochondria which is synthesized by Gila cells and taken up by neurons *glutamine can be obtained from global cells adjacent to neurons 2) Glu is removed from the synapse by high affinity reuptake into the nerve terminals and neighbouring glial cells 3) action of glutmate is not stopped by enzymatic breakdown as in other neurotransmitter systems but stopped by removal involving 2 transport pumps: first pump is located in the presynaptic neurons 3.2 second pumps are located in nearby glia cells 4) Glutamate is taken up by glial cells is converted to 3.1 first pump is located in the presynaptic neurons 3.2 second pumps are located in nearby glia cells4) Glutamate is taken up by glial cells is converted to glutamine which is inactive in the sense that it cannot activate glutamate receptors- released from global cells to extracellular fluid and nerve terminals take up glutamine and convert it back to glutamate first pump is located in the presynaptic neurons 3.2 second pumps are located in nearby glia cells4) Glutamate is taken up by glial cells is converted to glutamine which is inactive in the sense that it cannot activate glutamate receptors- released from global cells to extracellular fluid and nerve terminals take up glutamine and convert it back to glutamate glutamine which is inactive in the sense that it cannot activate glutamate receptors - released from global cells to extracellular fluid and nerve terminals take up glutamine and convert it back to glutamate |
|
|
|
Glutamate receptors |
-acts at different types of ionotropic receptors and at a family of gprotein coupled receptors 1) Ionotropic receptors 1.1 NMDA: receptors bind glutamate and N-methyl-D-aspartate 1.2 AMPA receptors: bind glutamate and AMPA 1.3 Kainate receptors: bind glutamate and kainic acid 2) mGluRs bind to glutamate an amino acid that functions as an excitatory neurotransmitter -members of group C family of GPCRs |
|
|
|
GABA |
1)most abundant inhibitory neurotransmitter in the CNS 2)does not penetrate the blood brain barrier and thus synthesized in situ 3)synthesized from glutamate using GAF and pyridoxal phosphate (active vitamin B6) as a co-factor 4) this process converts glutamate the principal excitatory neurotransmitter into the principal inhibitory neurotransmitter GABA 5) after release GABA is rapidly taken up by surrounding astrocytes and neurons and degraded by GABA transaminase 6)GABAnergic transmission is terminated by NaCl dependent uptake through GABA transporters -removed from synapse by GABS transporters located in neurons and glia -these transporters are highs affinity transporters allowing for collected GABA to be concentrated into synaptic vesicles for release |
|
|
|
GABA functions |
-important in maintaining a normal level of firing -treatment of anxiety disorders, insomnia and agitation -benzodiazepines or barbiturates are helpful in the treatment and prevention of seizures -bezodiazepines and ethanol use the same mechanism to influence GABA receptors |
|
|

Chromosomal distribution |
-human somatic cells have 46 chromosomes (22 pairs somatic and 1 pair of sex chromosomes) -male chromosomes decide the gender of the offspring (females are XX and males are XY) |
|
|
|
Male reproductive system |
1) Organs: 1.1 testes -produce the sperm and make sex hormone testosterone -paired oval glands which synthesize testosterone-development starts in the 7th minah of the scrotum (on embryo's posterior wall)-produce sperm and secrete steroid hormone (testosterone)-Testis divided into 250-300 lobules each consisting if 2-3 coil seminiferous tubules (transport plus synthesize sperm) which join and last into straight tubule tecti and coiled into epididymis -5 cm long-outer smooth muscle contracts and knives sperm plus fluid through the tubule-inner epithelial cell layer (Sertoli cells) control formation and development of sperm-lumen of tubule filled with fluid and a medium for development (between tubule is a connective tissue which supports tubules and contains clusters of Leydig cells: interstitial cells of Leydig which synthesize and secrete testosterone)-Sertoli cells: form blood-testis barrier, prevent immune response against sperm cell surface antigens, support development of sperm and release into the lumeb (secrete hormone called inhibit which regulates the effects of testosterone and FSH) 1.2 ducts: epididymis, ductus deferens, ejaculatory ducts and urethra -transport, store and assist in maturation if sperm 1.3 glands: secrete mist of the liquid portion of the semen and protective mechanisms 1.3.1 seminal vesicles: secrete 60% of clear, alkaline seminal fluid with fructose sugar and ATP and prostaglandins for sperm function and nutrition 1.3.2 prostate: secretes 30% of milky, slightly acidic seminal fluid with an antibiotic to kill bacteria 1.3.3 bulourethral glands: secrete clear and alkaline mucus to buffer and lubricate urethra 1.4 supporting structures: scrotum and penis -penis contains the urethra a passageway for ejaculation of semen and urine penis-penis contains the urethra a passageway for ejaculation of semen and urine 1.4.1 Scrotum: -sac of loose skin, fascia and smooth muscle divided into 2 pouches by a septum (which support the testes) - further division by raphe into 2 testes -dartis and scrotum maintain temperature (Sperm requires a temperature 2-3 degrees lower than the body temperature) 1.4.1 Scrotum:-sac of loose skin, fascia and smooth muscle divided into 2 pouches by a septum (which support the testes)- further division by raphe into 2 testes -dartis and scrotum maintain temperature (Sperm requires a temperature 2-3 degrees lower than the body temperature)1.4.2 Penis-contains urethra which is a passage for semen and urine-3 columns of erectile tissue (2 dorsal columns of corpora cavernosa) (1 ventral column of corpus spongiosum: faster movement of sperm and aloe flexibility)-foreskin of penis removal is circumcision -contains urethra which is a passage for semen and urine 1.4.2 Penis-contains urethra which is a passage for semen and urine-3 columns of erectile tissue (2 dorsal columns of corpora cavernosa) (1 ventral column of corpus spongiosum: faster movement of sperm and aloe flexibility)-foreskin of penis removal is circumcision -3 columns of erectile tissue (2 dorsal columns of corpora cavernosa) (1 ventral column of corpus spongiosum: faster movement of sperm and aloe flexibility) -foreskin of penis removal is circumcision *the urethra comes out of the penis and helps the sperm and urine travel *the urethra comes out of the penis and helps the sperm and urine travel*glands are important for the survival of the sperm *glands are important for the survival of the sperm *the support structures such as the Lewis help keep the urethra in place and allow passing of sperm |
|
|
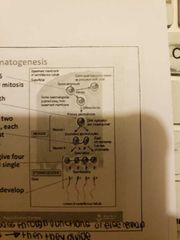
Spermatogenesis |
-spermatogium with 2n=46 chromosomes divide by mitosis -primary spermatocyte with 2n=46 -first meiosis division give 2 secondary spermstocytes with 23 chromosomes each -second meiosis gives 4 spermatids with 23 single stranded chromosomes each -each of 4 spermatids develop into sperm -some sperm cells remain as precursors and the active ones differentiate by mitosis |
|
|
|
Sperm structure |
-300 million per day in a lifetime -last 48 hours in the female tract -the head contains DNA and the acrosome with enzymes for penetrating the egg -middle contains mitochondria to form ATP for energy -tail is flagellum used for locomotion -sperm is 60 mm king |
|
|

Male sex hormones (androgens) |
-testosterone if the primary Male hormone in am androgen -leydig cells and adrenal glands (less often) produce testosterone and other androgens -sex hormone production is relatively constant with some diurnal variation -after birth the leydig cells become dormant until activated by gonodotropins during puberty -at puberty androgens cause sex organs to grow and sex characteristics to develoo |
|
|
|
Effects of testosterone in males |
-required for initiation and maintenance if spermatogenesis -decrease GnRH secretion via action ig hypothalamus -inhibit LH secretion via a direct action in the anterior pituitary (short loop) -male sex organ development and function -opposes breast growth and effects of estrogen -stimulates bone growth and protein anabolism -requiresmd for sex drive and may enhance aggression -stimulates erythropoietin by kidneys |
|
|
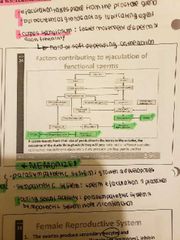
Semen |
-mix of sperm and seminal fluid -60% from seminal vesicles and 30% from prostate -slightly alkaline, milky and sticky -contains nutrients, clotting proteins and an antibiotic to protect sperm -typical ejaculate is 2.5 mL to 5 mL in volume -normal sperm count is 50 to 150 million/ mL -if less than 20 million per mL then one is sterile |
|
|
|
Female reproductive system |
1) Essential organs 1.1 Ovaries (gonads): produce secondary oocytes, progesterone, estrogen, inhibit and raxin -almond shaped paired glands weighing 3 g each -attached in pelvic cavity on each side of uterus (not directly attached to uterus) 1.1.1 Gerninal epithelium: covers surface of ovary 1.1.2 tunica albuginea: irregular connective tissue 1.1.3 connective tissue containing follicles surrounded by connective tissue that contain. fibres and fibroblasts (follicle/ oocyte plus surrounding cell) (surrounding cells nourish oocyte and secrete estrogen as follicles grow) -mature follicle: rupture and excel secondary oocyte (ovulation) -post ovulation: corpus luteum contains remains of a mature follicle which produces hormones such as relaxin, inhibin and progesterone until it regenerates into a scar known as albicans 2) Ducts or modified ducts 2.1 Uterine/Fallopian tubes: transport secondary oocytes to uterus and site of fertilization -ampulla is central region of tube -isthmus is narrowest portion that joins uterus ) -finbriae sweep secondary oocyte into tube (hand shaped)-oocyte moved by cilia lining wall-zygote reaches uterus in 7 days -oocyte moved by cilia lining wall -zygote reaches uterus in 7 days 2.2 Uterus: site of implantation of fertilized ovum and development of fetus and labor -maintenance and expulsion of fetus -site of menstruation -3 inches long by 2 inches wide and 1 inch thick -subdivided into fundus (dome shaped area above tubes), body and cervix (narrow opening into vagina) -cervix is location from where sperm enters (vagina to cervix) 2.3 Vagina: receives the penis during intercourse and passageway for child birth -10 cm fibrous muscle canal -pathway for sperm and passageway for delivery of baby from uterus -outlet for menstrual flow -fornix: recess surrounds cervix -acid environment prevents bacterial growth -extends from exterior to cervix-10 cm fibrous muscle canal-pathway for sperm and passageway for delivery of baby from uterus-outlet for menstrual flow-fornix: recess surrounds cervix -acid environment prevents bacterial growth -smooth muscular layer adjusts for intercourse or birth -selective for sperm only: hymen breaks after first intercourse or menstrual cycle -uterus contracts and cervix opens allowing birth (oxytocin) -smooth muscular layer adjusts for intercourse or birth -selective for sperm only: hymen breaks after first intercourse or menstrual cycle -uterus contracts and cervix opens allowing birth (oxytocin) 3) Sex glands 3.1 Breasts: synthesize, secrete and reject milk for nourishment of newborn & change size during pregnancy -hemispheric structures of variable size -milk secreting glands become active after child birth -internally: 15-20 lobules, into lobes, then alveoli -mature at puberty (lactation) -estrogen develops duct systems in breast (enlarge during pregnancy) -synthesize, secrete and eject milk (lactation)-estrogen develops duct systems in breast (enlarge during pregnancy)-progesterone: develop milk secreting glands called alveoli-prolactin: stimulate milk synthesis in the alveoli-oxytocin: stimulate milk ejection from he alveoli -progesterone: develop milk secreting glands called alveoli -prolactin: stimulate milk synthesis in the alveoli -oxytocin: stimulate milk ejection from he alveoli 4) External organs 4.1 vulva -female external genitalia -labia minor and majora: folds that surround vaginal and urethral openings -clitoris: small erotic structure located at anterior ends of folds of labia minora |
|
|
|
Oogenesis |
-formation of gametes in the ovaries -yolk sac is seen in the embryonic stage (yolk sac migrate to ovary and become potential egg cells in the oogonia) -200,000 to 2 million at birth, 40,000 at puberty, 400 mature -each month 2p oocytes become secondary oocytes but usually only one survived to be ovulated from graffian follicle 1) oocytes (egg forming cells) undergo 2 divisions 2) starts with 2n=46 primary oocyte tbat divides resulting in 2 n=23 cells but one is a large secondary oocyte Nd one is a small 1st polar body that may divide but uses 3) secondary division only occurs if secondary oocyte is fertilized a d results in one large n=23 ovum and one small n=23 2nd polar body 4) oogenesis results in one large fertilized egg and 3 small polar bodies |
|
|

Female sex hormones |
-estrogen and progesterone are produced by the ovaries -during infancy and childhood the sex hormone production is low -at puberty sexual maturation and sec characteristics develop -from puberty to menopause, sex hormones control the menstrual cycle and produces cyclically -hormones are higher during pregnancy, inhibit ovulation |
|
|
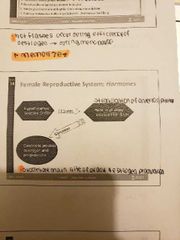
Effects of progesterone |

1) converts estrogen 2) induces thick, sticky cervical mucus 3) decreases contraction of fallopian tubes and myometrium 4) decreases proliferation of vaginal epithelial cells 5) stimulate breast growth, glandular tissues 6) inhibit milk inducing effects of prolactin 7) feedback effects on hypothalamus and anterior pituitary 8) increases body temperature |
|
|
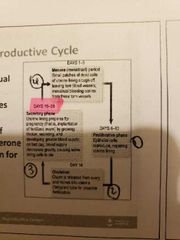
Female reproductive cycle |

-uterine cycle (menstrual cycle) -controllrd by hotmones from ovary -estrogens: growth of endometrium progesterone supports endometrium implantation - |
|
|
|
Interaugmentary system |
1) Skin 2) Hair 3) Nail 4) Glands -protects, abrasion, infection, dehydration and UV light, vitamin d production, sensory reception, raised eyebrows for communication, thermal regulation (insulation) |
|
|

The skin and hypodermis |
1) Epidermis -prevents entrance of microbial and provides a barrier function -made if keratinised, squashed epithelium -gives strength to the skin (varies in thickness) -nails are made up of epidermis -no blood supply so absorbs oxygen from blood vessels in dermis 1.1 Stratum Corneum -Stratum granulosum layer becomes stratum corneum -dead cells fill up keratin -cells lose buckets and fuse into sheets (shed in 2 weeks: desquamation) -thin vs. thick skin has different sizes of this layer -15 to 30 days for skin tk move to this layer and then 2 weeks to shed 1.2 Stratum lucidum (only in thick skin: palm, hands and feet) -only on the palms and soles -deep to the stratum corneum and superficial to the Stratum granulosum -thin layer provides protection from UV radiation 1.3 Stratum granulosum -as more cells made in basale, this layer is pushed up to become granulosum kaye -cells in this layer begin to die due to lack of nutrient source in dermis -the cells have s grainy appearance 1.4 Stratum spinosum -attached to each other by desmosomes (pointed) -cells stem alive but no longer divide in this layer -langerhans cells are white blood cells that function in the immune system -strength to epidermis 1.5 Stratum basale (deepest layer of epidermis) -4 types of cells: only keratonicyte makes the epidermis (protein, waterproof and strong) -merkel cells: sensory receptors for light and touch -macrophages: ingest debris -melanocytes: produce melanin (dark brown pigment) packaged in vesicles called melanosomes -everyone has the same number but different numbers are expressed *only layer of epidermis where cells are dividing (old cells get pushed up) *layers divided by mitosis, pushed up and replaced *merkel cells are sensory, cells ultimately become squashed 2) Dermis -sweat production and hair follicles 3) Hypodermis -hypodermic needle means a long hollow needle that reaches from epidermis to dermis -a hypodermic/subcutaneous injection goes into the dermis |
|
|
|
Skin injury and repair |
-4 stages of healing 1) Inflammation: increased blood flow and phagocytes attracted to site of injury -bleeding at site of injury attract mast cells and trigger an inflammatory response 2) Scab formation -after several hours, cells of stratum basale migrate to edges of wound -phagocytic cells remove debris and enhance circulation 3) Cell division and migration -phagocytic activity has ended and fibrin clot has disintegrated -one week after injury, scan has been undermined by elidermial cells migrating over mesheork produced by fibroblasts 4) Scar formation -after several weeks scab is shed and epidermis is complete -shallow depression marks injury site |
|
|

Burns |
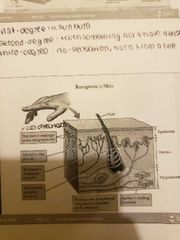
1) First degree: a sun burn 2) second degree burn: touched something warm and have a blister 3) Third degree burn: no sensation and burn from a fire |
|
|

Mechanoreceptors |
-2 types located close to the surface of the skin 1) Merkel receptor -fires continuously when stimulus is present -slowly adapting fibre and sense fine details 2) Meissner corpuscule -fire only when stimulus is first applied and when removed -rapidly adapting fibre -responsible for controlling hand grip 3) Ruffini cylinder -fires continuously to stimulation -slowly adapting fibre -aasociated with perceiving stretching of skin 4) Pacinjan corpuscule -fires only when stimulus is first applied and removed -rapidly adapting fibre -associated with sensing rapid vibrations and fine texture |
|
|
|
Free nerve endings |
-sense pain, itch, movement and temperature change -temperatures between 12 and 35 degrees activate cold receptors in epidermis -temperatures between 25 and 47 degrees activate warm receptors in dermis -below 10 and above 47 degrees stimulate nocioreceptors for pain -25 - 35 degrees both temperature receptors are stimulated |
|
|
|
Pain perception and types of pain |
1) Nocioceptive pain -single impending damage to the skin -mire launched receptors in centre of the body 2) Inflammatory pain -caused by damage to tissues and joints by tumour cells 3) Neuropathic pain -caused by damage to CNS (ex. Stroke, multiple sclerosis, diabetic neuropathy) |
|
|
Dermis |
1) Papillary layer: superficial layer (pimples) -loose connective tissue -ridges to increase SA for contact with epidermis to obtain nutrients - forms fingerprints - surgeons make incisions based in likes of cleavage formed by laljary lager 2) Reticular layer -dense irregular connective tissue -gives the dermis its strength -strongest layer of the dermis (epidermis strongest layer of skin and spinosum strongest of epidermis) -collagen and elastin - stretch marks are tears in collagen from dermis *transdermal patch diffuses from epidermis to dermis to reach blood vessels there (must be lipid soluble) 3) Meissner's corpuscle: nerve receptors for light and touch 4) Pacinian corpuscle: nerve receptors for vibration and pressure |
|
|
|
Hypodermis (subcutaneous layer) |
-loose connective tissue (adipose and areoli) -stabilized skin position (loosely attached to dermis and muscle) -contains many fat cells for thermal insulation and cushion |
|
|

Hair |
-filamentous strands of dead keratinuzed cells produced by hair follicles -contains hard keratin 1) root: imbedded in skin 2) shaft: projects above skin's surface (3 layers) 2.1 Medulla 2.2 Cortex 2.3 Cuticle -pigmented by melanocytes at the base of the hair 3) Functions of hair -maintain warmth -alert body to presence of insects on skin -guard scalp against physical trauma, heat loss and sunlight |
|
|
|
Hair follicle |
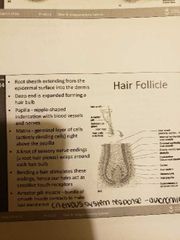
|
|
|
|
Sweat glands |
-2 to 3 million 1) Merocrine -distributed all over skin except nipples -secrete watery sweat for cooling -ducts lead to swear pore on surface 2) Apocrine -located in pubic and anal regions -larger than merocrine glands -duct opens into opening of hair follicle -secretes thicker sweat, high content of proteins and fats -scent molecules for sex and fear 3) Sweat -99% water with a pH of 4-6 -sweat glands produce 500 mL of insensible perspiration daily -diaphoresis: 1 L per hour when exercising or in hear (wet sweat) -2 sweat glands 1) Ceruminous: external ear canal; combine with sebum, and dead epidermal cells to form ear wax with bactericidal effect 2) Mammary: milk producing glands are modified apocrine glands |
|
|
|
Nails |
-scale like modification of epidermis of hard keratin -root, body, nail folds, cuticle and body (kerstinized cells) |
|
|
|
Oil glands (sebaceous glands) |
-over the entire body except palms and soles -simple alveolar glands (Secretes oily substance called sebum associated with a hair follicle) -functions of sebum: soften and lubricate hair plus skin, waterproofing, dirt collection |
|

