![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
is a single-stranded RNA virus of the Paramyxoviridae family whose genome includes 10 genes that encode 11 proteins |
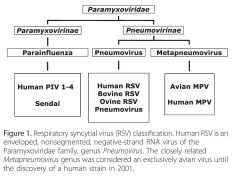
Humanrespiratory syncytial virus (RSV) |
|
|
|
Etiology of acute respiratory infections in children |
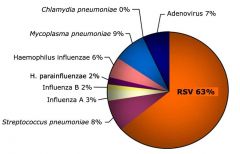
|
|
|
|
Transmission of RSV infection occurs through: |
- inoculation of the nasopharyngeal or conjunctival mucosa with respiratory secretions from infected individuals. - The virus remains viable on hard surfaces for up to 6 hours, on rubber gloves for 90 minutes, and on skin for 20 minutes. |
|
|
|
This constellation of acute inflammatory changes that form the immediate response to exponential viral replication in the bronchioles leads to airway obstruction and air trapping, producing the classic clinical triad of: |
- Polyphonic wheezing - Patchy atelectasis - Bilateral hyperinflation |
|
|
|
Auscultation in RSV reflects the vibration of conducting airways generated by turbulent airflow and is remarkable for: |
- Prolonged expiratory phase, - Diffuse polyphonic wheezing, and - Coarse crackles (rales) scattered throughout the lung fields. |
|
|
|
In the next few days, the clinical status evolves with involvement of the lower respiratory tract manifested by: |
Cough and increased work of breathing with use of accessory respiratory muscles to overcome the increased resistance of obstructed airways. |
|
|
|
Infants are usually more severely affected and may also develop: |
Lethargy, Fever, Poor feeding, and Otitis media, |
|
|
|
bilateral hyperinflation from air trapping, patchy atelectasis from airway plugging, and peribronchial thickening from lymphomonocytic infiltration. Patients with severe disease may also have features more consistent with pneumonia, with areas of interstitial parenchymal infiltration. |
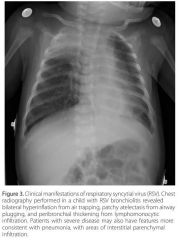
|
|
|
|
the mainstay of therapy remains supportive care, which includes: |
respiratory support combined with appropriate fluid and nutrition management |
|
|
|
Evidence-based management of bronchiolitis. |
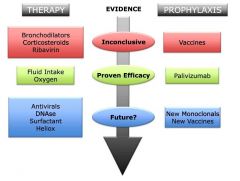
Passive prophylaxis is a safe and effective way of protecting infants at risk for severe respiratory syncytial virus (RSV) disease but is not cost-efficient. - Once the infection is established, the mainstay of current therapy remains supportive care because nosolid scientific evidence supporting the use of any conventional or experimental pharmacologic agent currently exists. - For the future, promising antiviral molecules and newgeneration humanized monoclonal antibodies are being investigated, and structural biology may overcome the challenges that have so far prevented the development of a safe and effective RSV vaccine. |
|
|
|
Children with oxygen saturations of 90% or less should receive: |
Warm, humidified oxygen. - Infants with hypoxemia refractory to supplemental oxygen, persistent respiratory distress, or evolving respiratory failure require either noninvasive support with nasal continuous positive airway pressure or endotracheal intubation |
|
|
|
is probably one of the most important factors that lead to the progressive decrease in mortality. |
Positive pressure mechanical ventilation |
|
|
|
significant adverse effects include tachycardia, tremor, hypokalemia, and hyperglycemia. |
Albuterol |
|
|
|
- Improves mucociliary clearance and is increasingly being used in airway diseases that involve mucous plugging (eg, cystic fibrosis). - It has also been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. - In particular, it is not effective in reducing hospitalization when used in emergency settings. |
Hypertonic Saline. Nebulization of 3% saline. |
|
|
|
a synthetic nucleoside analog with broad in vitro virustatic activity. |
Ribavirin |
|
|
|
The only antiviral agent ever licensed by the FDA for the therapy of severe RSV infections |
Ribavirin |
|
|
|
Palivizumab should be considered also for children with: |
hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular diseases that impair airway clearance, but no formal recommendation was made for patients with Down syndrome or cystic fibrosis because of insufficient data. |
|
|
|
The diagnosis of RSV bronchiolitis should be based on |
history and physical examination and does NOT require radiographic or laboratory studies. |
|
|
|
Severe respiratory failure requires: |
mechanical ventilatory support and occasionally high-frequency oscillatory ventilation or extracorporeal membrane oxygenation. (ECMO) |
|
|
|
Hypertonic saline may be used in hospitalized patients but |
NOT in the emergency setting |
|
|
|
On the basis of some research evidence, solid epidemiologic data suggest that early RSV bronchiolitis predisposes patients to: |
Recurrent wheezing and asthma during the first decade after birth. |
|
|
|
Prophylaxis (palivizumab, 15 mg/kg IM, for a maximum of 5 monthly doses) is recommended for: |
1. Infants born at <29 weeks 0 days of gestation without chronic lung disease of prematurity who are younger than 12 months at the onset of RSV season. 2. Infants with chronic lung disease of prematurity younger than 24 months who continue to require medical therapy within 6 months of the onset of RSV season. |
|
|
|
Prophylaxis (w/ palivizumab) may be considered for: |
1. Infants younger than 12 months with hemodynamically significant heart disease or children younger than 24 months who undergo cardiac transplantation during RSV season.
2. Infants younger than 12 months with airway abnormalities or neuromuscular disorder impairing cough.
3. Children younger than 24 months old severely immunocompromised during RSV season. |
|
|
|
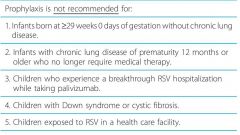
Prophylaxis (w/ palivizumab) is NOT recommended for: |
1. Infants born at >= 29 weeks 0 days of gestation without chronic lung disease.
2. Infants with chronic lung disease of prematurity 12 months or older who no longer require medical therapy.
3. Children who experience a breakthrough RSV hospitalization while taking palivizumab.
4. Children with Down syndrome or cystic fibrosis. 5. Children exposed to RSV in a health care facility. |
|
|

|

|
|

