![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
104 Cards in this Set
- Front
- Back
|
tissue necrosis is always followed by? |
Acute Inflammation (↑ in Neutrophils) |
|
|
Goal of acute inflammation? |
Eliminate pathogen
or
Clear Necrotic Debris |
|
|
How do Macrophages recognize G- bacteria? |
Recognize LPS on G- bacteria w/ CD14 receptor |
|
|
Describe pathway of TLR's |
1) PAMPs activate TLR's on macrophages/lymphocytes
2) Causes upregulation of NF-kB (transcription factor)
3) NF-kB -> production of multiple immune mediators |
|
|
TLR on B-cells importance |
TLR activation/signaling helps with IgM response (Tcell Indipendent) |
|
|
Arachidonic Acid Metabolites |
Arachidonic Acid released from cell membrane by Phospholipase A2
Acted upon by: COX => prostoglandins, prostocyclines, thromboxanes
5-lipoxygenase=> leukotrienes |
|
|
Factors that mediate Vasodilation and Increases Vascular Permeability |
PGD2, PGE2, PGI2 = DEI= "God"
vasodilation & increased vascular permeabiity are "prosto Gods (DEI)" of inflammation |
|
|
mediates pain and fever |
PGE2 - fEver and pain |
|
|
Neutrophil chemoattractants |
LTB4
IL-8
C5a
Neutrophil says, "I come only if I, "C 5 B4 I 8" - See 5 (o'clock) before I ate" |
|
|
Vasoconstriction, Bronchospasm, & Increased Vascular Permeability mediate by: |
* LTC4
* LTD4 * LTE4
Leukotrienes cause smooth muscle contraction -- bronchial muscles (Bronchospasm), smooth muscle in vessels (Vasoconstrict) and pericytes(edema) "To constrict, you have to TRY C, D, E, be4 giving up." |
|
|
Vasodilation occurs at the... |
arteriole ->slows down flow so that leukocytes can attach to wall |
|
|
increased vascular permeability occurs at... |
venule - once attached to wall leukocytes can extravasate, and swelling creates room for entering leukocytes |
|
|
Mast cells activated by |
1) tissue trauma,
2) C3a, C5a
3) cross linking by IgE on Fc receptors |
|
|
Mast cell immediate response |
Release Histamine -> vasodilation and increase permeability |
|
|
Mast cell delayed response |
Release Leukotrienes (LTC4, LTD4, LTE4)=> contract smooth muscle -> 1. vasoconstriction 2. bronchospasm 3. increase vascular permeability |
|
|
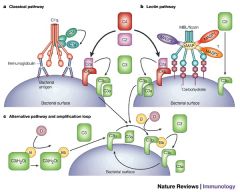
Complement System |
Classical Pathway -> C1 binds IgG or IgM that's bound to antigen
Alternative pathway -> microbial products directly activate complement
Mannose binding lectin pathway -> MBL binds to mannose on microorganisms and activates complement |
|
|
Review Complement pathway |
|
|
|
DIC Pathophysiology |
Tissue Factor (TF=Normally foundsuendothelially) release into circulation due to either
1) Damage 2) IL-1/TNF (normally endothelial cells do not express, only in response to these) 3) Endotoxin->
TF binds activated factor VIIa->Activates Extrinsic Pathway |
|
|
Cardinal Signs of inflammation |
1) Redness (rubor) = caused by Histamine mediated vasodilation
2) Warmth (calor) = also caused by Histamine mediated vasodilation
3) Swelling (tumor) = caused by Histamine mediated increase in vascular permeability
4) Pain (dolor) mediated by Bradykinin & PGE2 which sensitize nerve endings
5) Fever due to IL-1 & TNF -> ↑ cyclooxygenase activity in perivascular cells of the hypothalamus -> increases local PGE2 synthesis -> raises temperature setpoint |
|
|
Leukocyte margination occurs through? |
postcapillary venules!
Cells marginate from center flow to the periphery due to turbulence caused by slow down of blood flow from arteriolar vasodilation |
|
|
Leukocyte ROLLING occurs via? |
Selectin speed bumps (upregulated in endothelial cells)
Histamine -> release of P-selectin from Weibel-Palade bodies in endothelial cells
TNF and IL-1 -> release of E-selectins
Interaction of Sialyl Lewis X on Leukocytes w/ P-selectin and E-selectin causes leukocytes to roll along vessel wall |
|
|
What does selectin on endothelial cells bind on leukocytes (Neutrophils, Macrophages, etc) |
Sialyl Lewis X |
|
|
Leukocyte ADHESION to endothelial cells occur via? |
Adhesion molecules: ICAM and VCAM upregulated by TNF & IL-1
ICAM & VCAM bind to Integrins on leukocytes
TNF & IL-1 :
1) Rolling (Release E-selectins on Endothelial cells) 2) Adhesion (ICAM/VCAM expression on Endothelial cells) 3) Tissue Factor release from Endothelial cells 4) Fever: b/c ↑COX activity in Perivascular cells of Hypothalamus->↑PGE2 |
|
|
What causes upregulation of adhesion molecules? |
IL-1 & TNF |
|
|
What causes upregulation of selectins on Endothelial cells? |
Histamine causes release of P-selectin from Weibel Palade bodies
TNF & IL-1 for E-selectin
Selectins bind to Sialyl Lewis X on Leukocytes (Neutrophils, Macroph) |
|
|
What causes upregulation of integrins on leukocytes? |
C5a & LTB4, the chemotactic factors! (this is how they achieve Neutrophil chemotaxis)
|
|
|
MCC of leukocyte adhesion deficiency?
What is the clinical presentation? |
Lack of leukocyte extravasation from blood into tissues due to Defective CD18 (Integrin component)
NOTABLE CLINICAL SIGNS: 1) Failure of umbilical cord to detach 2) INCREASED circulating neutrophils because they can't be stored by attaching to vascular walls (marginated pool) 3) Recurrent bacterial infections that LACK pus formation
*pus=dead neutrophils sitting in fluid |
|
|
Opsonins...? |
IgG and C3b |
|
|
Chediak-Higashi syndrome |
Impaired phagolysosome formation
Pathophys: Microtubule polymerization disorder: "tracks" to get around the cell are broken->transport of phagosomes is deficient |
|
|
Giant granules in leukocytes and defective primary hemostasis, albinism |
Chediak-Higashi syndrome = Abnormal dense granules in Golgi apparatus->impaired primary hemostasis
Gant granules (in leukocytes) arise from fusion of granules in Golgi since they are NOT able to move |
|
|
Generation of bleach |
O2 -NADPH Oxidase (oxidative burst)->
O2- radical -Superoxide Dismutase->
H2O2 -MPO->
HOCl |
|
|
NTB dye |
O2-->O2 radical
If NADPH oxidase works, will stain blue |
|
|
Chronic Granulomatous Disease What organisms are you susceptible to? |
Poor O2 dependent killing due to NADPH oxidase defect
Vulnerable to Catalase + organisms: Reaction of catalase in the decomposition of hydrogen peroxide in living tissue: 2 H2O2 → 2 H2O + O2
Staph aureus Pseudomonas capacia Serratia marcescens Nocardia Aspergillus |
|
|
MPO Deficiency |
Defective conversion of
H2O2 -> HOCl
↑ risk for Candida infections |
|
|
Leukocyte O2 independent killing
What does it bind to? |
Reliant on lysozyme and major basic protein
Bind heparin sulfate proteoglycans |
|
|
Anti-inflammatory cytokines produced by macrophages? Pro-inflammatory cytokines produced by macrophages? |
Anti-inflammatory: IL-10 & TGF-ß
Pro-inflammatory: IL-8 |
|
|
Macrophage management of Acute Inflammation |
Resolve with IL-10 & TGF-ß
Continue inflammation with Neutrophil recruitment using IL-8
Abscess Chronic inflammation via CD4 lymphocytes |
|
|
Histological description of plasma cells |
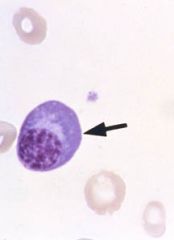
Plasma cells have eccentric nucleus with slight pale pallor next to nucleus
Not multilobulated like neutrophils. |
|
|
T cells have what receptors |
TCR and CD3 on all T cells. |
|
|
CD4+ TH-cell activation |
APC presents antigen on MHC II binds TCR
B7 on APC binds T-cell CD28 receptor |
|
|
Th1 cytokines |
IL-2 (T-cell growth factor and CD8+ activator)
IFN-γ
IL-6 (pyrogen, stimulates acute phase proteins) |
|
|
Th2 cytokines |
IL-4 (facilitates B-cell class switch to IgE & IgG)
IL-5 (eosinophil chemotaxis and activation, B cell maturation to plasma cells and class switch to IgA)
IL-10 (inhibits Th1 phenotype) |
|
|
CD8+ Cytotoxic T-cell activation how does it initiate apoptosis? |
Intracellular antigen presented on MHC I
IL-2 from TH1(CD4+) Tcell: confirmatory
CD8 induces apoptosis via perforin and granzyme GRENADES or Expression of FasL which binds to Fas receptor on cells inducing apoptosis |
|
|
B-Cell activation |
Ag binding by surface IgM or IgD->maturation to IgM or IgD secreting plasma cells
No second signal needed if enough Ag present to activate & cross link Ig's or Ag activates TLR
Alternate Pathway: B-cell presents Ag to TH2 T-cell via MHC II -- CD40 receptor on B cell binds CD40L on TH2 as 2nd signal -- Th2 secretes IL-4 and IL-5 to mediate isotype switching, hypermutation and plasma cell maturation |
|
|
A granuloma is characteristically defined by |
epithelioid histiocytes (Macrophages with abundant pink cytoplasm) usually surrounded by lymphoctes/giant cells |
|
|
Characteristic of non-caseating granuloma. Common causes? |
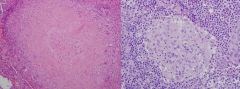
Lacks central necrosis
Usually caused by: 1)Sarcoidosis
2) Reaction to foreign material
3) beryllium exposure
4) Crohn's disease
5) Cat scratch disease(Bartonella hensellae) |
|
|
Characteristic of Caseating granulomas |
central necrosis characteristic of tuberculosis and fungal infections |
|
|
Steps for Granuloma formation |
1) Macrophage Processes Ag
2) MHC II on Macrophage presents Ag on MHC II to CD4+ cell
3) Macrophage secretes IL-12 differentiating CD4+ to TH1
4.) TH1 secretes IFN-γ converting macrophages to Epithelioid histiocytes and Giant cells |
|
|
DiGeorge Syndrome |
Failure of 3rd and 4th pouch development due to 22q11 microdeletion->
1) T-cell deficiency (lack of thymus) 2) Hypocalcemia (lack of parathyroids) 3) Abnormalities of Heart, great vessels, & face |
|
|
lack of thymus, hypocalcemia and abnormalities in heart and great vessels |
DiGeorge Syndrome (22q11 microdeletion) ->
1) Lack of thymus ->T-cell deficency (recurrent infections)
2) Lack parathyroids ->hypoparathyroidism (low PTF) causing hypocalcemia |
|
|
Bruton's Agammaglobulinemia or X-linked Agammaglobulinemia |
Complete lack of Ig due to disordered B-cell maturation->
Naive B cells can NOT mature into plasma cells ->No Ig secreted (Due to mutation in a tyrosine Kinase) |
|
|
Recurrent bacterial, enterovirus (polio, coxsackie), and giardia lamblia
after 6 months of life |
Bruton's agammaglobulinemia
Presents >6 months b/c Mother's Ig's present first 6 months.
Enterovirus infection due to lack of IgA |
|
|
Severe Combined Immunodeficiency |
Combined humoral and cell-mediated defect, so usually involves TH cells b/c they mediate both Can be due to multiple causes
1) Due to Cytokine receptor defect, leads to failure to development and differentiation of T and B cells (specifically IL-2R which promotes Tcell activation) - b/c IL-2Receptor located on X chromosome = called X-linked SCID
ADA (adenosine deaminase deficiency=Adenosine ) & MHC Class II deficiency ADA involved in breakdown of purines. Lack of ADA causes accumulation of dATP. This metabolite will inhibit the activity of ribonucleotide reductase, the enzyme that reduces ribonucleotides to generate deoxyribonucleotides. Without functional ribonucleotide reductase, lymphocyte proliferation is inhibited and the immune system is compromised.
MHC class II NECESSARY for CD4 to do anything |
|
|
most common selective Ig deficiency? a/w? |
IgA defficiency -> celiac disease and viral gastroenteritis
All upper respiratory diseases
this is why many people get anaphylactic rxns. w/ blood transfusions |
|
|
Hyper-IgM Syndrome etiology? |
Elevated IgM Mutated CD40L (T-cells) or CD40(B-cells) - as such second signal cannot be delivered for required class switching |
|
|
thrombocytopenia, eczema, recurrent infections |
defective humoral and cellular immunity due to WASP gene. This is X-linked. Called Wiskott-Aldrich Syndrome Small platelets that do not function properly are removed by the spleen causing the thrombocytopenia
WASp activates actin polymerization by binding Arp2/3 complex necessary for immunological synapse
In T-cells, WASp is important because it is known to be activated via T-cell receptor (TCR) signaling pathways to induce formation of immunological synapse
Immune deficiency caused by decreased Ab production & inability of T cells to form synapse (combined immunodeficiency) |
|
|
C5-C9 deficiencies |
increased risk for Neisseria infection |
|
|
hereditary angioedema, edema of the skin, periorbital edema and mucosal surface |
C1 inhibitor deficiency C1 inhibitor is a serine protease inhibitor that is the most important inhibitor of kallikrein in the body. decreased C1 inhibitor --> increased kallikrein --> increased bradykinin --> increased permeability |
|
|
What's a method of T-cell inactivation that occurs after mature T-cells leave thymus? |
Anergy - stimulation without second signal deactivates them |
|
|
Systemic Lupus Erythematosus is what type of hypersensitivity? |
TypeII & III hypersensitivity, Antibodies against host damage multiple tissues, and depositing immune complexes cause further damage |
|
|
Classic symptom of SLE |
Renal damage -- diffuse proliferative glomerulonephritis |
|
|
Small sterile deposits on both sides of heart valve |
Libman-sacks endocarditis secondary to SLE Caused by non-infectious inflammatory response to Ag-Ab caused by Lupus |
|
|
Lupus lab test findings |
ANA positive, anti-dsDNA (highly specific) |
|
|
anti-histone antibody Common associations? |
drug induced lupus, reversible common drugs, hydralazine, procainamide, isoniazid |
|
|
increased PTT time but hypercoaguable? |
think lupus anticoagulant or anticardiolipin(cardiolipin is present on mitochondrial membrane) |
|
|
recurrent pregnancy loss in female with fever and weight loss |
Anti-cardiolipin Ab or Lupus anti-coagulant cause frequent thrombosis in arteries and veins
ARTERIES AND VEINS causing DVT's
hepatic vein thrombosis (Budd-Chiari)
placental thrombosis(recurrent pregnancies)
stroke |
|
|
Unilateral enlargement of the parotid gland |
B-cell lymphoma in marginal zone secondary to Sjogren's |
|
|
CREST Scleroderma |
C - Calcinosis/anti-Centromere antibodies
R - Raynaud's
E - Esophageal Dysmotility
S - Sclerodactyly
T - Telangiectasias |
|
|
Lots of dental carries and corneal abrasions. What do you test for? |
ANA and anti-ribonucleoprotein (anti-SS-A/Ro and anti-SS-B/La) |
|
|
Sjogren's associated with? |
Auto-immune attack of salivary & lacrimal glands -> development of xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eyes) ->lymphocytic infiltration of the glands Iinflammatory process eventually severely damages or destroys the glands
If both parotid glands swell
If 1 parotid gets worse, think: B-cell lymphoma in the MARGINAL zone
|
|
|
Anti-U1 Ribonucleoprotein (Anti-U1RNP) |
mixed connective tissue disease features from SLE, systemic sclerosis and polymyositis |
|
|
Stem cells of lung... |
Type II Pneumocyte |
|
|
Stem cell of GI tract |
Mucosal Crypts |
|
|
Stem cell of bone marrow are positive for what molecule? |
CD34+ = HSC |
|
|
What type of collagen present in keloids? |
Type III collagen (that is why so soft) Also present in blood vessels and embryonic tissue and early wound healing |
|
|
Collagen in early wound healing |
Type III collagen |
|
|
What cofactor does collagenase require? |
Zinc |
|
|
TGF-α |
Epithelial & Fibroblast growth factor |
|
|
TGF-β |
1) Important fibroblast growth factor
2) Inhibits inflammation |
|
|
Platelet Derived Growth Factor (PDGF) |
Growth Factor for:
1) Endothelium
2) Smooth muscle
3) Fibroblasts |
|
|
Fibroblast Growth Factor (FGF) |
1) IMPORTANT FOR ANGIOGENESIS
2) mediates skeletal development |
|
|
what kind of signaling is wound healing mediated by? |
paracrine macrophages secrete growth factors that target fibroblasts |
|
|
Necessary factors for wound healing |
1) Zinc
2) Copper
3) Vitamin C |
|
|
What role does Vit C play in wound healing |
Hydroxylates proline residues so that they can cross link each other and form collagen |
|
|
What role does Copper play in wound healing? |
Cofactor for lysyl oxidase which cross-links lysine and hydroxylysine to form stable collagen (Hydrogen Bonding) |
|
|
What role does Zinc play in wound healing? |
Zinc is a cofactor for collagenase, which replaces type III collagen of granulation tissue with the stronger type I collagen |
|
|
African-American recently got an ear piercing
What should you be afraid of? |
Keloid formation. Characterized by type III collagen overproduction |
|
|
What collagen is a hypertrophic scar made out of? |
Type I Collagen |
|
|
Compare hypertrophic scar with keloid |
keloid = overgrowth of granulation tissue (type III collagen) at the site of a healed skin injury that grows far beyond the boundaries of the original wound (slowly replaced w/ Type 1 Collagen, but is HUGE!)
hypertrophic scar = excessive amounts of Type 1 Collagen which gives rise to a raised scar - but is limited to site of wound (due to excessive TGF-β) |
|
|
Treat antiphospholipid syndrome with? |
lifelong anti-coagulants |
|
|
Defects in Inteferon Signaling predispose to what kind of infection? |
Mycobacterium tuberculosis, b/c Macrophage will produce IL-12 upon phagocytosis of M. tuberculosis, and TH1 in turn, wil produce IFN-Gamama. IGN Gamma that induces Mphage to engage in phagocytic killing
Additionally IFN_Gamam induces upregulation of MHC. These pts will therefore need liefelong anti-mycobacterial antibiotics |
|
|
Thymus Cortex and Medulla: Tcell Maturation |
Cortex:Positive Selection Medulla: Negative Selection |
|
|
Low C1 esterase inhibitor is diagnostic for: |
Angioedema |
|
|
Angioedema |
Inherited Autosomal Dominant condtion painless, non-pitting, well circumscribed edema
Face neck lips, and tongue most often affected, but internal organs can swell as well
If affects tracheobronchial tree, can cause respiratory obstruction & is potentially fatal |
|
|
Types of Organ Rejection |
Host vs. Graft
Graft vs. Host |
|
|
Types of Host vs. Graft Rejection |
Hyperacute - Happens almost immediately. It is due to preformed Anti-ABO Ab's. The inflammation is so severe that it leads to acute thrombosis of vascular supply->infarct. Screening for ABO blood grp. incompatability has eliminated Hyperacture rejection.
Acute Rejection - Happens 1-4 wks following transplant. Host Tcells get sensitized to graft MHC Ag.
Chronic |
|
|
Prevention and Tx of Acute Graft Rejection. |
Precention - calcineurin Inhibitors: cyclosporine & tacrolimus
Calcineurin: When APC interacts with Tcell->Increase in Cytoplasmic Ca2+->Activating Calcienurin->Incr IL-2->IL-2 activates TH cells
Amount of IL-2 produced by TH cells significantly influences extent of immune response
Tx. Add corticosteroids to Calcineurin Inhibitors |
|
|
IL-5 |
Made by TH2 cells
Promotes Production/Activation of Eosinophils
Promotes Bcell synthsis of IgA production |
|
|
IL-4 |
Promotes IgE Ab production by Bcells |
|
|
IL-2 |
Stimulates TH1 cell proliferation |
|
|
IFN-Gamma |
Stimulates Macrophage Activation |
|
|
IL-10 |
Downregulates Immune responses. Produced by Macrophages if they enter after Acute Inflammation (Neutrophils) and that pathogen/necrosis is cleared |
|
|
IL-3 |
Supports growth/differentiation of BM stem cells |
|
|
IL-1 |
Produced by Macrophages (after CD4+ THcell TCR binds to MHC Class2 on Macrophage) and induces TH cells to proliferates/secrete lymphokines |

