![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
219 Cards in this Set
- Front
- Back
|
3 nuclear changes in irreversable cell injury are:
|
PYKNOSIS (irrev. condens. of chr. in nucleus of cell undergoing necrosis or apoptosis)
KARYORRHEXIS KARYOLYSIS |
|
|
Pyknosis
|
Pyknosis, or karyopyknosis
irreversible condensation of chromatin in the nucleus of a cell undergoing necrosis or apoptosis. fyi: from Greek pyknono meaning "to thicken up, to close or to condense" |
|
|
What is KARYOLYSIS?
|
loss of nucleus = KARYOLYSIS
|
|
|
karyorrhexis
|
karyorrhexis = fragmentation of nucleus
(follows pyknosis; or condens. of chr. in nucleus during apoptosis) |
|
|
Arthrogryposis
|
Arthrogryposis: rare congenital disorder characterized by multiple joint contractures (shortened); can include muscle weakness and fibrosis; non-progressive disease.
* from Greek, literally meaning 'curved or hooked joints |
|
|
Chondrodystrophy
|
any congenital disorder of cartilage formation (e.g. dwarfism)
literally, "cartilage mal-development" |
|
|
Encephalocele
|
failure of neural tube to close characterized by sac-like protrusions of the brain and the membranes that cover it through openings in the skull.
|
|
|
palatoschisis
|
cleft palate
|
|
|
T/F ATP depletion is consequence of both ischemic and toxic injury.
|
True
ATP depletion is consequence of both ischemic and toxic injury. |
|
|
How can ATP depletion lead to cell injury?
|
* Hypoxia attacks aerobic respir.- stoppage of ATP prod.-ATP decr. in cell membrane- causes Na pump failure results Na retention (Na enters cell and K diffuses out)-water gets in cell which results in cell swelling.
* also changes metabolism; accum. of lactic acid |
|
|
Reperfusion [tissue] injury refers to ?
|
Reperfusion inj. refers to tissue damage caused when blood supply returns to the tissue after a period of ischemia; caused by oxidative stress
|
|
|
Effects of increased Ca in the cell:
|
Activates a number of enzymes with deleterious effects
|
|
|
Name 4 examples of "deleterious" enzymes (released after intracellular Ca+ increase):
|
1. Phospholipases - damage cell membr.
2. Proteases 3. ATPase - hasten ATP depl. 4. Endonuleases (e.g. DNase) |
|
|
What is a free radical? How do they cause cell injury?
|
* single unpaired electron in an outer orbital
* unstable and extremely reactive and enters into reactions in cells with organic and inorganic chemicals. |
|
|
Sources of Ca+ rise?
(increased intracellular calcium) |
* Net influx of Ca across plasma membrane
* Release of Ca from mitochondria and ER |
|
|
Chemicals can induce injury in 3 ways:
|
1. Binding to cell membrane causing increased membrane permeability and inhibition of ATPase dependent transport.
2. Direct cytotoxic effects. 3. Reactive free radicles. |
|
|
Continued ATP depletion and increased calcium influx in cell causes: (2)
1. damage to ? 2. affects pH, how? why? |
1. Extensive damage to plasma membrane. Cell membrane damage is central factor in irreversible injury.
2. Lower pH- injury to mitochondria and lysosomes (release enzymes) |
|
|
Is Acute Cellular swelling a form of Reversible cell injury?
|
Yes
|
|
|
Acute Cellular swelling usually seen in what tissues/organs?
|
Usually seen in liver, kidney, muscles, glandular tissues, neurons, glial cells of brain and adrenals
|
|
|
How does the physical appearance of these organs change (w/acute cellular swelling)? cells?
|
Grossly - organ becomes pale, turgor., heavy.
Micro - Cytoplasm shows ground glass app., w/small vacuoles. Nuclear swelling. |
|
|
T/F In brain swelling is called ‘cytotoxic edema’. Swollen neurons fail to conduct nervous impulses and result in stupor or coma.
|
True
|
|
|
T/F Swollen epithelial cells in kidney impair absorptive and secretory function.
|
True - Swollen epithelial cells in kidney impair absorptive and secretory function.
|
|
|
T/F Endothelial cell swelling causes obstruction, worsening cellular hypoxia.
|
True - Endothelial cell swelling causes obstruction, worsening cellular hypoxia.
|
|
|
4 Forms of necrosis
|
Depends on cause and tissue involved.
1. Coagulative necrosis 2. Liquifactive necrosis 3. Caseous necrosis 4. Fat necrosis |
|
|
Coagulative necrosis Caused by:
|
type of accidental cell death typically caused by ischemia or infarction.
Also: * Toxic product of certain bacteria- necrobacillus. * Poison, burn, electricity, x-ray. * Zenker`s necrosis- |
|
|
Gross appearance of tissue undergoing coagulative necrosis ?
|
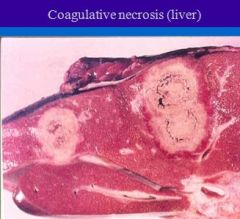
Gross appearance of coagulative necrosis - Gray or white, firm and depressed.
|
|
|
Microscopic appearance of coagulative necrosis?
|
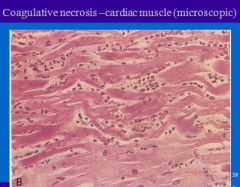
Microscopic appearance-
1. Outline of the necrosed cell is preserved, 2. Cytoplasm homogenous strongly acidophilic, 3. Nuclei show degeneration. |
|
|
What type of necrosis occurs in CNS (spinal cord lesions) and in abscess?
Causes? |
Liquefactive (colliquative) necrosis
Causes- same as for coagulative necrosis |
|
|
Gross appearance of tissue undergoing liquefactive (colliquative) necrosis ?
|
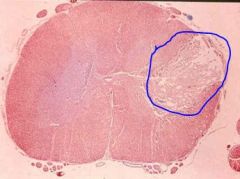
Gross lesions- small or large cavity containing yellow white opaque fluid, the walls of the cavity are irregular and soft.
|
|
|
What type of necrosis is caused by toxins of certain micro-organisms?
|
All 4 including caseous and peritoneal fat necrosis - caused by toxins of certain microorganisms
|
|
|
Where does caseous necrosis get its name?
|
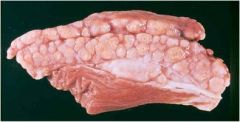
Because gross lesions- looks like “milk curd” or “cheese”
hence the name caseous. Necrosed tissue is dry, greasy and breaks easily. |
|
|
Cause: fat necrosis is often seen in what 2 places?
|
A) Fat of peritoneal cavity- enzymic fat necrosis or pancreatic fat necrosis. Hard masses form on omentum, mesentery and peritoneum.; intestinal obstruction & clinical signs.
B) fat under the skin and pelvic canal- traumatic necrosis of fat. |
|
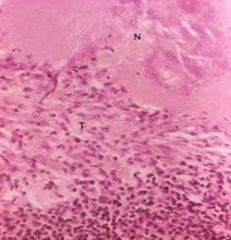
Which type of necrosis?
Mxscopic lesions- loss of cell line and normal arch. of tissue, necrosed tissue is granular and stains purple ~Inflamm. zone surrounds the necrosis ~calcif. is usually present in necrosed tissue. |
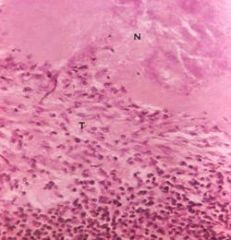
Caseous necrosis
|
|
|
Which type of necrosis?
Gross lesions- lose shiny appearance, become opaque, whitish, solid or granular. |
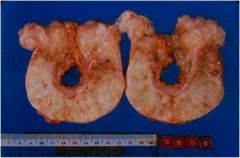
Fat necrosis (necrosis of fat cells)
- fat becomes opaque |
|
|
Type of necrosis seen in young cattle fed on tall fescue (grass) infected with fungus (Acremonium coenophialum)?
|
Peritoneal fat necrosis
“Summer fescue toxicosis” in cattle, sheep and horses. |
|
|
Refers to the death of single cells-through programmed ‘suicide’ path way.
|
Apoptosis
|
|
|
How are necrotic foci different from tissues undergoing typical apoptosis or post mortem autolysis? (4)
|
Necrotic foci is often surrounded by inflammatory rxn; can cause 1. sloughing 2. liquefaction w/abscess or autolysis of foci, 3. encapsulation by fibrous tissue or 4. calcification
|
|
|
Condition when necrosed tissue is invaded by putrifactive bacteria.
|
Gangrene
|
|
|
Three types of gangrene
|
Dry gangrene.
Moist gangrene Gas gangrene --Area of gangrene are separated from healthy tissue by a line of demarcation- seen as red zone of inflammation. |
|
|
Type of apoptosis triggered by-
* irradiation * virus infection * certain toxins * drugs |
Pathological apoptosis
|
|
|
What is physiological apoptosis?
3 examples where might find it? |
* normal cell turn over (liver, kidney, epithelium)
* Hormone induced atrophy- Involution of thymus * remodeling of embryonic development * tumors |
|
|
Chromatin condensation and fragmentation into seperate "bodies" is characteristic of apoptosis OR necrosis?
|
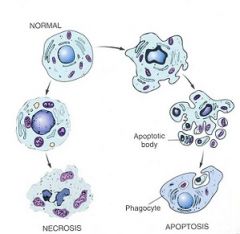
APOPTOSIS
- Cell shrinkage - Chromatin condensation and frag. into apoptotic bodies - Phagocytosis of apoptotic bodies by tissue macrophages |
|
|
____ is caused by gradual cooling of carcass.
|
Algor mortis: gradual cooling of carcass.
|
|
|
____ is caused by gravitational pooling of blood to the down side of the animal.
|
Livor mortis (hypostatic congestion): gravitational pooling of blood to the down side of the animal.
|
|
|
____ is caused by contraction of muscles occurring after death, because of depletion of ATP in muscle.
|
Rigor mortis; contraction of muscles occurring after death, because of depletion of ATP in muscle.
|
|
|
Bile imbibition refers to what post mortem changes?
|
Bile imbibition refers to yellow staining of tissues adjacent to gall baldder. Seen in 1-6 hours after death.
|
|
|
Pseudomelanosis refers to what post mortem changes?
|
Pseudomelanosis: blue green discoloration of tissues by iron sulfide (rxn of H2S from bacteria and Hb from RBC).
|
|
|
Abnormal or excessive accumulation of fat within the cell (fat changes) common in what tissues?
|
Fatty changes mostly occurs in LIVER, kidney and muscle.
|
|
|
T/F: Digested fat arrives at liver from intestine in form FFA
|
False: if from GI -> short chain FA
but if comes from fat tissue -> FFA |
|
|
Pathological glycogen deposition is occurs in what tissues? (4)
|
Pathological deposition occurs in LIVER, kidney, leukocytes, cardiac muscle and less often in spleen, smooth muscle, brain and lymph nodes.
|
|
|
3 possible causes of abnormal glycogen deposition?
|
Causes:
a) Hyperglycemia (in diabetes m.) b) Drug induced metabolic dz (corticosteroids) --> Glucocorticoid hepatopathy c) Glycogen storage diseases** |
|
|
**Glycogen storage diseases (lead to glycogen deposition) are caused by ?
|
GSD's are autosomal recessive inherited disorders characterized by deficiencies of various enzymes involved in the synthesis or degradation of glycogen.
|
|
|
How many forms of GSDs are there in animals? "Numbers" associated with them?
|
Many types in humans, but only of them in animals (Type 1, 2, 3, 4, 7, and PSSM)
|
|
|
Type I ; occurs in what breeds?
|
Type I ; occurs in toy breeds;
|
|
|
Type I due to ____.
|
Type I due to deficiency of glucose-6- phosphatase. Glycogen storage is more in the liver; hypoglycemia develops.
|
|
|
type II glycogenosis also known as ?.....what animals occur in ?
|
Type II = Pompe`s disease
* Reported in Brahman and shorthorn cattle calves, Corriedale sheep, Lapland dogs, cats, turkeys and Japanese quail. |
|
|
Pompe`s disease (type II glycogenosis) caused by ?
|
Pompe`s disease (type II) = Deficiency of lysosomal acid maltase (glucosidase).
|
|
|
type III glycogenosis known as ?.....what animals occur in ?
|
type III = Cori`s disease
Reported in German Shepherd dogs. |
|
|
type III (Cori`s disease) caused by deficiency in ?
|
Cori's dz = Deficiency of amylo 1,6- glucosidase, involved in breakdown of glycogen to glucose.
|
|
|
Cori's dz (type 3) causes glycogen to accumulate in ?
|
Glycogen accumulates in liver, nerve cells, heart, skeletal and smooth muscles.
|
|
|
T or F: Dogs affected with Cori's Dz (type III) have hyperglycemia
|
False: Affected dog develops hepatomegaly and hypoglycemia (low sugar in blood, b/c all in glycogen)
|
|
|
Type IV glycogenosis occurs in ?
|
Type IV glycogenosis occurs in Norwegian forest cats, and American painted / quarter horses
|
|
|
Type IV glycog. in NF cats caused by ?
|
Type 4 = Deficiency of glycogen branching enzyme;affected kittens die soon after birth or develop progressive muscle degen., if survive after 7-8 month.
|
|
|
Type IV deposits appear in what tissues?
|
Type 4 = Glycogen deposit in many tissues but more in skeletal and cardiac muscles.
|
|
|
Type IV glycog. in horses caused by ?
|
Inherited as autosomal recessive trait in quarter horses and American paint horses; Due to congenital lack of glycogen brancher enzyme.
|
|
|
Symptoms of type IV glycog. in horses
|
Affected foals are aborted, stillbirth or weak at birth.
|
|
|
Type IV glycog. in horses appear in what tissues?
|
Accum. of long un-branched chain of glucose within cardiac, skeletal myocytes, Purkinje fibers and to less degree in hepatocytes; that leads to abnormal glycogen formation and deposition.
|
|
|
Type VII glycogenosis found in what breeds?
|
Type VII glycogenosis
* Reported in English springer spaniel dog and American cocker spaniel. * |
|
|
Type VII glycogenosis caused by deficiency in ?
|
Deficiency of M type phosphofructokinase (PFK)
|
|
|
Dogs with Type VII glycogenosis show what signs? (4)
Clinical problems? (4) |
* Affected dogs show hemolytic anemia, intravascular hemolysis and hemoglobinuria.
* Clinical signs- exercise intolerance, muscle wasting and muscle cramping. |
|
|
Type VII glycogenosis affects what tissues/cells?
|
Affected dogs have 1-6% of normal Muscle PFK activity;
and 10-20% of normal RBC PFK activity, (normal RBC PFK is 80-90% of M-type, so a lot missing). Glycogen accum. in muscles |
|
|
PSSM stands for ?
|
Polysaccharide storage myopathy in horses
|
|
|
PSSM manifests itself in what tissues ?
|
Excessive glycogen storage in horses with PSSM involves skeletal muscle, which is 1.5 to 4 times greater than normal horses.
|
|
|
What horse breeds get PSSM?
|
A familial basis for PSSM has been reported in Quarter horses, Arabian morgan, Pony of Americas and draft related breeds.
|
|
|
PSSM caused by ?
Not caused by ? |
* Clinical, histological and biochemical similarities to PSSM is PFK deficiency.
* Although PSSM is classified as GSD or glycogenosis, NO signif. enzyme deficiency has been demonstrated. |
|
|
Lysosomal storage diseases are classified according to the major stored material; give 4 examples.
|
LSD::
a) mucoplysaccharidosis b) sphingolipidosis c) glycogenosis d) mucolipidosis. |
|
|
Characterized by extracellular deposition of abnormal proteinaceous substance in tissues.
|
Amyloidosis = extracellular deposition of abnormal proteinaceous substance in tissues.
|
|
|
Amyloid is seen morphologically uniform in tissues, but chemically it consists of two major forms of protein.
|
1. Amyloid light chain protein (AL)
2. Amyloid associated (AA) protein |
|
|
Amyloid light chain protein (AL) derived from ?
|
Amyloid light chain protein (AL) derived from plasma cells, contains light chain immunoglobulin
|
|
|
Amyloid associated (AA) protein, secreted by ?
|
Amyloid associated (AA) protein, secreted by liver cells in response to various cytokines.
|
|
|
5 types of Amyloidosis (Based on clinical settings, anatomic location and biochemical composition)
|
1. Primary 2. Secondary
3. Heridofamilial 4. Amyloid of aging 5. Localized amyloidosis |
|
|
Lesions of amyloidosis common in what tissues? (3 + 7)
|
Common sites- liver, spleen, kidney.
Other organs affected - pancreas, lymph gl., adrenals, heart, lung, brain and G.I.. |
|
|
Lesions of amyloidosis in liver have what appearance grossly?
|
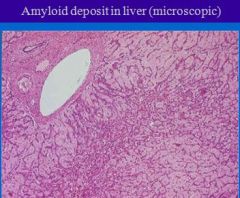
Lesions:
Liver- grossly not-noticed in small lesions. In advanced cases liver becomes hard, pale, firm and enlarged. External and cut surfaces are waxy. |
|
|
Amyloidosis causes protein deposits where in liver?
|
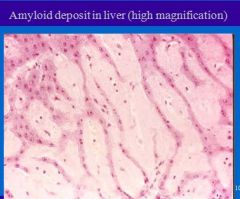
Deposition starts in space of Disse (or perisinusoidal space) and continue to sinusoids, pressing on hepatocyte, squeezing to death.
|
|
|
What is the space of Disse ?
|

Space of Disse (or perisinus. space) is in liver btw. hepatocytes and sinusoids; contains blood plasma. Microvilli of hepatocytes into space allowing proteins, etc. from sinusoids/plasma to be absorbed by hepatocytes. Fenestration of endothelium facilitates transport. Space may be obliterated in liver disease.
|
|
|
Lesions of amyloidosis in kidney have what appearance grossly?
|
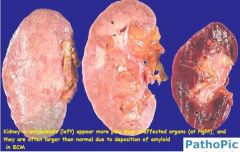
Kidney: Large gray and firm, or reduced in size.
|
|
|
Amyloidosis causes protein deposits where in kidney? in dogs? in cats?
|
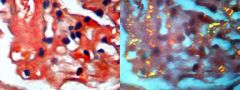
Deposit mainly in glomeruli in dogs and other species, but extend to peritubular tissue in cats.
"Glomeruli are affected in almost all cases of AA amyloidosis and in less than half of AL amyloidosis." //source: www.kidneypathology.com/English_version/Amyloidosis_and_others.html |
|
|
Lesions of amyloidosis in spleen have what appearance grossly?
|
Spleen: Depending on pattern of deposit- ‘sago spleen’ or ‘bacon spleen’. Organ in both cases is firm, pale, gray and waxy.
|
|
|
Amyloidosis causes protein deposits where in spleen?
|
Staining of amyloid: congo red stains it pink or red; toluidine blue stains blue.
|
|
|
True or False: Most cases of amyloidosis found at autopsy, without clinical signs.
|
True: Most cases of amyloidosis found at autopsy, without clinical signs.
But prognosis of amyloid patients is poor. |
|
|
Renal Amyloidosis causes what clinical problems/symptoms?
|
Renal involvement causes proteinuria and loss of anti-coag.,which results into thrombosis (blood clots). Adv. cases lead to RF and uremia (abn. high level of nitrogen-type wastes in blood).
|
|
|
Liver Amyloidosis causes ?
|
Liver Amyloidosis causes metabolic derangement.
|
|
|
Pancreatic Amyloidosis causes ?
|
* Pancreatic involvement causes non insulin dependent diabetes mellitus ( common in cat)
|
|
|
What are hyaline changes?
|
Hyaline change is any alteration within cells or in extracellular spaces or structures that give homogeneous, glassy pink appearance in tissue sections stained with H&E.
|
|
|
Example of intracellular hyaline change:
|
Intracellular hyaline change:
-Intracellular hyaline change is seen in renal tubular epithelium in proteinuria. --Proximal convoluted tubules in the kidney |
|
|
Example of extracellular hyaline change:
|
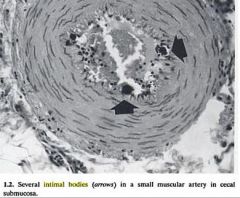
Collagen fibrous tissue in old scar
*Hyalinization of glomeruli in chronic renal diseases *Equine intimal bodies *Hyaline membrane disease *Hyalinization of arterioles in diabetes (humans) |
|
|
Is it normal to have deposition of calcium salts in tissues other than bone and teeth?
|
nope
|
|
|
What type of calcification is this?
* Deposition of Ca. salts in dead and degen. tissue! * Not related to increased conc. of calcium in blood.(10mg/dl of blood-normal) |
Dystrophic calcification
This occurs as a reaction to tissue damage! |
|
|
Which type of calcification is assoc. w/elevated levels of calcium in blood?
|
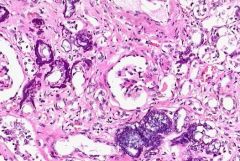
Metastatic calcification:
Deposition of calcium salts in tissues as result of incr. level of calcium in the blood. |
|
|
True or False: Serum calcium levels may be low, normal, OR high in dogs with CRF.
|
True; so treatment should adjusted accordingly
|
|
|
Serum calcium levels are normally tightly regulated by ___ and ____.
|
combined actions of calcitriol and
PTH For example, calcitrol promotes intest. abs. of calcium |
|
|
Causes of Metastatic calcification? (name five)
|
Causes:
1. Hyper-parathyroid activity 2. In RF, where phosphates retained in blood. 3. Hypercalcemia of malignancy 4. Granulomatous dz (TB and fungal diseases in dogs) 5. Feeding on plants rich in vitamin D or excessive medication and toxicity of Vitamin D. |
|
|
Lesions in pathological calcification mostly observed in what animals?
|
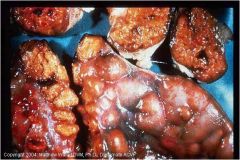
Mostly observed in cattle (see pic), sheep and goat.
|
|
|
Lesions in pathological calcification mostly observed in what tissues?
|
Mostly deposited in aorta and muscular arteries
|
|
|
What is gross appearance of Pathological calcification?
|
Gross- White or gray irregularly round particles, which gives gritty sound when cut with knife.
|
|
|
What is micro appearance of Pathological calcification?
|
Micro- seen as small irregular granule.
|
|
|
True or False: Dystrophic calcification is dangerous to body and metastatic is beneficial to the body.
|
False:
Dystrophic calcification is beneficial to body and metastatic is dangerous to the body. |
|
|
Which type of calc. affects any tissue?
|
Dystrophic
* Affects any tissue * considered beneficial to the body |
|
|
Which type of calc. affects affects mainly the blood vessels and is harmful to body?
|
Metastatic
*affects mainly the blood vessels * Injury to tissue not a prerequisite * Affects Mainly the blood vessels * Harmful to the body |
|
|
Is metastatic calcification common in dogs and cats?
|
Hypercalcemia in dogs and cats usually does NOT cause metastatic calcification in large blood vessels; but may see Ca+ deposits in epith. cells of kidney
|
|
|
What is calcinosis?
|
Calcinosis- calcification in or under the skin.
|
|
|
Calcinosis- seen in two forms:
|
1. Calcinosis cutis-
2. Calcinosis circumscripta |
|
|
Which calcinosis reported in large breeds; rare in cats. Deposit of calcium in CT of dermis, esp. at pres. points, and trauma?
|
Calcinosis circumscripta
seen at points of trauma, hence, considered dystrophic form (check this). |
|
|
Which calcinosis occurs in dogs with hyperadenocorticism. char. by mineralization of dermal collagen, epid. and follicular basement membranes?
|
Calcinosis cutis
occurs in dogs with hyperadenocorticism. |
|
|
Which calcinosis type can be either dystrophic or metastatic?
|
Calcinosis cutis
but most common cause of metastatic calcinosis cutis in dogs is overproduction of adrenal hormones (corticosteroids or “steroids”). . |
|
|
What is gout?
|
Gout = Deposit of uric acid and urates in tissues.
|
|
|
Gout common in ?
|
Common in birds, reptiles and occasionally in dogs and cats.
|
|
|
Two forms of gout:
(where is each found?) 1+3 |
1. Articular form- deposit in joints
2. Visceral form- serous membranes, liver, kidney |
|
|
Which might see around gout deposit (microscopically)?
|
Microscopically-granulomatous reaction around the deposit.
|
|
|
Exogenous pathological pigmentation formed by what compounds? (name 2 or 3)
|
Exogenous- pigment formed outside the body like carbon, dust, metal etc.
- characterized by fibrosis of tissue around granulomas (because body cannot remove) |
|
|
Endogenous pathological pigmentation formed by what compounds? (name 2 or 3)
|
Endogenous- pigment formed inside the body.
Like melanin (melanosis), blood pigments and lipogenic pigments. |
|
|
Five possible conditions which can lead to exogenous (dust, etc.) pigmentation?
|
1. Pneumoconiosis (dust in lung)
2. Anthracosis- carbon particles. 3. Asbestosis- dust from asbestos factories. 4. Silicosis- silicon from rock factories 5. Siderosis- iron dust from mines P-A-A-S-S |
|
|
Where might dog/cat get pneumoconiosis or anthracosis?
Which tissues? |
Dogs and animals exposed to <carbon particles> from coal mines and smoky cities. Mostly lungs, but anthracosis can be in lymph nodes also.
|
|
|
Which exogenous pigmentation does not stain and does not dissolve in any solvent?
|
anthracosis
I guess can try to treat pneumoconiosis with transtracheal wash? |
|
|
can get from mesotheliomas?
|
mesotheliomas is cancer of mesothelium from asbestos exposure
(asbestosis is just pigmentation from asbestos exposure) |
|
|
Melanin and melanosis result from endogenous pigmentation and usually seen in ?
|
Melanosis is pigmentation of melanocytes (bottom layer of epidermis) and is usually seen on lung, pleura, heart and meninges.
|
|
|
melanosis is harmful?
|
No, melanosis harmless; but tumors of melanocytes are life threatening.
|
|
|
tumors of melanocytes (melanoma) seen what spp.?
|
dogs and horses
e.g. melanoma on skin or liver |
|
|
CO poisoning causes problems how?
|
CO is inhaled, combines with Hb forming ‘Carboxyhemoglobin’ which deprives tissues of oxygen.
(Blood on CO poisoning is bright cherry red.) |
|
|
Poisoning by nitrites, chlorate, to less extent with acetoaminophen, local anasthetics and copper poisoning and other conditions cause ?
|
intravascular hemolysis and form methemoglobin, which is incapable of oxygen transport.
>ferrous iron (Fe-2) in hemoglobin is oxidized to ferric iron (Fe3) forming methemoglobin |
|
|
What is a porphyrin ?
|
Organic compounds, many naturally occurring. but best known is heme group (if u take away iron), what you have left is a porphyrin
(porphyrin + iron = heme) |
|
|
Hemochromatosis is ?
Caused by? |
Build up of iron pigment (bluish) in the cytoplasm of hepatocytes and epithelial cells of kidney.
- diet def. in copper/cobalt and rich in iron is pointed as cause. |
|
|
Hemochromatosis reported in what animals? (5)
|
Reported in cattle, buffalo, horse, goats and a few species of birds.
|
|
|
Formalin pigment (brownish) formed body by?
|
acid (low ph) and hemoglobin
e.g. usually seen in tissues fixed in acidic formalin. (does not stain with iron stain whatever that means) |
|
|
Parasite hematin?
|
believed to be excreted by parasites
|
|
|
Jaundice is the result of ?
|
either excess production (hemolytic condition) or reduced clearance of bilirubin such that it accumulates in the plasma.
|
|
|
3 Types of jaundice
|
1. Prehepatic
2. Hepatic 3. Post hepatic or obstructive jaundice |
|
|
Which type of jaundice caused by damage to hepatocytes and known as "toxic jaundice"?
|
Hepatic jaundice - damaged liver cells are incapable of conjugation process, and swollen liver cells block the bile flow.
|
|
|
Which type of jaundice char. by circulation of conjugated and unconjugated bilirubin?
|
Hepatic jaundice - increase in both conjugated and unconjugated bilirubin circulating in blood.
|
|
|
What does it mean that bilirubin is "conjugated"?
|
conjugated in liver (glucuronidation) so that it can be excreted
|
|
|
Laboratory tests for elevated bilirubin: (2)
|
1) Icterus index-spectrophotometry (quantitative test)
2) van den berg reaction- a qualitative test to differentiate type of jaundice. Specially in animals differentiation requires full investigation of blood and its chemical values. |
|
|
In dogs and cats- conjugated bilrubinemia can occur in hemolytic jaundice. How is this possible?
(check this) |
Maybe if hepatocytes able to conjugate even high levels of heme/bilirubin being produced by hemolysis, but hepatic duct cannot excrete efficiently as other animals.
|
|
|
Is bilirubin (in circulation) harmful?
|
Not in small quantities, but excessive amount causes itching and related conditions (esp. in young animals)
|
|
|
What is condition “porphyria”?
|
* Elevated levels of a photosensitizing pigment
* condition develops as a result of action of sunlight on fluorescent pigment present in body. |
|
|
condition “porphyria” reported in what animals?
|
All domestic animals except dogs
(congenital form in cats, pigs and cattle) |
|
|
What symptoms of “porphyria”?
|
* Lesions are dermatitis, starting as erythema, edema, developing to necrosis and sloughing.
* Strong effects are on part of skin unprotected by pigment, hair or fleece. |
|
|
Types of photosensitization?
|
1. Type I (primary photosensitization)
2. Congenital erythropoietic porphyria 3. Type II- Hepatogenous (secondary) |
|
|
Plasma flowing through kidneys stained dark red to reddish black and urine red, explain:
|
Excessive intravascular hemolysis, hemoglobin stains plasma pink
Similar condition is observed in myogolobinuria |
|
|
methemoglobin?
myoglobin? |
* formed when ferrous ion is oxidized to ferric ion (poisoning by nitrites, acetaminophen, anesth., etc.)
* product of muscle break down |
|
|
Caused by ingestion of some plant or drugs which give rise to photodynamic pigment in the body.
|
Type I (primary photosensitization):
|
|
|
Is Liver damage is necessary in type I (primary photosensitization)
|
No, liver damage is not necessary.
|
|
|
It is inherited metabolic defect in the synthesis of normal heme pigment.
|
Congenital erythropoietic porphyria (type of photosensitization):
inherited metabolic defect in the synthesis of normal heme pigment. |
|
|
Congenital erythropoietic porphyria seen in certain breeds of ?
|
Congenital erythropoietic porphyria seen in certain breeds of cattle, swine and short hair and siamese cats.
|
|
|
Cattle with congenital erythropoietic porphyria suffer from ?
|
Cattle suffer from dermatitis and anemia
|
|
|
Cats with congenital erythropoietic porphyria suffer from ?
|
cats have anemia and red coloration of bone/teeth (osteohemochromatosis <- misnomer)
|
|
|
Type II- Hepatogenous ( secondary photosensitization) seen in what animals?
|
Type II- Hepatogenous PS is seen in ruminants
|
|
|
Type II- Hepatogenous ( secondary photosensitization) caused by?
|
In ruminants develops as metabolic byproduct (phylloerythrin) from plant pigment chlorophyll, is removed by liver. Damaged liver fails to remove pigment, hence circulates in blood.
Note: this is simplified explanation |
|
|
Known as wear and tear pigment; or pigm. of brown atrophy, imparts color to affected tissues; mostly KIDNEY, brain, heart, skeletal and smooth muscles.
Believed to be formed from fatty acids derived from membranes of autophagocytosed organelles. |
Lipofuscin (a lipogenic pigment)
|
|
|
Because of its gross app. has been termed, "yellow fat dz” or “brown dog gut”.
Pigment is granular, yellow to brown in cell cytoplasm of heptocytes and in macrophages, fat cells, cardiac musc., smooth muscles of spleen and intestine. |
Ceroid pigment (a lipogenic pigment)
|
|
|
Seen in wasting disease, senility or emaciation.
|
Lipofuscin (a lipogenic pigment)
|
|
|
* Stains positive for fat, but resistant to fat solvent
* Reported in dog, horse, cattle, pig and rat. |
Ceroid pigment (a lipogenic pigment)
|
|
|
Pigment accumulation attributed to the deficiency of choline or vitamin E
|
Ceroid pigment (a lipogenic pigment)
|
|
|
Two types of extravascular fluid accumulation ?
|
Transudate (edema)
Exudate (inflammatory) |
|
|
Two types of vascular clots ? (masses/abnormal constituents in the blood/blood vessels)
|
Thrombus
Embolus |
|
|
Increase in volume of blood in tissues. It may be active/passive, local/general, acute/chronic.
|
Hyperemia
|
|
|
Abnormal accumulation of arterial Blood in an organ/tissue; can be physiological and pathological.
can be local and acute; affected organ/tissues are swollen and bright red. |
Active hyperemia:
|
|
|
Accumulation of blood in veins and capillaries; may be local or generalized. may be acute or chronic; affected part is dark red/blue and swollen
|
Passive hyperemia (passive congestion, congestion)
|
|
|
Which type of hyeremia always pathological?
|
Passive hyperemia (passive congestion, congestion)
|
|
|
Local passive hyperemia cause ? (1+3)
|
Cause: Obstruction of venous drainage due to:
a) organ misalignment (telescopy, volvulus (twisted on itself), torsion, herniation). b) venous thrombosis or embolism. c) compression. |
|
|
Effects of local passive hyperemia: (2)
|
1. Edema because of increased venous pressure.
2. Anoxia- degeneration and necrosis. Consequences depend on the organ involved. |
|
|
Intussusception is ?
|
part of the intestine has invaginated into another section of intestine
|
|
|
Causes of general passive hyperemia
|
Causes:
1. Cardiac failure- valvular, myocardial disorders. 2. Impeded venous return- caval thrombosis, hydropericardium. 3. Increased pulmonary resistance- hydrothorax etc. |
|
|
Effects of general passive hyperemia (3)
|
Effects of general passive congestion:
* reduced output (forward effect) * increased venous pressure (backward effect) * long term effects are in lungs and liver. |
|
|
area of tissue death (necrosis) due to a local lack of oxygen caused by obstruction of the tissue's blood supply
|
Infarction
|
|
|
Various terms used in connection with _____:
* _ by rhexis * _ by diapedesis * petechiae (1-2mm) * ecchymosis (>1cm) * purpura (bruise) * hematoma |
hemorrhage
|
|
|
Edema, abnormal accumulation of fluid in tissue spaces or body cavities, may be classified into what categories? (2&2)
|
Edema, accum. of extravascular fluid, may be:
1. Inflammatory OR non-inflammatory 2. Local OR generalized |
|
|
Which type of edema caused by loss of (renal disease) or decr. formation of protein (nutritional edema)?
|
Non-inflammatory edema
*renal diseases (renal edema) *certain parasitic infections |
|
|
How can parasitic infections cause non-inflammatory edema?
|
* loss of protein (nutrional edema)
* obstr. to lymphatic drainage |
|
|
Retention of Na&H2O - seen in congestive HF, nephritis and nephrosis - causes local OR generalized edema?
|
Retention of sodium and water causes 'generalized edema'
|
|
|
What type of edema is confined to a particular organ, e.g. hydrocephalus, pulmonary edema?
|
Local edema
|
|
|
Generalized edemas DO have predilection sites in different spp., what organ in cat?
|
cat - thoracic cavity (hydrothorax)
|
|
|
Generalized edemas DO have predilection sites in different spp., what organ in dog?
|
dog- peritoneal cavity (ascitis)
|
|
|
Generalized edemas DO have predilection sites in different spp., what organ in sheep/cattle?
|

sheep/cattle- sub-mandibular/peritoneum
|
|
|
Generalized edemas DO have predilection sites in different spp., what organ in horse?
|
horse- limbs
|
|
|
Anasarca is ?
|
generalized edema under the skin
|
|
|
edema in peritoneal cavity
|
Ascites
|
|
|
edema fluid in scrotal layers
|
Hydrocele
|
|
|
What might one see microscopically w/appearance of edema?
|
extracellular space is enlarged. It stains light pink homogenous.
|
|
|
extravascular fluid with:
- low protein content - low specific gravity (< 1.013) - lack of inflammatory cells |
transudate
|
|
|
extravascular fluid with:
- high protein content - high specific gravity (>1.018); - presence of inflammatory cells and clotting factors |
exudate
- high protein content - high specific gravity |
|
|
a mass formed from constituents of the blood in blood vessels or heart during life
|
Thrombus
|
|
|
What will this cause? Name?
1) alteration in the vascular endothelium 2) alteration in blood flow 3) alterations in the constituents of blood. |
Virchow`s triad --> thrombosis
|
|
|
Alterations in blood flow types/causes: (2)
|
Stasis
Turbulence - sites of branching of arteries, in arteriosclerosis, venous valves and congenital heart diseases. |
|
|
What type (color) thrombi formed in rapidly moving blood- usually in heart and arteries?
|
White or pale thrombi: fast blood
|
|
|
What type (color) thrombi formed in slow moving blood- usually in large veins?
|
red thrombi: in slow moving blood- usually in large veins.
|
|
|
Mural thrombi:
|
attached to a side of the vessel, not occluding the lumen completely.
|
|
|
Occlusive thrombi:
|
completely blocking the vessel.
|
|
|
In cattle thrombi in the vena-cava results from ?
|
the liver abscesses (skip until know other stuff)
|
|
|
resolution of thrombi via ___
|
fibrinolysin
|
|
|
Laminations form in post mortem clots OR thrombi?
|
Lamination of red and white thrombi; not PM
|
|
|
Prevents blood from clotting normally. May cause excessive (thrombosis) or bleeding (hemorrhage) throughout body and lead to shock, organ failure, and death.
|
Disseminated intra-vascular clotting
|
|
|
Disseminated intra-vascular clotting occurs in ?
|
DIC occurs in microvasculature.
|
|
|
___ is solid material carried by blood from origin to distant site within circulation.
|
embolism
|
|
|
Types or examples of emboli
|
Pieces of thrombus, fat, tumor cells, gas, bacteria and parasites.
|
|
|
Effects of emboli:
|
It mostly leads to infarction.
|
|
|
Infarcation more common in arteries or veins?
|
arteries (95% of cases)
both can in veins or both |
|
|
Infarctions cause what type of necrosis?
|
coagulative necrosis
|
|
|
clinical entity of body caused by inadequate perfusion of organs & tissue
|
Shock
|
|
|
secondary disorder (conseq.) resulting from large scale platelet activation a/o thromboplastin release for any reason (tissue damage)
|
Disseminated Intravascular Coagulation:
if fibrolytic protein used up in tissue, may get coagulation elsewhere |
|
|
DIC usually seen in what tissues?
|
microvasculature
|
|
|
Another name for DIC?
|
consumption coagulopathy
- if fibrolytic protein used up in tissue, may get coagulation elsewhere |
|
|
How to differentiate PM clot from thrombus?
|
PM clot not attached to vessel wall (unlike thrombi)
|
|
|
Clinical effects of DIC depend on balance btw. what 2 proteases?
|
thrombin (clot forming) and plasmin (fibrolytic; favors bleeding)
|
|
|
Shock often due to 1 of 3 events; list them:
|
1. Cardiogenic: Reduced cardiac filling or emptying
2. Hypovolaemic (reduced BP; blood loss) 3. Vasculogenic |
|
|
3 causes of vasculogenic shock?
|
1. neurogenic (dilation, deep anesth.)
2. endotoxic (infection) 3. anaphylactic (histamine) |
|
|
A piece of liver (from dead animal) floats when placed in formalin. What does this indicate?
|
fatty changes
|
|
|
What GSD is char. by deficiency in debranching enzyme?
|
Type 3 (Cori's Dz)!!!
>Don't confuse with branching enz. (type 4); DEbranching enzyme = amylo 1,6 glycosidase |
|
|
what type of amyloidosis associated with long standing condition (e.g. chronic inflammatory dz)?
|
secondary amyloidosis
|
|
|
type of amyloidosis occurring with plasma cell dyscrasias (dysfunction) e.g. multiple myeloma and B cell prolif. disorders?
|
primary amyloidosis
|

