![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
712 Cards in this Set
- Front
- Back
|
What is the most common cause of acquired, physical etiology? |
Trauma |
|
|
Macule |
a discolored spot on the skin or mucosa that isn't elevated above the surface |
|
|
Papule |
small, circumscribed, superficial, solid elevation of the skin or mucosa, measuring less than 1.0mm in diameter |
|
|
Plaque |
a small, circumscribed, superficial, solid elevation of the skin or mucosa, equal to or greater than 1.0mm in diameter |
|
|
Wheal |
smooth, slightly elevated area on the skin which is redder or paler than the surrounding area; usually associated w/ itchiness (pruritus) |
|
|
Nodule |
a small, protruding bump that is felt on the surface of an organ, skin or mucosa |
|
|
Mass |
a collection of solid tissue; may be sessile or pedunculated; endophytic or exophytic; very general term; anything that's a big collection of tissue |
|
|
Cyst |
an epithelial line cavity that appears as a mass; it is a type of mass that is closed |
|
|
Vesicle |
a clear, fluid-filled blister that is less than 5mm in diameter |
|
|
Bulla |
a clear, fluid-filled blister that is greater than 5mm in diameter |
|
|
Pustule |
a visible collection of pus within or beneath the epithelium |
|
|
Ulcer |
a local defect of the surface of an organ or tissue, which is produced by the sloughing of inflammatory necrotic tissue |
|
|
Leukoplakia |
a white area on the oral mucosa that will not rub off |
|
|
Erythroplakia |
an erythematous (red) area on the mucosa |
|
|
Sclerosis |
an indurated or hardened piece of tissue or organ |
|
|
Pathognomonic |
a particular sign whose presence means that a particular disease is present beyond any doubt; diagnostic quality |
|
|
What is the pathognomonic sign of Erythema multiform |
Target lesion; the defining feature of this disease |
|
|
What is the Diagnostic Sequence for Differential Diagnosis |
1. Chief complaint 2. History of Present Illness (duration of process) 3. Medical, social, family histories 4. Complete clinical examination 5. Evaluation and classification of disease/lesion 6. Develop list of differential diagnoses 7. Develop working or tentative diagnosis 8. Lab and special exams 9. Formulate final diagnosis 10. Tx plan and Tx |
|
|
What is the Pathogenesis of a disease |
Sequence of events in cells or tissues in response to the etiologic agents from the initial stimulus to the functional and structural abnormalities that characterize the disease |
|
|
What are the four adaptive responses that cells can have to stress |
Atrophy Hypertrophy Hyperplasia Metaplasia |
|
|
What are the two characteristics that determine a cell type's stress/adaptive responses |
Ability to turnover Regenerative capacity |
|
|
What is a cell's adaptive response if it has high turnover ability and high regenerative capacity |
Hyperplasia - still divide and replace cells |
|
|
What cells of the body adaptively respond to stress with hyperplasia (have high turnover) |
Bone marrow Epithelium Hepatocytes Fibroblasts Oral cavity tissues (buccal mucosa, tongue) |
|
|
What cells of the body exhibit low turnover and are responsive to stimuli |
Endothelium (blood vessel lining) Supportive cells (in bone, cartilage, smooth muscle) |
|
|
What cells of the body exhibit little or no ability to turnover |
Neurons Skeletal myocytes Cardiac myocytes |
|
|
Atrophy |
loss of cellular organelles and cellular size
(a reversible and adaptive response of cell to reduce mass of its functional cytoplasm by decreasing number/volume of organelles; decrease in cell size and then cell number) |
|
|
What is involution of the uterus after birth an example of |
Physiologic Atrophy (due to loss of hormonal stimulation after parturition) |
|
|
What is the loss of secondary sex characteristics with age an example of |
Physiologic atrophy (due to decrease in hormonal stimulation) |
|
|
What is decrease in muscle size of your calf after breaking your ankle an example of |
Pathologic atrophy (due to reduced functional demand) |
|
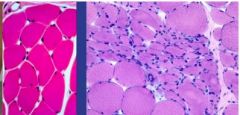
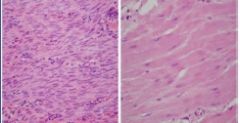
What has happened to the myocytes on the right slide if the cells on the left are normal |
Cells in the center have atrophied due to reduced functional demand (Pathologic Reversible Atrophy) |
|
|
Is it possible to reverse physiologic or pathologic atrophy |
Yes, you can reverse either through hypertrophy |
|
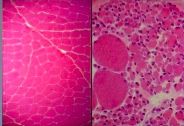
What has happened to the myocytes on the right if the myocytes on the left are normal |
Neurogenic atrophy (due to loss of innervation); Some myocytes have atrophied and some have hypertrophied (a compensatory mechanism)
atrophy --> hypertrophy of adjacent tissue |
|
|
Autophagy |
not getting an adequate supply of nutrients/O2 so cells respond by using less energy/use own energy |
|
|
What are the possible causes of pathologic atrophy |
Reduced functional demand Loss of innervation Inadequate supply of O2 Inadequate nutrition Loss of endocrine stimulation (testicular, breast) Aging (senile atrophy - brain/heart) |
|
|
What are the possible causes of physiologic atrophy |
Developmental change (thymus) Physiologic change (uterus after parturition) Aging change (involution of secondary sex traits) |
|
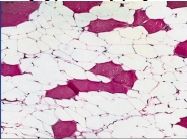
What has happened to these muscle cells |
Irreversible/Extreme atrophy after long period of immobilization; cell loss/death; white areas = fat inside muscle that was washed away |
|
|
What are the mechanisms of atrophy |
Decreased protein synthesis Increased protein degradation (ubiquitin ligase activation, proteasome digestion) Organelle recycling via Autophagy Cell death (extreme) |
|
|
What is the mechanism that underlies atrophy |
Autophagy - self-eating |
|
|
What is hypertrophy |
increase in organelle/cell SIZE
(increase in cell size leading to increase in organ size due to increase in organelle number/size; result of increased functional demand, hormonal stimulation; can be physiologic or pathologic; reversible if stimulus removed) |
|
|
What are the possible causes of hypertrophy |
Increased functional demand (lifting weights) Hormonal stimulation (increase in uterus size during pregnancy) |
|
|
What are the mechanisms behind hypertrophy |
Increased protein synthesis |
|
|
What can happen to the heart as a result of systemic hypertension |
Hypertrophy; (increased functional demand due to HTN leads to heart muscle hypertrophy in order to get blood to the system, which eventually leads to reduced functionality b/c of too much demand on the heart and CHF) |
|
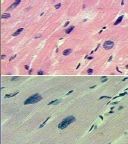
What has happened to these cardiac myocytes from the top slide to the bottom slide and why |
Hypertrophy; the cells on the bottom are larger and the nuclei are farther apart; due to increased functional demand |
|
|
Hyperplasia |
increase in cell NUMBER
(due to proliferation; cell growth both physiologic and pathologic; it is a controlled process, even if proliferation is abnormal; cells respond to a signal; when the signal is gone cells will stop proliferation; it is NOT cancer, but you do have increased risk of cancer) |
|
What two adaptive stress responses does the uterus undergo during pregnancy |
Hypertrophy and Hyperplasia (increase in cell size and increase in cell number) |
|
|
Neoplasia |
Uncontrolled proliferation of cell number; CANCER; you have increased risk of this with hyperplasia |
|
|
What are the possible causes of hyperplasia |
Hormonal Stimulation (uterus during pregnancy, lactating breast) Compensatory (blood donation, liver regeneration) Excessive Hormonal Stimulation (pathologic; benign prostatic hyperplasia) Excessive Growth Factor Stimulation |
|
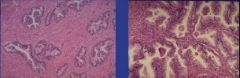
What has happened to these prostate cells |
Benign Prostatic Hyperplasia; pathologic; NOT cancer, but increased risk of cancer; expanded glandular elements; uneven hyperplastic growth areas (the whole tissue is not enlarged evenly like in lactating breast hyperplasia) |
|
|
Metaplasia |
replacement/reprogramming of cells
(replace of one adult cell type with another through an adaptive process - reprogram stem cells of normal tissue; reversible to an extent) |
|
|
What are the possible causes of metaplasia |
Chronic Irritation (smoker's tracheal epithelium, esophagus w/ GERD) Vitamin A deficiency (essential for bronchial epithelium) |
|
|
What adaptive cellular response often occurs to the tracheal epithelium of smokers or those with a Vitamin A deficiency |
Metaplasia; changed from ciliated columnar epithelium with functional goblet cells and cilia to stratified squamous epithelium (tougher but lacks functionality - more prone to infections of the lung) |
|
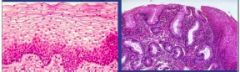
What adaptive cellular response has occurred from the esophageal epithelium on the left to that on the right |
Metaplasia due to GERD (gastroesophageal reflux disease); change from normal stratified squamous epithelium to epithelium that looks like glandular lining of the gut
can lead to Barrett's esophagitis if left untreated Tx with Prilosec |
|
|
What is chondroplasia |
Type of metaplasia where you have cartilagnious growth in place of normal tissue; e.g. - extra growth on gingiva when you remove denture (cartilaginous growth); chronic irritation from denture makes tissue that's stronger |
|
|
What are intracellular accumulations |
cells deal with materials they're unable to eliminate by intracellular digestion |
|
|
What are the possible causes of intracellular accumulations |
Abnormal metabolism (fatty liver in alcoholism - fat accumulates inside cells) Defect in Protein Processing (accumulation of proteins) Lack of Enzyme Processing (accumulation of endogenous materials) Ingestion of Indigestible materials (anthracosis - breathe in CO2 - coalminers - black lung) |
|
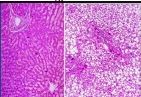
What has caused the normal liver cells on the left to change to those on the right in someone with chronic alcoholism/alcohol poisoning |
Intracellular Accumulations/Fatty Change/Steatosis
triglyceride accumulation in parenchymal cells; appears as clear vacuoles in the cells |
|
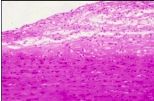
What has occurred here to the macrophages in smooth muscle in someone with atherosclerosis |
Intracellular Accumulations - phagocytic cells ingesting cholesterol but it cant be digested; clear cells formed with nuclei and nothing else; left with foam cells - major contributor to atherosclerosis |
|
|
What are four pigments that frequently accumulate in cells - intracellular accumulation |
1) Carbon - exogenous pigment; anthracosis
Endogenous: 2) Melanin (from oxidation of tyrosine in melanocytes; brown-black) 3) Lipofuscin ("wear and tear" - golden-brown; increases with age; in terminally differentiated, neuron, or infrequently cycling cells, hepatocyte) 4) Hemosiderin (excess iron - yellow/brown) |
|
|
What are five common types of intracellular accumulations |
1) Fatty Change (steatosis; triglycerides) 2) Protein (Russell Bodies - IgG accumulation in plasma cells; hyaline change) 3) Glycogen (hyperglycemic/uncontrolled diabetes) 4) Inherited lysosomal storage disease (incomplete breakdown of complex lipids b/c of lysosomal enzyme issues - Gaucher (cerebrosides), Tay-Sachs (gangliosides), Hunter diseases (mucopolysaccharides) 5) Pigments |
|
|
What are heat-shock proteins |
stress proteins made to help protect the cell from a particular stress (heat, oxidants, etc); some help to re-fold denatured proteins, some unfold proteins to allow transport into organelles; limits tissue necrosis by rescuing cells
Examples: chaperones = folding proteins ubiquitin - facilitates degradation of proteins denatured beyond repair |
|
|
What three things cause Cellular Aging |
1) DNA damage (ROS - oxidative stress; mutations in DNA repair enzymes) 2) Decreased replicative capacity/Cellular Senescence (telomere length/reduced telomerase activity) 3) Defective protein homeostasis (unfolded protein /ER/integrated stress response activated w/ defective protein folding - lose this with age) |
|
|
What are three possible ways to reverse cellular aging |
1) Reduce insulin/IGF signaling (stop eating) 2) Reduce activation of kinases (TOR and AKT) 3) Activation of Deacetylase
Increased metabolism leads to aging |
|
|
What is a reversible injury |
Injury where you can remove the injurious agent and the cell returns to normal |
|
|
What is an irreversible injury |
Cell does not return to normal after an injurious stimulus; either necrosis or apoptosis occurs |
|
|
Ischemia |
Loss of blood supply |
|
|
Which cell death pathway stimulates an inflammatory response: necrosis or apoptosis |
Necrosis - always pathologic and stimulates inflammatory response
(apoptosis can be pathologic or physiologic - does NOT stimulate an inflammatory response) |
|
|
What are the steps of a reversible injury |
1) ER and mitochondrial swelling, chromatin condense, form membrane blebs, form myelin figures 2) Recovery (when injurious stimulus removed) |
|
|
What are the steps of an irreversible injury that leads to necrosis |
1) ER and mitochondrial swelling, chromatin condense, form membrane blebs 2) Myelin figures form (phospholipid growths), amorphous densities in mitochondria, lysosome releases proteases 3)Plasma membrane ruptures, Nucleus contents leak (Cell death/Necrosis) - causes inflammatory response |
|
|
What are the steps of an irreversible injury that leads to apoptosis |
1) NO SWELLING; chromatin condense, form membrane blebs; cell SHRINKS 2)Cellular fragmentation - form apoptotic bodies (Apoptosis) 3) Apoptotic fragments Phagocytosed - NO inflammatory response |
|
|
What are the possible causes of Cell Injury |
Oxygen deficiency/Hypoxia Chemical agents Physical agents Nutritional Imbalances Infectious agents Immunological reactions Genetic factors Aging |
|
|
What is the one cause of Cell Injury that usually results in apoptosis rather than necrosis |
Genetic factors |
|
|
What is the most important cause of hypoxia |
Ischemica (loss of blood supply) causes hypoxia (oxygen deficiency) |
|
|
What three factors about a cell injury does the cellular response depend on |
Type Duration Severity |
|
|
How does ischemia effect the skeletal muscle and cardiac muscle differently |
2-3 hr for skeletal muscle cell injury could be reversible; 20-30 mins for cardiac muscle cells is irreversible |
|
|
What are the four main mechanisms behind cell injury and do they result in necrosis or apoptosis |
Result in Necrosis: 1. Mitochondrial damage - ability to generate ATP and ROS 2. Disturbance to Ca++ homeostasis 3. Membrane damage (plasma, mitochondrial, lysosomal)
4. Genetic damage; DNA damage and misfolding of proteins (only one that can result in apoptosis) |
|
|
What are the two major effects of mitochondrial damage that result in cellular necrosis |
Decrease in ATP generation Improper control of ROS |
|
|
What two injurious effects result from increase in Ca++ entry/disturbances in Ca++ homeostasis |
1) Increased mitochondrial permeability (decreased ATP production) 2) Activation of multiple cellular enzymes (membrane damage, nuclear damage, decreased ATP production) |
|
|
What results from plasma membrane damage and what results from lysosomal membrane damage |
Plasma membrane damage - loss of cellular components
Lysosomal membrane damage - enzymatic digestion of cellular components |
|
|
What is the name of the major event that causes mitochondrial damage, and in extreme cases, necrosis |
Ischemia (loss of blood supply)/Hypoxia (decreased oxygen supply) - mitochondria need nutrients/O2 to function |
|
|
What are three main examples of ROS |
superoxide anion (O2-) hydrogen peroxide (H2O2) hydroxyl anion (OH-) |
|
|
What is formed as a natural byproduct of normal metabolism of oxygen, but can damage the cell if not properly controlled |
Reactive Oxygen Species (ROS) |
|
|
When does a phagosome form, how, and what is its funtion |
Formed in response to bacterial infection; forms when a neutrophil engulfs bacteria; contains phagocyte (NADPH) oxidase, which generates radicals that kill the microbes in the phagosome |
|
|
What is the process of a phagosome (ROS) killing microbes known as |
Respiratory/oxidative burst |
|
|
How are the ROS from phagocytic leukocytes (phagosomes) different from the ROS in mitochondria |
Phagosome ROS function is to kill microbes Mitochondria ROS function is to produce energy |
|
|
Under what circumstances and how do ROS cause tissue injury |
Depends on rate of production and rate of removal |
|
|
How are ROS's normally removed |
By several enzymes in the mitochondria; eventually all converted to either H2O2 or H2O and then removed |
|
|
What are three different ways that free radicals cause injury |
1. Lipid peroxidation - membrane damage 2. Protein damage - breakdown, misfolding 3. DNA strand breaks - mutations, breaks |
|
|
What is it called when you observe small, clear vacuoles in the cytoplasm and cell swelling; is this reversible or irreversible |
Hydropic Swelling/Vacuolar degeneration; Reversible |
|
|
What is it called when you see lipid vacuoles in the cytoplasm and what cells does this typically occur in; is this reversible or irreversible |
Fatty change; occurs in cells that have fat metabolism, like hepatocytes and myocardial cells; Reversible |
|

What are the names of the two processes that have occurred in these liver slides from a reversible injury (normal liver is leftmost slide) |
Hydropic Swelling and Fatty Change |
|
|
What are the distinguishing features of cells that have undergone reversible injury (as opposed to normal cells and cells undergoing irreversible injury) |
Normal cells - homogenous, uniform spaces, nuclei and cell size
Reversible injury cells - empty spaces due to water/fluid, membrane blebs, lots of protein, cells swollen/larger
Irreversible injury cells - plasma membrane wouldn't be intact |
|
|
What are distinguishing morphological traits of a reversible cell injury manifested at the organ level |
Increased weight (Swollen/larger) - fluid accumulation in many cells Pallor (Lighter color) - cells compressing on capillaries so blood supply compromised |
|
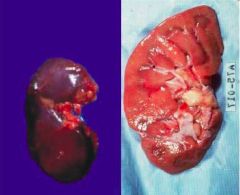
What has occurred to this kidney |
Reversible cell injury at organ level - increased weight (larger) and change in pallor (lighter) |
|
|
Damage to what parts of the cell signal that the damage is no longer reversible, and now irreversible |
Mitrochondrial or plasma membranes |
|
|
What two major things make a cell injury irreversible |
Mitochondria damage Plasma membrane ruptured |
|
|
What are the three types of Nuclear/DNA breakdown that indicate cell death |
Karyolysis Karyorrhexis Pyknosis |
|
|
What is karyolysis |
Nucleus has undergone lysis and no contents are observed; indicates cell death |
|
|
What is karyorrhexis |
Fragmentation of DNA within the nucleus; indicates cell death |
|
|
What is pyknosis |
Nucleus becomes smaller and stains very dark; indicates cell death |
|
|
In which of the following instances is the cell dead: pyknosis, karyorrhexis, karyolysis |
Cell is dead in all cases |
|
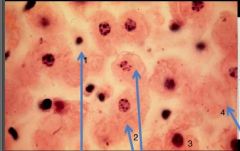
What nuclear status is depicted in cell #1 |
Pyknosis (small cell and nucleus) |
|
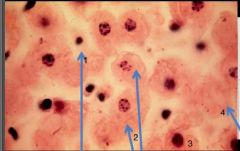
What nuclear status is depicted in cells #2 |
Karyorrhexis (DNA present but fragmented) |
|
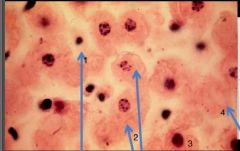
What nuclear status is depicted in cell #3 |
Normal; Alive |
|
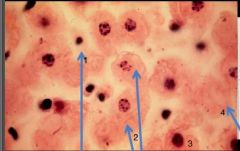
What nuclear status is depicted in cell #4 |
Karyolysis; no DNA |
|
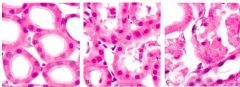
Three different states of kidney tubules are depicted. Describe each one |
Left: Normal Center: Reversible Injury (membranes still intact, swelling, lots of protein, empty spaces/fluid) Right: Irreversible Injury (most cells in karyolysis - no nuclei; segment of kidney is dying) |
|
|
What is the type of necrosis most commonly associated with hypoxia |
Coagulative necrosis |
|
|
What are two causes of hypoxia aside from ischemia |
1. Hypoxemia - deficient oxygenation of blood 2. Hemoglobin problems |
|
|
What is a localized area of coagulative necrosis due to loss of blood supply called |
Infarct (ischemic necrosis) |
|
|
What is a defining structural feature of coagulative necrosis |
Architecture/structure remains intact (ex: can still tell kidney tubules are tubules in coagulative necrosis); can occur in any tissue except brain tissue |
|
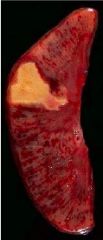
What has happened to this kidney |
Coagulative necrosis; in white, triangular area, pale and firm, tissue retains basic outline |
|
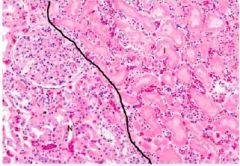
What has happened to the right side of this slide if the left is normal kidney tubules |
Coagulative necrosis: Right shows tubules missing nuclei, but can still determine the architecture of the cells/tubule
Left shows normal part of kidney with glomeruli, tubules, and neutrophils |
|
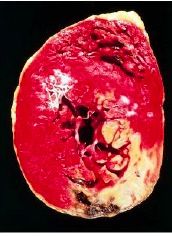
What has happened to the white area of this heart |
Coagulative necrosis - due to occlusion of the coronary artery and resulting ischemia; white area is dead tissue |
|
|
What type of necrosis involves the complete digestion of tissue; autolysis and heterolysis |
Liquefactive Necrosis; occurs following microbial infection and also in brain tissue/infarct |
|
|
In which type of necrosis is the tissue no longer recognizable: Coagulative or Liquefactive |
Liquefactive - tissue converted into a semi-liquid mass |
|
|
Which type of necrosis is often associated with microbial infection and a strong inflammatory stimulus |
Liquefactive |
|
|
What is the difference between neutrophil function in Coagulative vs liquefactive necrosis |
Coagulative - neutrophils remove dead cells and then repair tissue
Liquefactive - neutrophils release proteolytic enzymes and ROS and liquefy the tissue, you form an abscess |
|
|
In what type of necrosis do you form an abscess |
Liquefactive |
|
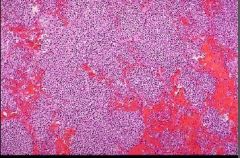
What type of necrosis is pictured here |
Liquefactive - VERY strong inflammatory response |
|
What type of necrosis is pictured here |
Liquefactive - VERY strong inflammatory response |
|

What has occurred to this lung tissue |
Liquefactive necrosis - has many abscesses |
|
|
What two types of necrosis does gangrene include |
Coagulative - on bottom (dry) Liquefactive - on top (wet) |
|
|
What part of gangrenous necrosis is associated with a foul smell |
Wet gangrene - liquefactive necrosis with bacteria |
|
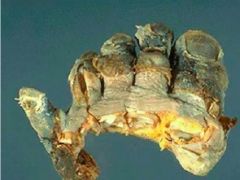
What condition is depicted here and what disorder does it often result from |
Gangrene (dry) Diabetes |
|
|
What characteristics describe caseous necrosis |
Immune injury; seen in granulomatous diseases (Tb and some fungal infections); cheese-like, white appearance; has a necrotic center enclosed within a granuloma; tissue architecture obliterated |
|
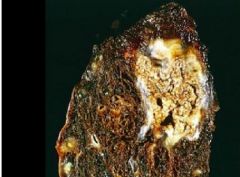
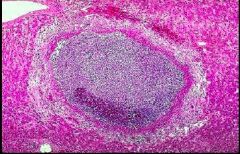
What condition is shown here resulting from tuberculosis |
Caseous necrosis |
|
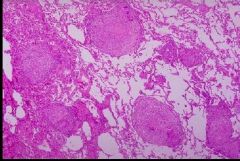
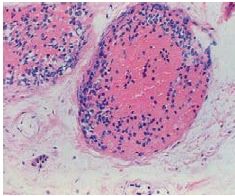
What type of necrosis is shown here and in what tissue type does it occur |
Caseous necrosis; in lung tissue; pictured are lung alveoli; large pink areas are granulomas with necrotic center |
|
|
Why is fibrinoid necrosis not actually a type of necrosis |
cell death doesn't occur; walls of arteries are damaged, allows plasma proteins to leak out; associated with immune injury and malignant HTN |
|
|
What type of necrosis only occurs in fat tissue and what is the mechanism behind it |
Fat Necrosis Results from injury that releases lipases that attack the plasma membranes of fat cells, hydrolyze triglycerides yielding free fatty acids, become calcium soaps usually associated w/ pancreatitis |
|
|
What are the two types of pathologic calcification and where do they occur |
Dystrophic Metastatic
Occur in necrotic tissue |
|
|
Where does dystrophic calcification occur and how does it occur |
locally, at areas of necrosis in presence of normal calcium-phosphorous metabolism; interferes w/ normal organ fxn formation of calcium phosphate mineral (similar to bone) |
|
|
Where does metastatic calcification occur and how does it occur |
in normal tissue, when there's hypercalcemia (e.g. - in hyperparathyroidism) occurs globally/not at one site formation of calcium phosphate mineral (similar to bone) |
|
|
What materials are released into the surroundings/ECM in apoptosis |
NOTHING; everything remains contained within apoptotic bodies then macrophages come to phagocytose the bodies |
|
|
Can necrosis and apoptosis be: physiologic, pathologic or either |
Necrosis - only pathologic Apoptosis - can be physiologic or pathologic |
|
|
What are the physiologic causes of apoptosis |
Embryogenesis Involution of hormone-dependent tissues Elimination of cells that served their purpose Removal of potentially self-reactive lymphocytes Killing of virus infected and neoplsatic cells by cytotoxic T cells |
|
|
What are the pathologic causes of apoptosis |
Cells damaged beyond repair (DNA damage and Misfolded proteins) |
|
|
What is involved in the extrinsic pathway of Apoptosis |
death-receptor initiated; cell surface receptors TNF and Fas ligand activated, activated adapter proteins, activate caspase cascade (eventually results in formation of apoptotic bodies) |
|
|
What is involved in the intrinsic pathway of Apoptosis |
mitochondrial pathway; activated by cell injury, activates Bcl-2 family, activates Bax and Bak (regulated by Bcl-2 and Bcl-x), interact w/ mitochondria to increase permeability, releases cytochrome C and pro-apoptotic proteins, both activate parts of caspase cascade (eventually results in formation of apoptotic bodies) |
|
|
What is the result of the caspase cascade |
Initiator caspases activated, then executioner caspases activated, then endonucleases activated which results in DNA fragmentation AND cytoskeleton breakdown (eventually results in formation of apoptotic bodies) |
|
|
What are the cardinal signs of inflammation |
Redness Swelling Pain Heat Loss of Function |
|
|
What are the two responses involved in inflammation, which comes first |
Vascular then Cellular |
|
|
What is the most common and most important cause of inflammation |
Infection (bacterial) |
|
|
What must tissue be in order to undergo inflammation |
Living |
|
|
What are the two most important reasons for inflammation |
Control Infection (destroy and eliminate cause/remove dead tissue) Initiate wound healing |
|
|
What is the purpose of inflammation when caused by bacteria versus necrotic tissue |
Bacteria - destroy and eliminate Necrotic tissue - remove dead tissue |
|
|
What are two reasons why inflammation might backfire and hurt the host, give clinical examples |
Reaction too powerful (bacterial meningitis, staph pneumonia)
Inappropriate response (hay fever, asthma, anaphylaxis, arthritis, autoimmune diseases) |
|
|
What are the steps that occur during the vascular phase of inflammation |
Injury/Stimulus Transient, reflex vasoconstriction Vasodilation (due to mast cells/histamines) Increased blood flow (through arterioles) THEN Increased blood volume (hyperemia) - leads to redness and heat AND Increased vascular permeability (to protein) Edema/Exudate in extravas spaces - leads to swelling |
|
|
What cells are most important during the vascular response of inflammation |
Mast cells |
|
|
What cells predominate the cellular phase of inflammation |
Neutrophils |
|
|
What role do mast cells play in inflammation |
Activated almost immediately by anything that causes inflammation; have granules, histamines, and leukotrienes and release contents VERY rapidly - cause vasodilation and increase vascular permeability |
|
|
Why are women more likely to develop gingivitis when pregnant than when not |
Increased hormonal effect; hormones cause an increase in bacteria in gingival crevices |
|
|
What is the difference in blood flow in microvascular beds in normal versus inflamed tissue |
Normal - blood flow is intermittent; arteriolar/pre-capillary sphincters normally opening and closing in response to mediators
Inflamed - sphincters remain open, blood volume increases (active hyperemia) |
|
|
What is it called when more blood is brought to an affected area due to dilation of arterioles |
Hyperemia |
|
|
What does Starling's Law explain and what is the purpose |
Exchange of substances between blood and tissues:
Hydrostatic Pressure = Osmotic Pressure + Lymphatic Drainage (in normal tissue)
Keeps water from accumulating in tissues |
|
|
What are normal capillaries freely permeable to |
water, gases and electrolytes (only semi-permeable to proteins; about 90% retained) |
|
|
What do capillaries become permeable to during inflammation that they are not normally freely permeable to |
Plasma proteins |
|
|
What morphological characteristics of inflammation result from hyperemia |
Redness and Heat |
|
|
What is edema primarily caused by |
the Loss of Barrier Functions of capillaries and venules |
|
|
What is exudate |
protein-rich edema fluid; occurs when plasma proteins accumulate in tissues due to increased permeability of venules and capillaries to protein (result of inflammation) |
|
|
What components of Starling's law change during inflammation |
Hydrostatic pressure - marked increase; forces more fluid out from capillaries into tissues
Osmotic pressure - decreases due to leakage of plasma proteins into tissues (draw more fluid out of vessels and favor fluid retention in tissues)
Lymphatics try to compensate for changes but can't
Result is protein-rich edema fluid/exudate in tissue |
|
|
What are the three different types of exudate |
Serous Fibrinous Purulent |
|
|
What is the content of serous exudate |
High in fluid Few proteins No WBCs |
|
|
What is the content of fibrinous exudate |
High protein number Some fluid Few or no WBCs |
|
|
What is the content of purulent exudate |
High WBC number Some proteins Some fluid |
|
|
What are the physical characteristics of serous exudate/inflammation |
yellow, straw-like color; ex: skin blister resulting from a burn |
|
|
What are the physical characteristics of fibrinous exudate/inflammation |
fibrin is dominant feature; whitish material, like wet paper; ex: bread and butter pericarditis |
|
|
What are the physical characteristics of purulent inflammation |
Yellow-green color; PUS; usually associated w/ bacterial infection within a thin membrane; filled with neutrophils; mostly liquefactive necrotic tissue; ex: bacterial meningitis - in meninges of brain |
|
|
Which type of exudate is associated with death |
Purulent; usually occurs in brain cavity (bacterial meninges) - too much pressure causes death |
|
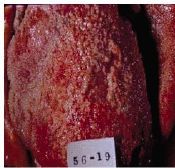
What condition does this heart depict |
Fibrinous exudate/inflammation (bread and butter pericarditis) |
|
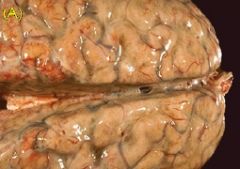
What condition does this brain depict |
Purulent exudate/inflammation (due to bacterial meningitis) |
|
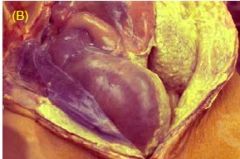
What condition does this ruptured colon depict |
Purulent exudate/peritonitis |
|
|
What is an abscess |
Localized collection of pus (purulent exudate is NOT localized) - caused by pyogenic organisms or infection from necrotic tissue (liquefactive necrosis) |
|
|
What is transudate |
Edema associated with non-inflammatory conditions; has NO protein (no vascular permeability) |
|
|
What are some of the mechanisms that result in transudate |
Abnormal Fluid Dynamics: Increased hydrostatic pressure (not compensated for by osmotic pressure or lymphatics) Problems with lymphatic drainage Problems with osmotic pressure |
|
|
What are five clinical conditions that have production of transudate |
1) Congestive Heart Failure 2) Kwashiorkor/Malnutrition 3) Liver Cirrhosis 4) Breast Cancer (scarring OR obstruction of lymph nodes) 5) Filariasis/Elephantiasis |
|
|
Why is transudate produced in congestive heart failure |
Increased hydrostatic pressure (b/c of left or right sided heart failure) |
|
|
Where does transudate manifest in left and right sided heart failure respectively |
Left: transudate in lungs Right: transudate in lower limbs |
|
|
Why is transudate produced in Kwashiorkor and Liver Cirrhosis and what are the clinical manifestations of transudate in these conditions |
Malnourishment and liver damage result in low level of plasma proteins - leads to reduced Osmotic pressure
Ascites - fluid accumulation in peritoneal cavity (can also include exudate accum from infection) |
|
|
Why is transudate produced in lymphatic obstruction (either from breast cancer tx and damage of lymph nodes or obstruction of lymph nodes by cancer) |
Lymphatic drainage cannot function effectively |
|
|
Why is transudate produced in Filariasis/Elephantiasis |
Lymphatic drainage cannot function effectively Filariasis = worm infection that invades lymph nodes results in fluid drainage to lower extremities (Elephantiasis) |
|
|
What is the difference between active and passive hyperemia |
Active - blood accum due to dilation of arterioles/mast cells and mediators
Passive - blood accum due to increased blood pressure and gradual accum of fluid |
|
|
What important mediator of vascular permeability is preformed in secretory granules |
Histamine |
|
|
What are the two most important cell types that produce histamine |
1) Mast cells 2) Basophils |
|
|
What are the four primary cell-derived mediators of vascular permeability |
Histamine Prostaglandins Leukotrienes Cytokines |
|
|
What are the primary plasma protein-derived mediators of vascular permeability |
Complement (C3a, C5a, C3b, C5b) Factor XII (Kinin and Plasmin system) |
|
|
What cell(s) produce prostaglandins/leukotrienes |
All leukocytes Mast cells |
|
|
What cell(s) produce cytokines |
Macrophages, lymphocytes, EC, mast cells |
|
|
From what organ are plasma proteins typically derived |
Liver |
|
|
In what order are the five major mediators of vascular permeability released |
1) Histamines 2) Prostaglandins/Leukotrienes 3) Plasma 4) Cytokines 5) ROS |
|
|
Which mediators of vascular permeability take the longest to to be synthesized of prostaglandins/leukotrienes, cytokines and histamines |
Cytokines |
|
|
During what phase of the inflammation process does histamine play the largest role |
earliest during permeability change; during acute inflammation |
|
|
Where are mast cells typically located in the skin |
dermal layer; surrounding blood vessels and close to nerve cells |
|
|
What stimuli trigger the release of histamine from mast cell granules |
Physical injury/trauma Neuropeptides/Substance P Complement fragments |
|
|
What are anti-microbial peptides (AMPs) |
peptides released by epithelial cells to kill microbes; provide first layer of defense during infection; also activate mast cells to release mediators |
|
|
What two substances play a role in immediate defense after initial infection and help to activate mast cells/histamine release |
Anti-microbial peptides Complement (C3a and C5a) |
|
|
In what scenario is histamine release unfavorable |
In response to an allergen/synthesis of IgE |
|
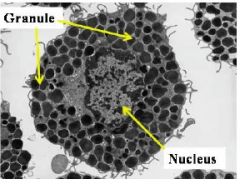
What type of cell is this |
Mast cell - prominent nucleus; contains granules; tissue resident |
|
|
What is the mechanism of a mast cell |
First - rapid, transient release of histamine
released contents, other released mediators, cause increased dilation of arterioles, active hyperemia, increased vascular permeability to proteins, formation of exudate |
|
|
How is arachidonic acid formed |
formed when phospholipases activated and hydrolyze membrane phospholipids |
|
|
What are the two pathways by which arachidonic acid is metabolized |
Cyclooxygenase - produce prostaglandins and thromboxanes Lipoxygenase - produce leukotrienes |
|
|
What are the names of the two lipid-derived mediators of vascular permeability that follow release of histamine, and what additional functions do they have |
Prostaglandins - Vasodilation (same effects as histamine, just later); inhibits platelet aggregation Leukotrienes - Neutrophil chemotaxis; bronchospasm |
|
|
What are the potent inhibitors of prostaglandins and leukotrienes |
Corticosteroids |
|
|
What is the function of aspirin and NSAIDs |
anti-inflammatory drugs; inhibit all upstream cyclooxygenase activity - no inflammation |
|
|
What is the purpose of drugs that block cysteinyl leukotrienes (LTC4) |
Manage asthma - prevent bronchospasms |
|
|
What are the three major plasma protease systems |
1) Kinin 2) Fibrinolytic/Plasmin 3) Complement |
|
|
What do plasma protease systems depend on |
Formation of biologically active peptide fragments in body fluids by the action of proteolytic enzymes on their substrates
Normally proteases are inactive; upon activation they produce peptide fragments that mediate permeability and other inflammatory changes |
|
|
What is the name of the proteases (and their inactive form) that act to form bradykinin |
Kallikreins (from inactive Pre-K) |
|
|
What is the name of the proteases (and their inactive form) that act to form peptides |
Plasmins (from inactive plasminogen) |
|
|
What does Factor XII do and where is it produced |
Activates the proteases (forming kallikrein and plasmin) that eventually form bradykinin and peptides
Liver |
|
|
What does bradykinin do in the inflammation process |
Causes pain and increased vascular permeability |
|
|
What do peptides do in the inflammation process |
Increase vascular permeability, mediate WBC chemotaxis, activate the complement system |
|
|
What are the two major cytokines involved in endothelial activation during inflammation |
Tumor necrosis factor-alpha (TNF-alpha) Interleukin-1 (IL-1) |
|
|
What is the principle function of cytokines in inflammation |
Endothelial activation - cause upregulation of cell surface adhesion molecules, promotes adhesion of neutrophils and monocytes |
|
|
What cells are TNF-alpha and IL-1 mainly produced by |
Mast cells and macrophages |
|
|
What is an inflammasome |
a molecular complex involved in the activation of inflammatory caspases resulting in processing of immature pro-IL-1 into mature IL-1 |
|
|
Describe the two pathways that result in the production of cytokines |
1) Bacterial cell wall lipids activate Toll-like receptor (on plasma membrane, endosomal membrane or in cytosol), (e.g. produces TNF) 2) Inflammasome pathway activated by extracellular pathogenic ATP - inflammatory caspases activated by K+ efflux or production of ROS (e.g. converts immature pro-IL-1 to mature IL-1) |
|
|
What are the primary functions of Vascular Endothelial Growth Factor (VEGF) |
Increases vascular permeability: transcytosis of proteins across the endothelium AND stimulates formation of new microvessels (inherently leaky) |
|
|
In what blood vessels do most of the vascular permeability changes occur |
Venules (except when cause is direct injury - could be any blood vessel) |
|
|
What is the most important cause/mechanism of vascular permeability |
Gaps/Endothelial Contraction; involves the rapid, transient response of histamines and leukotrienes; endothelial cells move away from one another, creating gaps in the venules |
|
|
What growth factor increases transcytosis of proteins across the endothelium AND stimulates formation of new microvessels |
Vascular Endothelial Growth Factor (VEGF) |
|
|
What is a clinical example of a delayed prolonged mechanism behind vascular permeability |
mild sunburn (sun damage to endothelial cells releases cytokines which take a long time to appear)
thermal injury, UV radiation |
|
|
What mechanism of vascular permeability causes an immediate transient response |
Gaps/Endothelial Contraction; immediate release of histamines and leukotrienes
Short-lived, after mild injury; reversible |
|
|
What mechanisms of vascular permeability cause an immediate prolonged response |
Endothelial contraction, Endothelial retraction and Leukocyte-dependent injury
response to bacterial infection; biphasic because you have histamine release, cytokine release, and neutrophil recruitment
moderate injury; can last for hours or days |
|
|
What mechanism of vascular permeability causes and immediate sustained response |
Direct injury; injury to the blood vessel causes leakage that will be sustained until the blood vessel is repaired
severe injury; can last for hours or days |
|
|
What mediator system only produces vascular permeability |
None; all mediator systems have multiple inflammatory effects |
|
|
What are the four major systemic protective effects of inflammation |
1) Fever (cytokines work on brain) 2) Increased plasma levels of acute-phase proteins (cytokines work on liver) 3) Leukocytosis (cytokines work on bone marrow) 4) Increased HR and BP |
|
|
What mediator is primarily responsible for the systemic effects of inflammation |
Cytokines |
|
|
Does most antimicrobial defense occur in the bloodstream or in the tissues |
Tissues - most infections originate in the tissues outside the bloodstream - inflammatory response goes to tissue and attempts to prevent microbial spreading beyond initial site of infection |
|
|
What are the major systemic pathologic effects of inflammation |
Low cardiac output Low peripheral resistance Blood vessel injury Thrombosis ARDS
SEPSIS |
|
|
What is the purpose of producing fever, more acute-phase proteins in plasma and more neutrophils (leukocytosis) during inflammation |
All systemic protective effects of inflammation
Fever - tells body something is wrong Acute-phase proteins - help fight infection Neutrophils - help fight infection |
|
|
What is the mechanism behind a fever |
LPS released from cell wall - causes release of TNF-alpha and IL-1, metabolize AA, produce Prostaglandin E2 - releases NTs in hypothalamus - resets body's thermostat to a higher temp |
|
|
What happens during rouleaux and what is the purpose |
(+) Fibrinogen binds to (-) RBCs, RBCs clump together
this increases Erythrocyte Sedimentation Rate (ESR) |
|
|
What is the normal number of WBCs in the blood and what number does this increase to when fighting infection |
4,500-11,000 to 15,000-20,000 |
|
|
What are some of the systemic effects of increased HR and BP during inflammation |
Decreased sweating Shivering (reduced blood supply to skin) Malaise Anorexia |
|
|
What are examples of acute-phase proteins |
C-reactive Proteins and Serum Amyloid (microbe elimination)
Fibrinogen (rouleaux)
All work to fight microbial infection; number increased 100-fold during infection |
|
|
In what stage of the inflammatory response are neutrophils most important |
Acute inflammation |
|
|
What are the most predominant cell type found in the blood |
Neutrophils |
|
|
What types of cells stain pink and blue respectively |
Eosinophils (pink), Basophils (blue) |
|
|
What three cell types are considered granulocytes |
Neutrophils, basophils, eosinophils
All contain granules; but have different nuclei and granule characteristics |
|
|
What color do neutrophils stain |
Don't stain/neutral |
|
|
What characterizes an eosinophil and what percent of WBCs do they constitute |
stain pink; lobulated nucleus, horseshoe shape; granulocyte
1-4% of WBCs |
|
|
Besides fighting infection/inflammatory what other roles do eosinophils play in the body |
fight infection against worms and protozoa; impt role in allergic diseases and asthma |
|
|
What characterizes a neutrophil and what percent of WBCs do they constitute |
Polymorphonuclear Leukocyte - multilobular nuclei; granulocyte
40-60% of WBCs
|
|
|
What characterizes a basophil and what percent of WBCs do they constitute |
stain blue with H&E stains; share many characteristics of mast cells (release histamine when activated) - associated wtih allergic rxn; granulocyte
0.5-1% of WBCs |
|
|
What two cell types are considered mononuclear/round cells |
Monocytes Lymphocytes |
|
|
What characterizes a monocyte and what percent of WBCs do they constitute |
prominent nucleus, kidney-bean shaped; largest cell; eventually leave bloodstream and become macrophages; impt in induction of immune response and wound healing
2-8% of WBCs |
|
|
What type of cell is capable of differentiating once it leaves the bloodstream and enters the tissue |
Monocyte (to macrophage) |
|
|
What types of cells besides macrophages do monocytes serve as precursors to |
Kupffer cells in liver Osteoclasts in bone |
|
|
What characterizes a lymphocyte and what percent of WBCs do they constitute |
Subtypes: T & B cells, plasma cells, NK cells, T helper cells, T suppressor cells; regulate immune response and guard against mycobacteria, viruses; primarily involved in chronic inflammation
20-40% of WBCs |
|
|
What "cells" are not true cells/leukocytes |
Platelets; involved in clot formation and wound healing |
|
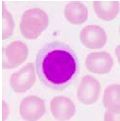
What type of cell is pictured here |
Lymphocyte - round nucleus |
|
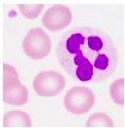
What type of cell is pictured here |
Neutrophil - multilobular nucleus |
|
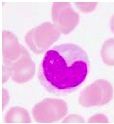
What type of cell is pictured here |
Monocyte - kidney bean shaped nucleus |
|
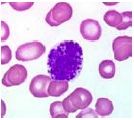
What type of cell is pictured here |
Basophil - blue stained granules |
|
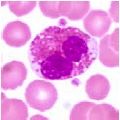
What type of cell is pictured here |
Eosinophil - pink stained granules |
|
|
Which of the cardinal signs of inflammation does vasodilation cause |
Redness |
|
|
Which of the cardinal signs of inflammation does increased blood flow (hyperemia) cause |
Heat |
|
|
Which of the cardinal signs of inflammation does fluid exudation cause |
Swelling |
|
|
What is the purpose of: red and white blood cells transported as a lubricated column and positioned toward the center of the stream |
Laminar Flow |
|
|
Why does bloodflow slow down in the microcirculatory bed during inflammation |
RBC concentration in the vessels increases b/c of increased vascular permeability and mvmt of proteins and fluid to tissue - blood becomes more viscous |
|
|
What is the presence of numerous dilated small blood vessels packed with RBCs and slow flowing blood called |
Stasis |
|
|
What is it called when RBCs stick together to form clumps and what does this lead to |
Rouleaux, leads to increased Erythrocyte sedimentation rate (ESR) |
|
|
What is the space between columns of blood cells and platelets called and what does it do |
Plasma - acts as a lubricant to assist laminar flow |
|
|
What acute phase protein clumps together with RBCs during rouleaux |
Fibrinogen |
|
|
What action by RBCs assists with the margination of WBCs |
Rouleaux - clumping of RBCs pushes WBCs toward the endothelial wall |
|
|
When vascular permeability is coming to an end, what can get through still and what can't |
Cells/leukocytes can get through, proteins can't anymore |
|
|
What is diapedesis and what cells undergo this process |
Transmigration - crossing from blood vessel to tissue; Leukocytes do this |
|
|
What are the three major steps of the leukocyte-endothelial cell adhesion cascade |
Rolling Adhesion Transmigration |
|
|
What are the three types of selectin, where do they occur, and what is the purpose of selectin |
E-selectin: endothelium P-selectin: platelets and endothelium L-selectin: lymphocytes
Adhesion molecules |
|
|
Why is the initial adhesion process of leukocytes to the endothelial surface described as rolling |
Leukocytes bind to selectins via carbohydrate ligands (sialyl-lewis X) through low-affinity interactions with a fast off-rate; adhesion easily disrupted by blood flow; leukocytes detach and bind again - "roll" along endothelial surface |
|
|
What type of selectin is first stimulated to go to the endothelial cell surface by mediators like histamine, thrombin and platelet activating factor (PAF) in order to recruit leukocytes |
P-selectin - redistribute from W-P bodies (intracellular store in granlues) to surface of endothelial cell |
|
|
What type of selectin is expressed within 1-2 hours after injury on endothelial cells by macrophage/mast cell release of TNF-alpha and IL-1 (cytokines) |
E-selectin |
|
|
What are the two endothelial adhesion molecules of the immunoglobulin family and what is their function |
ICAM-1 and VCAM-1 (intracellular/vascular cell adhesion molecules) - found on endothelial cells
serve as ligands for integrins found on leukocytes |
|
|
What is Sialyl-Lewis X and where is it found |
Glycoprotein found on leukocytes; selectins bind to this - leads to rolling/slight adhesion |
|
|
What integrins bind to ICAM-1 |
Beta-2 integrins; LFA-1 (CD11a and CD18) and Mac-1 (CD11b and CD18) |
|
|
What integrins bind to ICAM-2 and where is this integrin expressed |
Beta-1 integrins; VLA-4
mostly in granulocytes, but also in monocytes and macrophages |
|
|
What are integrins |
glycoproteins made of alpha and beta chains that are expressed on many cell types and bind to ligands (adhesion molecules) on endothelial cells |
|
|
What are Sialyl-Lewis X and integrins attached to vs what are adhesion molecules (selectins and ICAM/VCAM) attached to |
Sialyl-Lewis X and integrins presented on leukocytes
Adhesion molecules presented on endothelial cells |
|
|
Integrins are normally expressed on leukocytes in a low-affinity state, what induces their expression in a high-affinity state |
Chemokines - produced at site of injury that enter the blood vessel, bind to the endothelial cells, and are displayed at high concentrations on endothelial cell surface - induce change to high affinity state (receptor shape changes) |
|
|
What do integrins presented on leukocytes have affinity for |
ICAM-1 and VCAM-1 (on endothelial cells) |
|
|
What is the difference between affinity and avidity |
Affinity is a single protein-protein interaction
Avidity is interaction between multiple ligands and receptors at the same time (tighter overall interaction) |
|
|
What do chemokines do to induce a higher affinity state of integrins for adhesion molecules |
Change conformation/shape of integrin receptor (increased avidity) |
|
|
What does the increased avidity between integrins and adhesion molecules do to the leukocytes attached to the endothelial cells |
Stop rolling, create firm adhesion, leukocytes flatten |
|
|
What mediators act on adherent leukocytes to stimulate the cells to migrate through interendothelial spaces toward site of injury/infection |
Chemokines |
|
|
What is the name of homophilic adhesion molecules and where are they located |
PECAM-1 (platelet endothelial cell adhesion molecules)/CD31
Intercellular junction of endothelium AND Leukocyte surfaces |
|
|
Must there be vascular permeability in order for leukocytes to transmigrate |
No. But they do typically correlate |
|
|
What happens to leukocytes after PECAM-1 molecules bind to each other |
Migrate across endothelium |
|
|
How do leukocytes most likely pierce the basement membrane of endothelium |
secrete collagenases |
|
|
What is the net result of leukocyte transmigration |
leukocytes accumulate where they're needed |
|
|
What do leukocytes do temporarily after they transmigrate/leave circulation |
Surround/Cuff the vessel |
|
|
All the leukocytes express integrins and endothelial cells have ligands for them, which cells appear first and which appear later |
Depends on age of inflammatory response and nature of etiology
In acute inflammation: neutrophils appear first (6-24 hrs) As injury ages: monocytes appear (24-48 hrs) Infection by pseudomonas organism: neutrophils may continue to be recruited for several days Viral infection: lymphocytes Allergy/asthma: eosinophils |
|
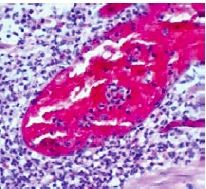
What stage of an inflammatory response is this/what stage of transmigration are the leukocytes in and what type of WBCs are they |
Cuffing the BV - after transmigration Neutrophils |
|
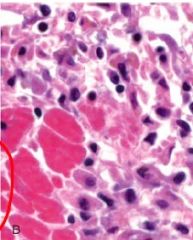
The large pink cells are myocytes that have undergone karyolysis; what other cells are present here and why/what stage of inflammatory response is this |
Mostly monocytes (prominent, round nuclei) - later stage of inflammatory response because monocytes have replaced neutrophils and come to clean up the mess |
|
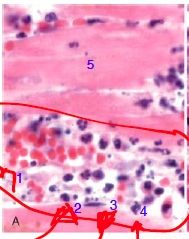
What types of cells are (1) and what is happening to them What types of cells are (2-4) and what is happening to each What type of cell is (5) and what has happened to it |
(1) - Red blood cells; in Rouleaux (2-4): Neutrophils (2): marginating/rolling (3): firm adhesion (4): just transmigrated (5): myocyte - karyolysis |
|
|
Which disease is a rare autosomal recessive disease that causes reduced expression of CD18 on the surface of leukocytes; and what is the pathogenesis |
LAD-I; decrease in firm adhesion to the endothelium |
|
|
What disease manifests as: delayed separation of the umbilical cord, recurrent bacterial infection, gingivitis/periodontitis, absence of pus formation and impaired wound healing, leukocytosis/neutrophilia |
LAD-1 (severity depends on extend of CD18 deficiency) |
|
|
What percent CD18 deficiency constitutes severe deficiency |
98% deficiency in CD18 |
|
|
What percent CD18 deficiency constitutes mild/moderate deficiency |
70-98% deficiency in CD18 |
|
|
How are neutrophils in tissue usually dealt with after they have served their purpose |
Efferocytosis of apoptotic neutrophils in tissue by macrophages (after 1-2 hours) |
|
|
What is the pathway of mediators that acts as a thermostat and is inhibited when neutrophils are undergoing efferocytosis |
IL-23 to IL-17 to G-CSF (which results in production of neutrophils in bone marrow and release of mature neutrophils to circulation) |
|
|
With LAD-1 what happens to the cytokine pathway that mediates the production and release of neutrophils |
In LAD-1 there are no/few neutrophils in the tissues because they are unable to transmigrate from the blood b/c deficient CD-18; therefore no neutrophils are undergoing efferocytosis, and the cytokine mediator pathway cannot be inhibited, so there's an increased production of neutrophils from the bone marrow that are circulating in the blood (Neutrophilia) |
|
|
What is the result of the upregulation of IL-17 in the gingival areas (due to LAD-I) |
Bone resorption/Periodontal bone loss; TH-17 cells secrete cytokine IL-17 which produces increased amounts of neutrophil in the blood but they can't function properly |
|
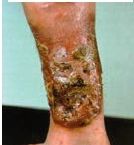
This person has LAD-I, what is the clinical diagnosis of their tissue |
Dysplastic Eschar - dead tissue sloughing off; develops b/c there's a wound and neutrophils can't get there to remove dead tissue |
|
What is the clinical diagnosis of this situation - elevated neutrophil count in the blood but neutrophils unable to leave blood vessel |
LAD-I (gingivitis/periodontitis) |
|
|
What are proposed treatments for LAD-I and how would they work |
Antibiotics - antibody against IL-17 to protect from bone loss
Bone marrow/hematopoetic cell transplantation; replace the dead bone marrow tissue |
|
|
What is the disease that is a rare autosomal disease in which the expression of Sialyl-Lewis X is completely deficient, meaning they fail to interact with selectins |
LAD-II |
|
|
What results from LAD-II deficiency of expression of Sialyl-LewisX mechanistically |
Defective rolling; E and P selectins don't adhere, but neutrophils still able to adhere and transmigrate via integrins/ICAM under conditions of reduced sheer force; allows for some defense against bacterial infections |
|
|
What are the clinical manifestations of LAD-II |
Less severe than LAD-I; no delay in umbilical cord separation; bacterial infection NOT life-threatening
Add'l: children have delayed motor development, mentally retarded, short in stature |
|
|
What is disease is a rare genetic disease in which there is a genetic loss in activation of beta-integrin by chemokines (mutation in kindlin) and in which patients have a defect in chemokines |
LAD-III |
|
|
What are the clinical manifestations of LAD-III |
Similar to LAD-I b/c defect in neutrophil adherence mechanism; (add'l defect in platelet aggregation can cause cerebral hemorrhage at birth and bleeding disorders) |
|
|
In diagnosis, how can you distinguish LAD-I from LAD-III |
In LAD-III: integrin expression is intact but activation impaired; genetic analysis for mutation in kindlin |
|
|
What is granulopoiesis |
when neutrophils produced in the bone marrow are released into circulation |
|
|
What are all of the steps neutrophils must accomplish in order to kill bacteria and prevent infection |
Adherence Transmigrate Chemotaxis Phagocytosis |
|
|
In what organ are neutrophils produced |
bone marrow |
|
|
What percent of neutrophils are reserved in bone marrow; where are the remaining neutrophils located |
93% in bone marrow 3% circulating in blood 4% marginating on surface of endothelial cells |
|
|
About how many neutrophils does a normal individual produce in a day and how many does an infected person produce |
100 billion - normal Over 1 trillion - serious infection (mobilized reserve of mature neutrophils) |
|
|
What two factors stimulate bone marrow to produce neutrophils |
G-CSF and GM- CSF (glycoproteins) |
|
|
What are the cells stages involved to produce a mature neutrophil |
Stem Cell Myeloblast Promyelocyte Myelocyte Metamyelocyte Band PMN Segmented PMN |
|
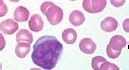
What is the name of this cell in the neutrophilic series |
Myeloblast - originates from stem cells; relatively undifferentiated with a high nuclear:cytoplasmic ratio; prominent nucleoli (immature nucleus) |
|
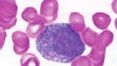
What is the name of this cell in the neutrophilic series |
Promyelocyte - the biggest cell in the neutrophilic series; characterized by the production and accumulation of primary (azurophil) granules plus MPO; nucleus is still immature (can see nucleoli) |
|
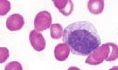
What is the name of this cell in the neutrophilic series |
Myelocyte - appearance of MPO-negative specific secondary granules, smaller and finer than primary granules |
|
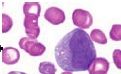
What is the name of this cell in the neutrophilic series |
Metamyelocyte - looks like a mature neutrophil except for its nucleus (larger and kidney bean shaped - looks like a monocyte); cell surface receptors begin to be expressed |
|
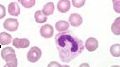
What is the name of this cell in the neutrophilic series |
Band PMN - smaller than the metamyelocyte and nucleus appears U-shaped; begin synthesis of gelatinase |
|
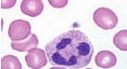
What is the name of this cell in the neutrophilic series |
Segmented PMN - a mature neutrophil |
|
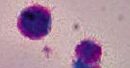
What is the name of this cell in the neutrophilic series |
Stem cell - completely undifferentiated |
|
|
At what stage in the neutrophilic series does granule formation begin |
Promyelocyte (continues to segmented cell stage) |
|
|
During what stages of the neutrophilic series can the cells proliferate and at what stage do the cells become end cells |
Proliferation possible from stem cells to myelocyte stage; Ends cells at Metamyelocyte stage |
|
|
About how many days does it take for neutrophils to be produced and mature in bone marrow |
12 days |
|
|
What is the neutrophil lifespan in the blood |
5-10 hours; then apoptose and removed by monocyte/macrophage system |
|
|
What is the neutrophil lifespan in tissue |
1-2 hours |
|
|
What causes a "shift to the left" |
Depletion of reserve pool of mature neutrophils; less mature neutrophils (band cells) enter the blood
Bad - immature cells not as efficient/effective at attacking microbes as mature cells |
|
|
What is the name of the cytokines that form a neutrophil production regulatory system |
Colony-stimulating factors (CSFs); e.g. G-CSF |
|
|
What neutrophil blood count means a person is susceptible to infection |
less than 1500 mm^3; host defense mechanism compromised, won't be able to fight infection if encountered |
|
|
What neutrophil blood count means a person has an infection |
less than 500 mm^3; have infection, can't fight it |
|
|
What are the possible causes of neutropenia |
Neutropenia is decrease in blood neutrophil count
Bone Marrow Pathology (congenital, malignancies, radiation, drugs - anti-cancer antibiotics, infection - hep a and b, measles) Ineffective Production of Folic Acid or Vitamin B12 Enhanced Destruction (Septicemia, Hypersplenism - neutrophils removed and stored in spleen/overactive spleen) |
|
|
What are the two most common clinical signs of infection in Neutropenia |
Oral Ulcerations (Stomatitis) Severe Gingivitis/Periodontitis |
|
|
During neutropenia, is there a way for oral mucosa breakdown to be reversed |
Yes, when neutrophil count begins to rise again the oral status usually begins to improve |
|
|
What part of the oral mucosa is most effected by decrease in neutrophil number |
Gingiva - gingival sulcus heavily populated with neutrophils in health b/c of great bacterial load; decrease in neutrophils - loss of protection |
|
|
What are the different ways to treat oral manifestations of neutropenia |
Antibiotics Improved Oral Hygiene G-CSF supplementation |
|
|
What parts of the body are very susceptible to bacterial infection because of neutropenia |
Middle ears, Oral cavity, Perirectal area, Lungs |
|
|
How does cyclic neutropenia differ from neutropenia |
Recurrent, opportunistic infection; have 3-6 days of severe neutropenia in 21 day period; asymptomatic during off days; on days - ulceration of tongue, gingivitis, stomatitis, cellulitis, sometimes death (10%), severe perio bone loss |
|
|
What is the neutrophil count during the on days of cyclic neutropenia |
<200 mm^3 |
|
|
What causes cyclic neutropenia |
mutation of gene elastase; neutrophil arrested at promyelocyte stage |
|
|
How do you diagnose neutropenia and how do you diagnose cyclic neutropenia |
Neutropenia - blood count Cyclic neutropenia - observe period of normal neutrophil count and abnormal - have to take sequential WBC count 2-3x/week for 8 weeks |
|
|
What is agranulocytosis |
Granulocytes (particularly neutrophils) are absent; due to decreased production or increased destruction |
|
|
What are most cases of agranulocytosis caused by |
Anticancer chemotherapeutic agents - inhibit normal mitosis and division
some cases are idiopathic |
|
|
Following transmigration, what process causes leukocytes to emigrate toward the site of injury |
Chemotaxis - trigger/stimulus causes neutrophils to move against concentration gradient (stop when there is no longer a gradient) |
|
|
What are the exogenous triggers for leukocyte chemotaxis |
1) Bacterial products 2) N-formyl-methionyl peptide |
|
|
What are the endogenous triggers for leukocyte chemotaxis |
1) Complement pathway (C3a and C5a) 2) Lipid-derived mediators (leukotriene B4) 3) Cytokines - esp in chemokine family |
|
|
What may cause abnormal chemotaxis and what is the result |
Bacterial toxins (e.g. leukotoxin from P. gingivalis); prevents migration and can cause increased risk of aggressive infection
P. gingivalis has proteases that break down C5a - causes perio disease |
|
|
What do granules contain that are very important in neutrophil ability to phagocytose |
Lysozymes |
|
|
How do neutrophils produce energy |
Glycolytic pathway; very short-lived so don't need complicated protein synthesizing apparatus or a lot of mitochondria |
|
|
What is the function of the ROS produced by neutrophils |
Help kill microbes (NOT for ATP production) - respiratory burst |
|
|
What is degranulation |
Release granules (part of digesting microbes) |
|
|
Activation of Toll-like receptors by what on microbes leads to the amplification of the inflammatory response |
Pathogen-associated molecular patterns (PAMPs) - induces cytokines |
|
|
What must neutrophils do in order to phagocytose microbes |
Adhere |
|
|
What can bacteria have to make it difficult for neutrophils to adhere |
Capsules - prevents neutrophil recognition; S. pneumonia/pneumococci, H. influenza, P. gingivalis |
|
|
What happens to bacteria to prepare them to be phagocytosed |
Opsonization - complement and antibodies (opsonins) bind to bacteria and prepare it to be phagocytosed |
|
|
What are the two types of opsonin used in the process of phagocytosis |
Complement (C3b) Antibodies (IgG) |
|
|
How does opsonization work |
Opsonins bind to bacteria and neutrophils must have receptors to be able to recognize the opsonins in order to phagocytose |
|
|
What are the two types of neutrophil opsonin receptors |
Fc (antibody) C3b |
|
|
What is formed when a neutrophil phagocytoses bacteria |
Phagosome |
|
|
What is present in the phagosome membrane that generates ROS and what is it sometimes known as |
NADPH oxidase/phagocyte oxidase |
|
|
What do NADPH oxidase and oxygen produce |
H2O2 (hydrogen peroxide) - oxygen-dependent process |
|
|
What is the oxygen-independent pathway necessary to produce HOCl- |
Granules contain MPO, combined with Cl- |
|
|
When you combine the oxygen-dependent and oxygen-independent pathways of phagocytosis, what do you produce |
HOCl- (causes fragmentation of bacteria and killing) |
|
|
Phagolysosome |
Phagosome including HOCl- combined with lysosome |
|
|
What are the two proteins produced by neutrophils that assist with phagocytosis |
Bacterial Permeability Increasing protein (BPI) LL-37 (cationic protein) they bind to bacteria and create holes in it to facilitate killing |
|
|
What are the four major components that come together to kill/degrade bacteria |
1) NADPH oxidase 2) Granules 3) Additional proteins (BPI and LL-37) 4) Lysozyme - degrades bacterial coat
occurs intracellularly - inside phagolysosome |
|
|
What is produced during phagocytosis and contributes to killing some types of bacteria, like pneumococci |
Lactic acid |
|
|
What is the extracellular mechanism by which bacteria are killed by neutrophils |
Neutrophil Extracellular Traps (NETS) - beneficial suicide; formation of ROS intracellularly - disintegration of nuclear and granular membranes and mixing/release of contents - release of NETs - trap bacteria and kill
fail-safe mechanism if intracellular mechanisms aren't functioning properly |
|
|
What are the potential negative consequences of NETs |
may cause autoimmune diseases - possible source of antigens |
|
|
What disease involves abnormal lysosome formation due to mutation, is autosomal recessive, has melanocytes with large granules, has neutrophils with giant azurophilic granules |
Chediak-Higashi Syndrome |
|
|
What diseases involves clinical manifestations identified in infancy/childhood, oculocutaneous albinism, photophobia, grey hair color, recurrent bacterial infection (gingivitis, oral ulceration, perio diseases) |
Chediak-Higashi Syndrome |
|
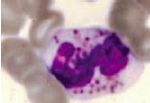
What is happening to this cell/what are the clinical implications |
Chediak-Higashi Syndrome; multilobular nucleus - so cell is neutrophilic, but has prominent granules |
|
|
What disease involves a defect in NADPH oxidase due to mutation and formation of a granuloma |
Chronic Granulomatous Disease; genetic defect in NADPH oxidase, don't generate sufficient amount - catalase positive bacteria remove H2O2; impaired ROS production; granuloma formation from reduced efferocytosis and activation of monocytes |
|
|
What disease clinically manifests as recurrent bacterial infection, is most susceptible to catalase positive bacteria, has superficial skin infections, abscesses, cellulitis, gingivitis |
Chronic Granulomatous Disease - have normal recruitment of neutrophils, they just don't function properly; have abnormal respiratory bursts |
|
|
What do long-term repeated infections, like CGD, likely result from |
lack of NETs formation |
|
|
What disease involves the mutation and loss of cathepsin C gene (LL-37 not produced), skin defect, and neutrophil function, keratosis in palms and soles |
Papillon-Lefevre Syndrome |
|
|
What type of bacteria produces leukotoxins and is assoicated with perio disease |
A. actinomycetemcomitans (Aa) |
|
|
What bacteria produces proteases that inactivate the complement pathway and prevent neutrophil from being recruited to site of infection |
P. gingivalis |
|
|
What bacteria produces protein A, which binds to the antibody receptor of neutrophils so the bacteria can't be opsonized |
S. aureus |
|
|
What does DNAse do |
dissolves NET (produced by some bacteria) |
|
|
What do leukocytes change their lipoxygenase-derived products from and to in the change from inflammation to resolution of inflammation |
COXs (pro-inflammatory) to lipoxins (resolve inflammation) |
|
|
How do lipoxins work to resolve inflammation |
Bind to specific cell surface receptors on neutrophils to inhibit chemotaxis and ROS generation and promote neutrophil apoptosis
ALSO - promote monocyte chemotaxis and do NOT induce cytokine production by monocytes/macrophages (don't wan to recruit inflamm cells) |
|
|
What is largely the cause of the extensive cellular and tissue destruction seen in purulent exudate |
proteolytic enzymes and other factors released by neutrophils |
|
|
What are the two process that can heal injured tissue |
Regeneration Repair |
|
|
What determines whether injured tissue undergoes regeneration or repair |
The type of cells the tissue is made of (labile, stable, permanent) |
|
|
What happens to injured tissue in regeneration vs repair |
Regeneration - replace damaged or lost cells with identical cell type (via stem cells in basement membrane)
Repair - replace injured/dead cells with fibrous/collagenous scar |
|
|
When does fibrosis occur and what is it |
Extensive deposition of collagen, occurs in tissues as a consequence of chronic inflammation (usually in lung, liver, kidney or heart) |
|
|
When does scar tissue interfere with organ function |
If the scar tissue is extensive (e.g. in infarcts - may lead to thinning of muscle walls and complications/loss of function) |
|
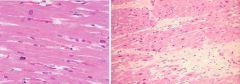
What has occurred from the slide on the left to that on the right; this is heart tissue |
Left: cells undergoing karyolysis following inflammatory response Right: replacement of dead tissue with fibrotic tissue; white areas where cells lost due to severe damage and replaced by collagenous scar |
|
|
Do you get regeneration or repair with: labile, stable and permanent cells |
Labile - most likely regenerate; usually cells that turnover rapidly (skin) Stable - can either regenerate or repair, depends on severity of injury (liver, kidney) Permanent - almost always repair with scar tissue (neuron, heart) |
|
|
What percent of cells are dividing/undergoing mitosis in tissues that contain labile cells; what are examples of these tissue types |
1.5% - give you regeneration
hematopoetic tissue, lymphoid tissue, surface epithelium (skin, mucous membranes, GI, urinary and respiratory tracts, salivary glands) |
|
|
How do stem cells, which are labile cells, divide |
They divide continuously; with injury - one daughter cell becomes another daughter cell and the other becomes a terminally differentiated cell |
|
|
What two things does regeneration of any tissue require |
1. Intact basement membrane 2. Sufficient stem cells to promote regeneration (need asymmetrical division to maintain integrity of basal cell layer/have enough stem cells) |
|
|
In what clinical situations will you develop a scar if the basement membrane of the surface epithelium is damaged, but won't scar if basement membrane left intact |
Chicken Pox Acne |
|
|
What cell type is long-lived, has low turnover, are quiescent and have minimal replicative activity in their normal state |
Stable cells - proliferate in response to injury or tissue loss |
|
|
What are examples of stable cells |
Parenchymal cells - liver, pancreas, thyroid, salivary glands, adrenal cortex, renal tubular epithelium
Connective tissue and mesenchymal cells - osteoblasts, fibroblasts, vascular endothelial cells, smooth muscle cells |
|
|
What percent of cells are dividing/undergoing mitosis in tissues that contain stable cells |
less than 1.5%; but this number does not determine an organ's ability to regenerate; always have ability to divide with appropriate stimulus/injury |
|
|
What biological action does the mythical story of Prometheus and the eagle give an example |
Regeneration of liver cells (stable cells) with damage to the liver |
|
|
What must not be damaged in order for liver cells to regenerate instead of repair |
Extracellular matrix (like basement membrane can't be damaged in epithelial cells) |
|
What has happened to the liver here |
Undergone cirrhosis (can happen with chronic alcoholism); liver nodules and fibrosis, it's hard and has lots of connective tissue; smaller than normal liver because the scar tissue contracts
normal liver is soft and tender, normal size is fairly large |
|
What has happened to these liver cells |
Surrounded by collagen because of damage to ECM - has undergone repair due to a severe injury |
|
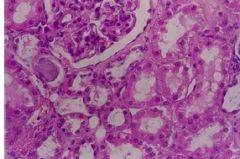
What has happened to some of the kidney cells/tubules pictured here |
Coagulative necrosis/karyolysis; cells are dead and you can't distinguish nuclei, can also see much protein stained pink by eosin, but basic architecture of the tubules is still intact |
|
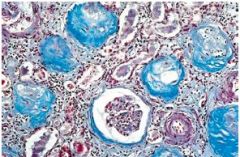
What has happened to the kidney cells here, and what types of cells have caused this |
Glomeruli surrounded by blue-stained collagen (undergo fibrosis more readily than tubules); caused by severely damaged stable cells - lost regenerative capacity |
|
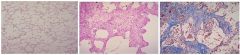
If the first slide is normal lung parenchyma, what has most likely occurred to the lung parenchyma in the other two slides |
1) Empty spaces, no fluid in cells; probably due to pneumonia; lots of collagen/fibrosis (H&E stain) 2) Fibrosis - due to persistent injury (Trichrome stain) |
|
|
What type of cell cannot replicate after birth, give examples |
Permanent cell (neurons, skeletal muscle, cardiac muscle); once destroyed - lost forever; scar formation occurs |
|
|
Why does the heart increase in size with age |
Due to Hypertrophy (increase in cell size) NOT hyperplasia (increase in cell number) because heart cells can't multiply/divide |
|
|
What are the three things that exit the blood vessels and enter the extravascular space during inflammatory response, and in what order |
1) Edema - with vascular/cellular response 2) Neutrophils - early acute inflammation 3) Monocytes/macrophages - late acute inflammation
|
|
|
What is a clinical example of exudate resolution after injury (etiology promptly destroyed, no or minimal cell necrosis) |
Mild heat injury |
|
|
What is a clinical example of exudate organization after injury (etiology promptly destroyed, no or minimal cell necrosis) |
Fibrinous exudate - Fibropurulent Pericarditis; have fibrotic layer on top of exudate which leaves a fibropurulent scar on top |
|
|
What is a clinical example of the framework remaining intact (after etiology NOT destroyed, in tissue of labile/stable cells) |
Lobar pneumonia
Basement membrane of skin intact, ECM of liver cells intact |
|
|
What is a clinical example of the framework being destroyed (after etiology NOT destroyed, in tissue of labile/stable cells) |
Liver Cirrhosis - scarring when ECM of liver cells destroyed |
|
|
What is a clinical example of necrosis of the tissue of permanent cells (after etiology NOT destroyed) |
Myocardial Infarct - scarring (from ischemic necrosis - permanent damage to cardiac myocytes) |
|
|
What does regenerative medicine involve, give an example |
regeneration and repopulation of damaged organs using stem cells; induced pluripotent stem cells (iPS cells) - expose genes that confer stem cell properties to pt's differentiated cells - resulting iPS cells can be induced to differentiate |
|
|
What are the predominant examples used to describe labile cells and stable cells |
Labile - Skin/epithelial cells Stable - Liver cells |
|
|
What are the three things that must be resolved in order for proper healing to occur |
1) Bleeding 2) Loss of tissue 3) Infection |
|
|
What are the four phases of wound healing |
1) Early 2) Intermediate 3) Late 4) Terminal |
|
|
What happens during the early phase of wound healing and how long does it last |
Platelets activated - form clot Neutrophils and macrophages recruited
rapid, transient |
|
|
What happens during the intermediate phase of wound healing |
Activation of resident cells - cells normally present in the tissue (fibroblasts, mast cells, endothelial cells) Proliferative response of resident cells - form new blood vessels (angiogenesis), re-epithelialization |
|
|
What happens during the late phase of wound healing |
Collagen synthesis |
|
|
What happens during the terminal phase of wound healing |
Remodeling; increase wound strength by replacing original collage deposited with stronger collagen
takes months |
|
|
What are the two major categories skin wounds can be grouped into |
Healing by First Intention - edges held close together by sutures, clips or tape to help heal
Healing by Second Intention - edges are widely separated |
|
|
What occurs during healing by first intention |
Minimal tissue loss, wound edges held close together, no infection and little scarring |
|
|
What occurs during healing by second intention |
Extensive tissue loss, ulcer/large wounds, extensive scarring |
|
|
What is the response that occurs within seconds after skin incised by a scalpel blade |
Hemostatic response - arrests hemorrhage and initiates healing
platelets release 5-HT from granules (plug defects in vessel wall and release factors into immediate wound area and activate clotting cascade), TxA2 activated, fibrin and fibrinogen (clotting proteins) |
|
|
What protein forms a clot to stop bleeding |
Fibrin |
|
|
What functions does a clot have |
Stabilizes platelet plug so it can't be dislodged Cements cut margins of wound Generate chemotactic peptides to attract leukocytes, resident fibroblasts and endothelial cells Forms a scaffold of fibrils - facilitate migration of leukocytes and mesenchymal cells to wound Discharge vasoactive compounds into wound (prostaglandins, PAF, proteolytic enzymes, VEGF) |
|
|
Are platelets required for wound healing |
Yes, must have platelets to stop bleeding |
|
|
In what type of wounds are neutrophils important |
Infected wounds - eliminate contaminating microbes |
|
|
What is a clinical example of a situation where neutrophils can't be recruited to site of injury and won't heal |
LAD-I - defect in neutrophil transmigration process so neutrophils don't get to wound and it can't heal (e.g. Dysplastic Eschar, Stump of umbilical cord) |
|
|
What is the difference between macrophage recruitment in acute inflammation and that in wound healing |
In acute inflammation - macrophages do NOT secrete cytokines, don't want to initiate an inflammatory response; cytokines required in wound healing for chemotaxis of fibroblasts AND angiogenesis and remodeling |
|
|
When do macrophages appear and how long do they remain during wound healing |
24-72 hours after injury; remain for 5-7 days |
|
|
What type of cell is recruited in wound healing that is not recruited in acute inflammation |
T-lymphocytes; produce cytokines that help with healing |
|
|
During wound healing, what process involves basal epidermal keratinocyte transformation from sedentary to migratory phenotype, under influence of cytokines |
Epithelialization |
|
|
What does re-epithelialization involve mechanistically |
Mitotic activity 4-5 cells from leading edge (where cells are exhibiting phagocytic activity and digesting their way through the clot); eventually the migrating cells contact and you have restoration of epithelium |
|
|
What is angiogenesis, in what stage of wound healing does it occur, and why is it important in wound healing |
formation of new blood vessels during intermediate stage - critical at injury site for collateral circulation and to bring in nutrients (plasma proteins) for next phase; process mainly mediated by VEGF |
|
|
What is the name of tissue that includes leaky, newly produced blood vessels during wound healing |
Granulation tissue - highly vascular; mass of migrating and proliferating fibroblasts and capillaries; contains many new blood vessels, fibroblasts, some monocytes, and few neutrophils; underneath the now thin epithelial layer - bleeds easy if traumatized |
|
|
What is degraded and then reformed in order to produce new blood vessels during angiogenesis in wound healing |
Basement membrane of endothelial cells |
|
|
What tissue changes occur from the intermediate stage of wound healing to the late stage |
Granulation tissue devascularized and turns into scar tissue |
|
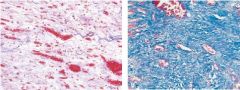
What two stages of the wound healing process do these two tissue slides depict |
Intermediate stage (granulation tissue) and Late stage (scar tissue/fibrosis) |
|
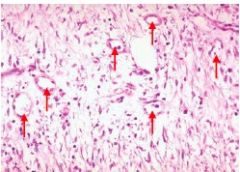
What do the arrows point to in this intermediate stage of wound healing |
Many blood vessels in granulation tissue |
|
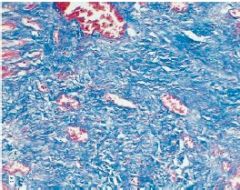
What stage of wound healing is depicted here |
Late stage - lots of collagen/scar tissue |
|
|
What are the most and second most abundant cells in connective tissue (i.e. scar tissue) |
Fibroblasts, and then mast cells |
|
|
What are the cells needed for wound healing (in order) and what is their primary function |
EARLY 1) Platelets - clot formation 2) Neutrophils - kill bacteria, digest ECM/basement membrane, remove dead tissue INTERMEDIATE 3) Macrophages - clean up mess, secrete cytokines for fibroblast chemotaxis; secrete cytokines for angiogenesis and epithelialization LATE 4) Fibroblasts - secrete collagen 5) Mast cells - interact with fibroblasts to stimulate collagen synthesis TERMINAL 6) Fibroblasts leave 7) Myofibroblasts - wound contraction |
|
|
What type of cells commonly cause tissue injury during inflammatory response |
Neutrophils |
|
|
What cells mediate healing by second intention |
Myofibroblasts - myocontractile and collagen synthesizing abilities |
|
|
What is wound contraction |
Process where wound edges begin to move towards each other to the center of the wound; results in mechanical reduction in size of wound; especially important in healing by second intention |
|
|
What does the terminal stage of wound healing involve |
maturation and increase in tensile strength of the wound (re-modeling) - replace original Type III collagen with Type I collagen - increased cross-linking between collagen fibrils increases strength |
|
|
Does tissue that undergoes wound healing ever reach the strength of normal skin |
No. 80% of original strength after ~6 weeks; 90% of original strength after ~6 months |
|
|
What replaces the epidermal and dermal layers of skin after the terminal stage of wound healing |
Epidermal - epithelial cells (regeneration - labile cells) Dermal - scar tissue (repair of basement membrane - stable cells) |
|
|
What type of collagen is originally produced in wound healing and what type of collagen replaces it during the terminal stage of wound healing |
Type III to Type I |
|
|
What is the defining feature, histologically, of a skin ulcer |
Loss of epithelial cell layer |
|

What type of skin condition is observed here |
Skin ulcer - no epithelial layer |
|
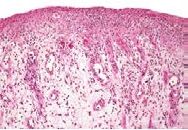
What stage of the wound healing process is shown here |
Intermediate - Granulation tissue with lots of blood vessels and thin epithelial layer (angiogenesis and re-epithelialization occurring) |
|
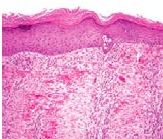
What stage of the wound healing process is shown here |
Intermediate/Late stage - Not a ton of blood vessels, wound contraction occurring in epithelial layer |
|
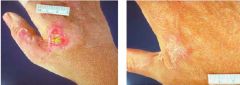
What process is depicted here in the change from the first picture to the second |
Wound healing by second intention - extensive scar tissue forms underneath epithelial layer; occurs with ulcers/large wounds |
|
|
What are the major differences in the vascular and cellular responses between inflammation and wound healing |
Inflammation: - Vascular response increases vascular permeability; initiated by mast cells - Neutrophils and macrophages impt for cellular response
Wound Healing - Vascular response causes angiogenesis - initiated by platelets/neutrophils - Macrophages for proliferative response - Fibroblasts for actual healing |
|
|
What is chronic inflammation |
a continuation of wound healing - if scar tissue becomes so big that it interferences with function of the organ then chronic inflammation occurs (e.g. myocardial infarct) |
|
|
How do growth factors function during wound healing |
proliferative response - promote survival, proliferation and migration
use cell surface receptors - activate signaling pathways and transcription factors in the nucleus through tyrosine-kinase activity (PDGF, VEGF) - except GPCR (no tyrosine-kinase activity) |
|
|
What type of receptor is utilized by certain chemokines (IL-8) and cytokines (IL-4, IL-2) |
G protein coupled receptors; receptors don't have tyrosine-kinase activity; they activate adapter proteins and result in transcription of genes that promote cell cycle entry, prevent apoptosis, etc |
|
|
What are the three different ways in which cytokines can function with receptors to initiation gene expression |
Autocrine - cell produces particular cytokines and has receptors for the cytokine on the same cell Paracrine - one cell produces cytokine that acts on receptors on a different, adjacent cell Endocrine - growth factors secreted into blood |
|
|
What are two examples of an autocrine system |
Lymphocyte proliferation by cytokines - T cells release cytokines and has receptors for those cytokines on the cell
Compensatory hyperplasia - part of liver removed, cytokines produced remaining liver cells act to produce more liver cells
|
|
|
What is an example of a paracrine system |
Recruitment of inflammatory cells - macrophage produces cytokines that act on other inflammatory cells to recruit them |
|
|
What growth factor initiates a healing response after clot formation during wound healing |
PDGF (platelet derived growth factor) - produced by platelets mostly, also macrophages, endo cells and keratinocytes; chemoattractant for neutrophils, monocytes, fibroblasts; important for synthesis and remodeling of ECM |
|
|
What does recombinant PDGF (regranex) treat clinically |
Diabetic ulcers |
|
|
What is the most important growth factor for epithelialization during wound healing |
EGF (epidermal growth factor) - promotes proliferation and migration of epithelial cells; also promotes proliferation of fibroblasts and induces granulation tissue formation |
|
|
What growth factor is the most important for angiogenesis during wound healing |
VEGF (vascular endothelial growth factor) - produced in response to PDGF, TGF-beta, and hypoxia by epithelial cells; also promotes migration and proliferation of endo cells (epithelialization) |
|
|
What growth factor should you develop antibodies against in order to stop growth of tumor cells in some cancers |
VEGF - reduce nutrient delivery to tumor |
|
|
What is the mesh beneath epithelial, endothelial or smooth muscle structures called |
Basement membrane |
|
|
What is the primary collagen type found in basement membrane |
Type IV - sheet-like collagen; doesn't form bonds between different fibrils; also includes proteoglycans and laminins |
|
|
What is the matrix between basement membrane and blood vessels called, what's included in this matrix |
Interstitial matrix - fibroblasts, integrins, adhesive glycoproteins, cross-linked collage (rope-like); reservoir for growth factors |
|
|
What is important for the production of collagen cross-linking and what happens if collagen can't cross-link |
Vitamin C - without cross-linking the area will bleed easily and the wound won't heal as well |
|
|
What growth factor is important for ECM deposition and scar formation |
Transforming growth factor beta (TGF-beta); chemoattractant for fibroblasts, promotes collagen/ECM synthesis, inhibits collagen degradation; induces TIMPs; results in fibrosis in kidney/lung/liver after chronic inflamm; has anti-inflammatory effects as well |
|
|
What does TIMP do during wound healing |
Tissue inhibitor of metalloproteinases - prevent metalloproteinases from degrading collagen |
|
|
In what situation is TGF-beta expressed transiently |
Fetal healing - healing occurs without scar formation; TGF-beta undesirable |
|
|
What can be administered to reduce scarring |
Antibodies to TGF-beta |
|
|
What three things does scar tissue formation depend on the balance between |
TGF-beta (promote collagen formation) MMPs - metalloproteinases (degrade Type III collagen during remodeling) TIMPs (inhibit degradation of Type I collagen)
|
|
|
In what types of tissues do you see labile cells/regeneration after wound healing |
Skin (epidermis) Bone Oral Mucosa Fetal wound |
|
|
In what types of tissues do you see stable cells/regeneration or repair depending on severity of injury during wound healing |
Liver Lung Kidney (regeneration in cortical tubules, scar tissue/repair in medullary tubules and glomeruli) |
|
|
In what types of tissues do you see permanent cells/repair and scar tissue formation during wound healing |
Heart Nervous System (except some parts of peripheral - regeneration of axons) |
|
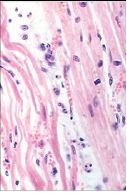
What type of necrosis do you here see (after myocardial infarct) |
Coagulative necrosis - nuclei look abnormal, but basic architecture still intact |
|
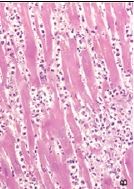
What is happening to this cardiac tissue |
Early acute inflammation: Karyolysis of cardiac myocytes (no nuclei); lots of neutrophils present |
|
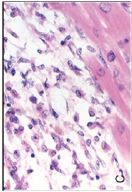
What is happening to this cardiac tissue |
Late acute inflammation: macrophages cleaning up mess from neutrophils |
|
What stage of wound healing is this |
Intermediate stage - granulation tissue with lots of new blood vessels |
|
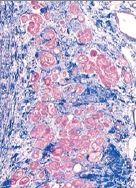
What stage of wound healing is this |
Late stage - lots of collagen produced, healing/repairing damaged tissue with scar tissue (replaced granulation tissue) |
|
|
What is a traumatic neuroma |
reactive proliferation of neuronal tissue after damage of nerve bundles (e.g. damaged axon); NOT a neoplasm; have scar tissue, can't reestablish innervation; tumor-like mass develops at site of injury |
|
|
What are the most common sites for traumatic neuromas |
mental foramen, tongue, lower lip |
|
|
What is the only case in which you have regeneration of neuronal tissue |
Traumatic neuroma - damaged axon |
|
|
What two types of ossification occur during bone formation/fracture repair |
Intramembranous ossification Endochondral ossification |
|
|
What occurs during intramembranous ossification and how do bones grow, what types of bones |
differentiation of mesenchymal cells into osteobalsts; osteoid formation (collagen and organic ECM), combined wtih Ca++ and phosphates results in mineralized bone matrix; also need osteoclasts for bone remodeling
bones grow in WIDTH (skull, maxilla, mandible) |
|
|
What occurs during endochondral ossification, how do bones grow, what types of bones |
differentation of mesenchymal cells into hyaline chondrocytes then replacement of hyaline cartilage with bone
Long bones grow in LENGTH |
|
|
What are the steps of bone regeneration after fracture |
1) Hematoma - clot 2) Provisional callus - angiogenesis/granulation tissue; fibrocartilage - soft 3) Hard callus - endochondral ossification; woven bone replaces soft callus 4) Remodeling - cortical bone replaces woven bone; healing without scar |
|
|
How can you tell a bone has been broken |
Bone more dense; but NOT scar tissue formed |
|
|
What are the two keys to successful bone repair/formation |
1) Alignment (screws, cast) 2) Good blood supply |
|
|
What are required in order to produce osteoblasts and form new bone |
Bone morphogenic proteins (BMPs) and Mesenchymal stem cells (MSCs) |
|
|
What other healing processes are nearly identical to that of skin |
Bone, socket healing |
|
|
What are six complications of wound healing and what causes them |
1) Alveolar Osteitis/Dry Socket - clot doesn't form 2) Delayed healing - from infection 3) Proud flesh/Pyogenic granuloma - too much granulation tissue 4) Dehiscence/Hernias/Scurby - deficient collagen deposition 5) Keloid/hypertropic scar - excessive collagen deposition (mutation) 6) Contracture - excessive contraction |
|
|
What typically causes dry socket formation |
Infection or trauma; involves dissolution of blood clot |
|
|
What are four predisposing factors to dry socket formation |
Oral contraceptives Site of extraction (mandible more prone than maxilla) Trauma Smoking |
|
|
Why does infection typically delay wound healing |
Healing by second intention with infection - results in more granulation tissue/scar formation
common cause of death from burn injuries |
|
|
What is a proud flesh, how does it occur, and how is it treated |
a protruding mass of granulation tissue; reason for development unknown; tx by cauterization with silver nitrate or surgical removal |
|
|
What is an exuberant proliferation of granulation tissue that occurs in oral tissues called, how does it occur and how is it treated |
Pyogenic granuloma (NOT actually a granuloma and NOT purulent); usually due to trauma or irritation |
|
What wound healing complication is seen here |
Proud flesh - protruding mass of granulation tissue |
|
What wound healing complication is seen here |
Pyogenic granuloma - protruding mass of granulation tissue in the oral mucosa |
|
|
Where are pyogenic granulomas located 75% of the time |
Gingiva |
|
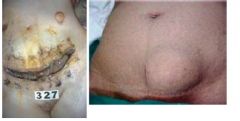
What wound healing complications are seen here |
Dehiscence/Incisional hernia - in abdominal wounds; caused by defect in collagen deposition |
|
|
What is a keloid |
excessive collagen formation that begins at wound site and then spreads to neighboring tissues; ratio of Type III:Type I collagen is high - "maturation arrest"; high rate of recurrence; common in african-americans and young people, common within families |
|
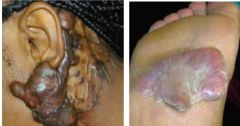
What wound healing complication is seen here |
Keloid - dark; excessive collagen formation that spread |
|
|
What is the pathogenesis of keloid formation |
Increased fibroblast proliferation and collagen synthesis due to overexpression of TGF-beta and VEGF |
|
|
What is a hypertrophic scar and how does it differ from a keloid |
Similar in appearance to a keloid; flatten and regress spontaneously over a period of months-years and do NOT spread beyond site of injury |
|
|
Can you treat keloids/hypertrophic scars |
Can treat hypertrophic scars but if you try to treat keloids they recur, so no |
|
What wound healing complication is seen here |
Hypertrophic scar - light, confined to one region |
|
|
What causes excessive wound contraction, and where does this typically occur |
Myofibroblasts; palms, soles, burns of skin, liver (cirrhosis) |
|
|
What must be removed from wounds in order to promote healing |
1) Necrotic tissue (promote bacterial growth, physical barrier, delays healing) 2) Foreign material (harbor bacteria, promote inflammation) |
|
|
What is a decubitis ulcer and how is it treated |
Bed sore; Pressure induced ulcer from ischemia (inadequate blood supply) - relieve pressure; use pDGF to initiate healing process |
|
What local factor in wound healing produced condition is shown here |
Decubitis ulcer/Bedsore - from pressure induced ischemia |
|
|
What are some of the local factors in wound healing aside from inadequate blood supply |
1) Endarteritis obliterans - due to post-irradiation scarring of blood vessels; reduces local blood flow 2) Atherosclerosis - restricts arterial perfusion, particularly in diabetics and elderly 3) Smoking - triggers vasoconstriction and may impair tissue oxygenation; also contributes to development of atherosclerosis |
|
|
What are some of the effects that local irradiation has on wound healing |
Curtains blood flow to wound Decreased vascularity limits platelet and leukocyte delivery to site Cytotoxic effects on fibroblasts and keratinocytes leads to increased fibrosis and thinning epidermis Dry skin due to damaged sebaceous and sweat glands |
|
|
What direction and location of a wound facilitate better healing |
Incisions made parallel to crease lines of skin |
|
|
What role does movement play in wound healing |
Immobilization important for bone fractures; excessive mechanical movement can produce additional trauma to skin wounds and may prolong healing |
|
|
Why do oral mucosa wounds tend to heal faster than skin wounds |
Moist environment promotes healing |
|
|
What systemic factors have a profound effect on wound healing |
Malnutrition Cancer Diabetes Mellitus |
|
|
What are the six major malnutrition involved systemic factors of wound healing and how do they effect wound healing |
1) Hypoproteinemia - decreased collagen synthesis 2) Reduced carb and fat intake - protein used as energy source instead 3) Vitamin C deficiency - abnormal collagen synthesis 4) Vitamin A deficiency - defective epithelialization 5) Vitamin D deficiency - defective bone healing 6) Zinc deficiency - decreased cell proliferation and granulation tissue formation |
|
|
Why does diabetes mellitus effect wound healing; how can you treat wounds that result |
Results in decreased growth factor - improper healing; treat with PDGF, antimicrobial, amputation |
|
|
What inflammatory process can follow wound healing |
Chronic inflammation - if etiologic agent is not removed |
|
|
When do you experience signs and symptoms of acute inflammation and of chronic inflammation |
Acute - almost immediately; redness, heat, swelling Chronic - not until late in process; indurated/hard |
|
|
What cells are involved in the first stages of acute inflammation vs chronic inflammation |
Acute - mast cells and neutrophils Chronic - Round cells (macrophages and lymphocytes) |
|
|
What is the biological response of acute inflammation vs chronic inflammation |
Acute - vascular/exudative Chronic - proliferative |
|
|
What are some of the main clinical examples of chronic inflammation |
Periodontal disease Pulpal/periapical lesion Alcoholic cirrhosis |
|
|
What is the most significant feature of chronic inflammatory diseases |
permanent or irreversible tissue/organ destruction |
|
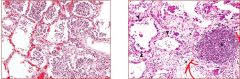
What two inflammatory responses are occurring here in the lung tissue |
1) Acute inflammation - normal alveoli and alveolar septae; blood vessels are dilated; lots of neutrophils
2) Chronic inflammation - empty spaces that used to have fluid replaced by fibrosis; foci of inflammatory "round" cells |
|
|
What two cell types interact in a bidirectional way during chronic inflammation |
Lymphocytes and Macrophages - Macrophages display antigens (MHC) to T cells, results in production of cytokines (like IL-12) that stimulate T cell responses - Activated T lymphocytes then produce cytokines (like IFN-gamma) that activate macrophages - Result is a cycle of cellular reactions that fuel and sustain chronic inflammation - Both macrophages and lymphocytes also produce other inflammatory mediators that result in leukocyte recruitment and inflamm |
|
|
What cell type produces plasma "cells" |
activated B lymphocytes - produce antibodies directed against persistent antigens in inflammatory site or against altered tissue components |
|
|
What cells in chronic inflammation go through almost the exact same process as neutrophil recruitment and transmigration in acute inflammation |
Monocytes |
|
|
Neutrophils can only survive in the blood for 4-5 hrs and in the tissues for 1-2 hrs; how long do monocytes/macrophages survive in both of these locations |
Blood - day or more Tissue - more than a year, VERY long-lived |
|
|
What does the foci of inflammatory cells seen in chronic inflammation consist of |
Macrophages and T cells |
|
|
In what cases might you see neutrophils present in chronic inflammation |
non-specific chronic inflammation resulting from presence of persistent microbes or necrotic cells |
|
|
What are the two main types of macrophages seen in chronic inflammation and how do they differ |
M1 - activated by IFN-gamma; produce ROS, lysosomal enzymes and cytokines that induce inflammation and microbial actions (similar fxn to neutrophils)
M2 - activated by cytokines other than IFN-gamma; NOT microbial; promote angiogenesis and collagen synthesis
Activated sequentially |
|
|
What inflammatory process is referred to as frustrated repair |
Chronic inflammation; b/c tissue destruction is occurring simultaneously
ex: cirrhosis - fibrous tissue repairs liver tissue but also prevents regeneration of normal hepatic lobules and interferes with normal blood supply to lobules - leads to portal hypertension |
|
|
What does fibrosis in kidney (chronic pyelonephritis) cause |
Loss of renal function/failure and hypertension |
|
|
What does fibrosis in the intestine during peptic ulceration cause |
Pyloric stenosis |
|
|
What does fibrosis in joint tissue cause |
Ankylosis |
|
|
What is most of the tissue destruction in chronic inflammation a consequence of |
activities and products released from inflammatory cells |
|
|
What are five different ways an etiologic agent is able to resist elimination by the host |
1) macrophages unable to kill bacteria (e.g. TB and Leprosy - delayed type hypersensitivity, DTH) 2) macrophages unable to degrade bacteria (certain strains of streptococci) 3) viruses and fungi (DTH) 4) cause by particulate or physical agents (uric acid, asbestos, gall stones, silica, talc) 5) immune complexes - deposits keep being dumped in lesion even when removed (e.g. joints, skin, kidney) |
|
|
What is delayed-type hypersensitivity |
An immune response produced by leukocytes in affected tissues that's incited by persistent infection (e.g. inability to remove TB or leprosy microbes; viral and fungal infection) |
|
|
What type of disease probably has an episodic progression of chronic inflammatory lesions (tissue destruction occurs in bursts) |
Periodontal disease - has active and inactive periods; loss of attachment and alveolar bone may be cumulative effects of one or more bursts of active disease; in quiescent periods, bacteria and leukocytes exist in harmony |
|
|
What are dysbiotic microbiota |
bacteria that already exist in the body become active and cause disease |
|
|
What does non-specific chronic inflammation usually result from |
Acute inflammation |
|
|
What are the four most common outcomes of acute inflammation |
1) Complete resolution 2) Fibrosis 3) Abscess formation 4) Progress to chronic inflammation |
|
|
Under what circumstances do tissue damage, acute inflammation, granulation tissue formation and fibrous repair occur at the same instead of sequentially |
when chronic inflammation follows acute inflammation (when the acute response can't be resolved because the injurious agent persists or problems with normal healing process) |
|
|
What are chronic peptic ulcers an example of |
Non-specific chronic inflammation; localized chronic ulcerations of the stomach or duodenum caused by the damaging effects of gastric acid secretion; reflect dynamic balance between tissue damage and repair |
|
|
What are the two sides that create homeostasis in the normal surface of duodenum (preventing ulceration/injury) |
1) Damaging forces - gastric acids, peptic enzymes 2) Defensive forces - mucus secretion, bicarbonates, mucosal blood flow, etc |
|
|
What two things can trigger injury in stomach/duodenum, breaking homeostasis, and causing an acute inflammatory response |
1) Increased damage - NSAID, H. pylori infection, aspirin, cigarettes, etc 2) Impaired defenses - ischemia, shock, etc |
|
|
What is bronchiectasis |
Chronic inflammation of the bronchi, associated with destruction of the wall and permanent dilation; caused by infection or obstruction by a tumor, cystic fibrosis or pneumonia; coexistance of tissue damage and repair |
|
|
What occurs during rheumatoid arthritis |
chronic inflammation; destruction of the synovial fluid and replacement by fibrotic tissue |
|
|
What is a pannus |
a formation of granulation tissue in a rheumatoid joint; includes, macrophages, plasma cells, dendritic cells, lymphocytes; eventually converted to fibrosis - causes loss of bone and cartilage |
|
|
What does the presentation of MHC Class II on microbes do during chronic inflammation processes |
activates T cells - producing cytokines - which activate B cells and more cytokines - eventually form immune complex |
|
|
What is inflammatory periodontal disease an example of and how and when does it occur |
Chronic inflammation - the gradual destruction of periodontium; usually before age 20; host response to dental plaque; first stage of disease is gingivitis - gingival tissue becomes inflamed and forms an exudate; involves deepening of the gingival sulcus resulting in development of a periodontal pocket; eventually bacteria products initiate a chronic inflamm response - result in resorbed bone due to osteoclasts |
|
|
What increases osteoclast activity and bone resorption |
Increased RANKL |
|
|
What type of inflammatory process is periodontal disease, why, and what is the result |
non-specific chronic inflammation - all phases are occurring at the same time; you get bone resorption |
|
|
What is the name for small, separate clusters of activated and differentiated macrophages |
granulomas |
|
|
What does the formation of a granuloma do |
provides a mechanism for dealing with particle materials that are difficult to eliminate (e.g. M. tuberculosis, particulate materials) |
|
|
What are the components of a classic granuloma |
Organized collection of mononuclear cells (T and B lymphocytes and macrophages) = round cells, and proliferating fibroblasts |
|
|
In what type of infection do granulomas contain eosinophils |
Chronic parasitic infection |
|
|
All of the cells that participate in non-specific chronic inflammation are present in what particular structure |
Granuloma |
|
|
What characterizes granulomatous inflammation |
Distinct pattern of chronic inflammation characterized by the presence of granulomas Epithelioid cells Surrounded by a collar of "round cells" and fibroblasts |
|
|
What is the name for an activated macrophage |
Epithelioid cell |
|
|
What is contained in a granuloma |
Giant cells and Epithelioid cells in the center; lymphocytes, fibroblasts and fibrosis in the periphery |
|
|
What are giant cells |
a committee of fused epithelioid cells; different types: Langhans, Foreign body, Schaumann body, Aschoff, Asteroid body; >50 micrometers in diameter; have abundant cytoplasm |
|
|
What characterizes epithelioid cells |
Pink granular cytoplasm Indistinct cell boundary Elongated nucleus May fuse to form giant cells |
|
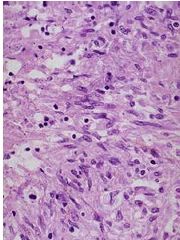
What types of cells are these |
Epithelioid cells - lots of pink cytoplasm, indistinct boundaries, elongated nuclei |
|
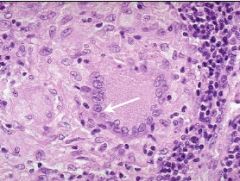
What type of giant cell is this |
Langhans - nuclei arrange in periphery like a horseshoe; often seen in TB |
|
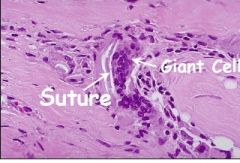
What type of giant cell is this |
Foreign body - can see suture next to giant cell; nuclei arranged haphazardly like pepperoni on a pizza |
|
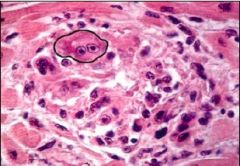
What type of giant cell is this |
Aschoff - look like owl eyes; often see in rheumatic fever |
|
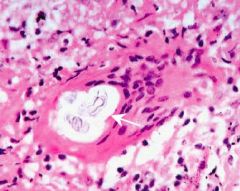
What type of giant cell is this |
Schaumann Body - calcified secretion |
|
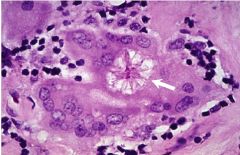
What type of giant cell is this |
Asteroid body - star shaped |
|
|
When/why does granulomatous inflammation occur |
when the cellular infiltrate of a tissue that is chronically inflamed is arranged in a distinct pattern (usually it's spread fairly evenly over a large area); creates clusters of granulomas - allows you to deal with particle materials or pathogens that are difficult to eliminate |
|
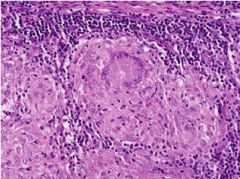
What is pictured here |
Granuloma - giant cell in the center (Langhans), surrounded by epithelioid cells; collar of lymphocytes/round cells surrounding the granuloma |
|
|
What must you see upon biopsy in order to consider something granulomatous inflammation |
Granulomas (NOT granulation tissue) |
|
|
Is pyogenic granuloma a granulomatous inflammation or a granulation tissue |
Granulation tissue - no granulomas seen in biopsy |
|
|
What is tuberculosis |
Chronic inflammatory disease/granulomatous disease, mostly of the lung, caused by mycobacterium; cant digest mycobacterium so granulomas formed in a miliary pattern; granulomas often caseating (b/c due to infection) |
|
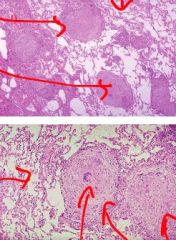
What has occurred to this lung tissue |
Most likely TB - can see granuloma formations close together that appear to be caseating adjacent to normal lung parenchyma |
|

What does this lung depict |
TB - miliary formation of granulomas |
|
|
What is a ghon complex |
Represents beginning phase of TB in lung - see granulomas forming at site of nucleation and hillar lymph nodes; immune system trying to keep bacteria under control |
|
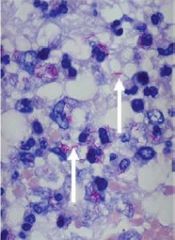
What is shown here to distinguish TB and fungal infection histologically, which is it |
Acid-fast stain; acid fast bacilli, like that in TB stain red |
|
|
Is Tuberculosis a granulomatous inflammation or granulation tissue |
Granulomatous inflammation |
|
|
Is Crohn's disease a granulomatous inflammation or granulation tissue |
Granulomatous inflammation |
|
|
Is a Proud Flesh a granulomatous inflammation or granulation tissue |
Granulation tissue |
|
|
What chronic granulomatous disease mainly involves the small intestine; transmural inflammation, fissures, lymphoid aggregations and non-caseating epithelioid granuolmas |
Crohn's Disease |
|
|
What are the signs/symptoms of Crohn's disease in the gut/small intestine and proximal colon |
Abdominal cramping, diarrhea, nausea |
|
|
In what percent of Crohn's disease cases do you see oral lesions before GI lesions and how do they present |
~30%; cobblestone buccal mucosa, swelling of lips |
|
|
What is the pathogenesis of Crohn's Disease |
Loss of autophagy - paneth cells contain granules with lysosomal enzymes and anti-microbial peptides that usually keep bacteria at bay; deletion/mutation of gene for autophagy - lose granular contents - macrophages uncontrolled |
|
|
What are common examples of causes of non-infectious granulomatous inflammation |
Foreign body particles: breast implant silicone leakage, sutures and restorative dental materials in oral tissues, talc injected by iv drug users |
|
|
What multisystem chronic granulomatous disease has unknown etiology |
Sarcoidosis; blacks more frequently effected than whites; 90% of cases involve the lung; 25% of cases have cutaneous manifestations (lupus perio lesions); often have red macules on hard palate/lower labial mucosa |
|
|
What types of giant cells are frequently found in patients wtih sarcoidosis |
Schaumann bodies - calcified secretions |
|
|
What does diagnosis of sarcoidosis usually depend on |
Radiograph - bilateral hilar lymphaenopathy OR Pulmonary fibrosis Biopsy of affected tissue Positive kveim test (inject sarcoid tissue into someone with suspected sarcoidosis) Raised serum levels of ACE in active phases |
|
What chronic granulomatous disease does this patient most likely have |
Sarcoidosis - lupus perio (lesions on face) |
|
What chronic granulomatous disease does this patient most likely have |
Sarcoidosis - macules on hard palate |
|
|
What is the typical treatment for sarcoidosis |
60% of cases resolve within 2 years Corticosteroids Methotrexate TNF-alpha antagonist |
|
|
What is the syndrome that involves unilateral facial paralysis, facial swelling (of one or both lips) and fissured tongue |
Melkersson-Rosenthal syndrome (type of orofacial granulomatosis) - rare triad of signs |
|
|
What is the syndrome that involves swelling of the lips and chararacteristic microscopical appearances |
Miescher's syndrome/Chelitis Granulomatosa (type of orofacial granulomatosis) - ONLY lips involved |
|
|
What type of infection can you compare a tooth/pulp infection to, and what are the differences |
Appendix - both enclosed - appendix infection due to obstruction - tooth/pulp infection due to breakdown in dentinal area - appendix infection can move sideways - tooth/pulp infection can only spread down |
|
|
What type of pulp infection is virulent/pyogenic |
Periapical abscess |
|
|
What type of pulp infection is less virulent/non-pyogenic |
Periapical granuloma |
|
|
What are the three major causes of pulp pathology |
1) Mechanical - trauma, iatrogenic, attrition, abrasion 2) Thermal/Chemical - dental procedure, acidic materials 3) Bacterial - caries |
|
|
What are the three protective responses against caries (make sure bacteria doesn't get into pulp) |
1) Decreased permeability of dentin 2) Formation of new dentin 3) inflammatory and immune reactions |
|
|
What is the most common cause of pulp disease |
Dental caries - localized, progressive destruction of tooth structure |
|
|
How does dental caries cause infection of the pulp |
Products of bacterial metabolism (organic acids and proteolytic enzymes) cause destruction of enamel and dentin - metabolites diffuse from lesion to the pulp and elicit an inflammatory reaction - eventually have extensive involvement of dentin to cause bacterial infection of pulp |
|
|
What is the most common response to dental caries |
Dentinal sclerosis - dentinal tubules become partly or completely filled with mineral deposits (either caries crystals or peritubular dentin formation - results from tooth abrasion) |
|
|
What is the primary effect of dentinal sclerosis |
Decreasing dentin permeability |
|
|
What types of cells must be present/vital in tubules for dentinal sclerosis to occur |
Odontoblasts |
|
|
What type of dentin forms during tooth development |
Developmental/Primary |
|
|
What type of dentin forms following the completion of tooth development throughout the life of the tooth |
Physiological/Secondary |
|
|
What type of dentin forms only in response to a specific stimulus/irritant at the base of dentinal tubules |
Reparative (Irregular secondary or Tertiary) |
|
|
What is the purpose of reparative dentin |
Defense mechanism against loss of enamel, dentin or cementum |
|
|
What happens to reparative dentin in response to an aggressive insult |
Swiss-cheese pattern - laid down very haphazardly, areas of soft tissue become entrapped in developing matrix |
|
|
What two factors determine the quality of reparative dentin |
1) nature/magnitude of the stimulus/irritant 2) status of the pulp - healthy pulp, better quality
Generally less tubular and less well calcified |
|
|
What two factors determine the severity of pulpal inflammation |
Depth - of bacterial penetration Degree - to which dentin permeability has been reduced by dentinal sclerosis/reparative dentin formation |
|
|
What appears clinically at the pulp if a lot of neutrophils accumulate |
Abscess - pus formed when neutrophils release their lysosomal enzymes and surrounding tissue digested (liquefactive necrosis) |
|
|
Why are abscesses at the pulp often painful |
the area where tissue digestion is occurring has greater osmotic pressure than the surrounding tissue; the pressure differential increases the sensitivity of sensory nerve endings |
|
|
What are the anatomic features of a tooth that affect pulp inflammation |
Unyielding calcified walls - limits swelling Constricted blood source - limits blood supply, subject to strangulation Tooth surrounded by bone - bone infection common |
|
|
How do you classify pulpitis |
Reversible or irreversible |
|
|
What type of pulpitis is characterized by a mild/moderate, short duration, easily localized, elicited pain response to cold |
Reversible |
|
|
What type of pulpitis is characterized by a sharp/severe, long duration, unable to localize, spontaneous response to heat |
Irreversible |
|
|
What is the response of reversible vs irreversible pulpitis to electric pulp testing (EPT) |
Reversible - response at low current Irreversible - response at high current or no response |
|
|
What is the histopathology of reversible pulpitis and how is it treated |
Hyperemia/Edema Reparative dentin
Tx by removing irritation |
|
|
What is the histopathology of irreversible pulpitis and how is it treated |
Congestion of venules/necrosis Necrotic tissue Chronic inflammation/fibrosis
Tx with extraction or root canal |
|
|
Which form of pulpitis (reversible or irreversible) has mobility/sensitivity to percussion |
Neither usually; pulp hasn't spread to underlying bone |
|
|
What other tissues is pulp similar to and why |
Brain, bone marrow, nail bed; housed within mineralized tissue, so in a low compliance environment - no room to swell with inflammation - can create a marked increased in intrapulpal pressure |
|
|
What does the pulp depend on for its blood supply |
Arterioles entering the apical foramen (no collateral circulation) |
|
|
What is the most common cause of pulp death |
Bacterial infection |
|
|
How does the pulp die in presence of pyogenic bacteria |
Liquefactive necrosis - production of pus/abscess |
|
|
What are the three types of pulpal necrosis |
Liquefactive - heterolysis of cell components by neutrophils; pus and abscess formation
Coagulative - interference with blood supply, hypoxia; NO infection
Gangrenous - necrosis with saprophytic bacteria; putrefaction - very extensive degradation of pulp; produce nitrogenous products, smelly |
|
|
What type of tissue is the connective tissue of the pulp continuous with and what does this connection do |
Periodontal ligament tissue adjoining the root apex - connection facilitates spread of disease from pulp to periapical tissues |
|
|
What determines whether a periapical granuloma or an abscess will form from an inflammatory lesion in the periapical tissues |
Virulence of bacteria in the root canal Status of host defense system
Cell mediated response - periapical granuloma pyogenic organisms predominate - periapical abscess |
|
|
What is the clinical term for periapical granuloma formation from increased number and pathogenicity of pyogenic organisms |
Acute suppurative response |
|
|
What type of pulpitis is characterized by infection, necrotic pulp, pain, swelling, elevation of tooth, sensitivity to percussion, fever, malaise, no response to thermal or EPT, lots of PMNs, liquefactive necrosis, early on thickening of PDL and late diffuse bone loss |
Periapical abscess |
|
|
What are the characteristics of a periapical abscess that develops as a direct extension of suppurative pulpitis |
develops rapidly, associated with pain, swelling, elevation of tooth in socket; may not be radiographic evidence of periapical bone loss for a week or more after symptoms first appear |
|
|
If a periapical lesion has been present for a while, what might a radiograph show |
Diffuse area of bone loss that has a "moth-eaten" appearance |
|
|
What is it called when an acute periapical abscess develops within a preexisting granuloma |
Phoenix abscess - resembles a granuloma radiographically |
|
|
What has occurred at the root apex with acute exacerabtion or a flare-up |
results after endo treatment; trauma from instrumentation and introduction of pyogenic bacteria into chronic inflammatory tissue at root apex results in suppuration and pain |
|
|
What is the purpose of abscess drainage and where does it occur |
pus seeks pressure release; may occur through root canal of affected tooth; more common to have a lateral drainage pathway or sinus tract that's created through adjacent tissues - by penetrating the alveolar process at its thinnest point and allowing infection to spread - progression to chronic stage |
|
|
What is a small sessile nodule on the mucosa adjacent to affected (periapical abscess) tooth |
parulis (gum boil) - pus flow from abscess to here through sinus tract |
|
|
What happens when a parulis ruptures and pus escapes, the lesion regresses and then swells and ruptures again (cyclical) |
Ludwig's Angina - bilateral infection in floor of mouth; can also involve submental and sublingual; need to resolve quickly, otherwise difficulty breathing/swallowing |
|
|
What is a periapical granuloma |
Mass of chronically inflamed granulative tissue at the apex of a non-vital tooth; NOT true granuloma; results in radiolucency visible on radiograph; large number of macrophages; fibroblasts and collagen enclose on periphery in capsular arrangement; usually asymptomatic |
|
|
How do small vs large periapical granulomas appear |
Small - thickening of PDL Large - well-circumscribed radiolucency |
|
|
How do you successfully treat a periapical granuloma |
eliminate microorganisms; root canal filling if tooth restorable; periapical surgery if lesion >2cm or tooth not appropriate for endo; non-restorable teeth must be extracted |
|
|
What is a periapical cyst |
Formed if pulpal canal of periapical granuloma containing necrotic tissue not treated - transforms in several months to a year; epithelium lined cavity within a tissue; commonly in the jaw; usually no symptoms; appears identical to periapical granuloma radiographically and tx is the same; cysts contain mostly fluid |
|
|
What does a Phoenix abscess appear like radiographically |
Looks like a periapical granuloma; presents with radiolucency |
|
|
What is osteomyelitis |
infection of the bone marrow space; uncommon in normal, healthy patients; can be acute or chronic; very serious condition that causes destruction of large amounts of bone; usually from odontogenic infection or jaw fractures; more common in males; mandible more susceptible than maxilla |
|
|
What is bone devoid of osteocytes called |
Sequestrum - dead bone |
|
|
What is sequestration |
Dead bone wont be resorbed by osteoclasts, spreads to surface and exfoliated |
|
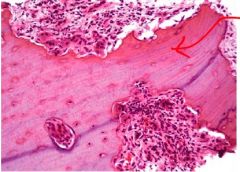
What inflammatory response in the jaw is seen here |
Acute osteomyelitis - bone has no osteocytes; it's sequestra |
|
|
What are the clinical manifestations of acute osteomyelitis |
severe pain, swelling, malaise, elevated WBC count; radiographs show an ill-defined radiolucency and sequestration of necrotic bone |
|
|
When is chronic osteomyelitis of the jaws formed |
if acute osteomyelitis left untreated or in absence of clinically detectable acute phase; inflammation is less intense and clinical symptoms milder than acute; bone appears moth-eaten |
|
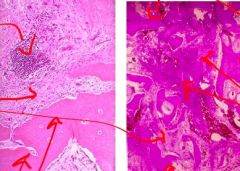
What inflammatory response in the jaw is seen here |
Chronic osteomyelitis - foci of chronic inflammatory cells (not seen in acute), fibrosis, sequestra, pockets of abscess |
|
|
Why is chronic osteomyelitis more difficult to treat than acute; how must it be treated |
Dead bone and microbes are shielded by fibrous tissue; limited blood supply
Surgical intervention mandatory - remove all infected tissues; also high dose, long-term iv antibiotics |
|
|
What is condensing osteitis |
localized lesion; represents reactive hyperplasia of bone (increase in cell number); considered a mild form of chronic inflammation that develops in response to long-standing, low grade pulp infection; more common in patients under 30; usually develops in mandible, near first molar; tx with endo or extraction |
|
What type of inflammatory response in the jaw is seen here |
Condensing osteitis - appears radiopaque radiographically in periapical region; all other forms of osteitis and periapical lesion appear radiolucent |
|
|
Exophytic |
Lesion growing above the surface, or presents this way (e.g. bump on the skin) |
|
|
Sessile |
Mass that you can't really move around; appears as a bump on mucosa that you can feel and see; hard to remove |
|
|
Pedunculated |
Can move the mass around; attached to the tissue by a stalk (like a leaf attached to the treat by a stem - you can move the leaf around); easy to remove the entire lesion at the stem |
|
|
Biopsy |
Surgical procedure; almost all physical pathology warrants a biopsy for diagnosis |
|
|
What are the two types of biopsy |
Excisional - entire lesion/mass surgically removed, circumferentially/at the base
Incisional - take part of the lesion/piece of tissue and leave rest of mass intact |
|
|
What results from excisional vs incisional biopsy |
Excisional - could be diagnostic or therapeutic; if mass is benign then this is the complete treatment
Incisional - do this when you're not sure what you're dealing with clinically or if lesion too large to completely remove; diagnosis guides treatment (cancer - excise the tissue; benign lesion - localized procedure) |
|
|
What are two different incisional biopsy techniques |
punch biopsy - use a tool with a circular blade at the end, like a hole puncher - punch out the tissue area and send to the lab for analysis
scalpel - take a piece of the mass, put in 10% formaldehyde, send to lab for analysis, diagnose, determine how to treat patient |
|
|
What is a differential diagnosis |
process of listing two or more diseases with similar signs and symptoms; initially list 5-7 possible diagnoses then narrow list to no more than three
determines additional tests that you can use to diagnose (e.g. blood test, biopsy, stool sample) |
|
|
What are the three causes of tissue injury |
Physical Chemical Trauma |
|
|
What is linea alba and is it considered physiologic or pathologic |
chronic friction (between teeth and mucosal tissue) leading to epithelial hyperplasia and hyperkeratosis; asymptomatic unilateral or bilateral white lines on buccal mucosa at level of occlusal plane
technically a pathology because it's not found in all mouths; but not really considered clinical pathology, just a variation of normal tissue |
|
|
What are three examples of factitious trauma |
Factitious injury - self-induced (deliberate or non-deliberate)
Morsicatio buccarum - cheek Morsicatio labiorum - lip Morsicatio linguarum - tongue |
|
|
What can be observed clinically/histologically from morsicatio labiorum |
lip biting; considered a deliberate injury; clinical pathology, but no significant histological changes, just some acute inflammatory cells, no great tissue change |
|
|
What can be observed clinically/histologically from morsicatio buccarum |
cheek biting; more chronic in presentation than lip biting injury, may see tissue changes in biopsy
most chronic change is hyperplastic - see more epithelial cells than in normal tissue |
|
|
What are the four categories of bruising attributable to trauma |
Petechiae - pinpoint Purpura - slightly larger than petechiae Ecchymosis - true bruise; more than 2cm in diameter Hematoma - large swelling, palpable
first three are macular, hematoma is raised (mass filled with blood) |
|
|
What happens if a bruise persists |
If it's a normal bruise related to trauma it should resolve on its own in a 10-14 days; if pathology lasts for more than a couple weeks, should determine cause, probably underlying systemic disease |
|
|
What are the three differential diagnoses for Petechiae and what tests do you do to determine the cause |
Trauma - take pt history, learn if it suggests trauma Systemic - blood testing Viral - add'l testing to rule out viral infection |
|
|
What are the most common causes and sites of Petechiae |
Trauma - forceful cough, bulimia, BJs Systemic - coagulation type disorders (hemophilia, thrombocytopenia) Viral infections - epstein-barr virus, mononucleosis; measles, HIV
Commonly occur on soft palate in oral petechiae |
|
|
What is a bruise |
trauma-induced extravasation of blood into surrounding tissues |
|
|
What are purpura, how do they differ from petechiae |
slightly larger area of subdermal or submucosal hemorrhage; can be induced by trauma, or by systemic disease (e.g. thrombocytopenia) |
|
|
What is an ecchymosis |
hemorrhagic accumulation of over 2 cm; usually induced by trauma with or without underlying systemic disease (e.g. hemophilia) |
|
|
What is the most common cause of intraoral ulceration |
Trauma |
|
|
What are non-specific ulcers, how do they occur and when do they usually heal |
intraoral ulceration caused by trauma; sudden onset, usually induced by a sharp cusp or restoration, fractured appliance, etc; non-specific because they have no distinct histologic features except ulceration of epithelium and acute/chronic inflammation |
|
|
What is an example of an ulcer that may persist for an extended period of time even with treatment |
Eosinophilic ulcer |
|
|
What is the clinical presentation of an eosinophilic ulcer |
irregular, solitary ulcer surfaced by a fibrinous membrane an surrounded by a zone of erythema; margins typically raised and indurated; may emanate pus; any mucosal surface may be affected, but tongue = ~60% of cases |
|
|
What can many cases of eosinophilic ulcers and reactive hyperplasias resemble |
Cancer |
|
|
How do eosinophilic ulcers appear histologically |
mixed inflammatory reaction - large mononuclear cells, numerous eosinophils, and lymphocytes |
|
|
What types of ulcers should ALWAYS be biopsied and what should the biopsy include |
Non-healing ulcers; differential diagnosis is trauma, infection, cancer
Biopsy of eroded or ulcerated area should ALWAYS include a portion of adjacent, intact epithelium |
|
|
What is the disease in which a form of eosinophilic ulcer develops in infants and why does this occur |
Riga-Fede disease; usually occurs on anterior ventral tongue; associated with developing anterior primary teeth and nursing; usually resolved by using a protective shield over damaged tissue |
|
|
What are often given to provide temporary relief/comfort when someone has an eosinophilic ulcer |
NSAIDs or topical anesthetics, topical corticosteroids with Orabase |
|
|
What are examples of caustic chemicals that dentists frequently use in clinical practice and what do they do |
Methyl methacrylate - keep component of acrylic (may develop contact dermatitis or contact stomatitis) Sodium hypochlorite/bleach - used in root canal therapy (chemical burn)
Chemical burn lesions usually resolve without scarring in 10-14 days if patient discontinues use of agent; if necrotic - surgical debridement and antibiotics |
|
|
What is a common example of a thermal burn |
pizza burn - appears as area of erythema and ulceration with focal necrosis; usually heal within a few days |
|
|
What is an extreme example of a thermal burn and what occurs |
Electrical burn; Usually when mucosa contacts exposed wire; lips most frequently affected; involves charring of area, marked edema, and eventually necrosis; treatment includes tetanus immunization, antibiotics
Extensive scarring may develop during healing - microstomia (want to prevent this) |
|
|
What is nicotine stomatitis an example of and what occurs |
reactive hyperplasia; benign, asymptomatic condition of palate in smokers; develops in response to the heat generated by the tobacco product, not to the carcinogens; usually in pipe smokers (more heat); presents as red dots (dilated or obstructed salivary glands) and then gray-white papules (inflamed minor salivary glands); see hyperplastic, hyperkeratotic epithelium over mild, chronically inflamed CT; completely reversible if pt stops smoking, healing within 1-2 weeks |
|
|
What is denture-induced reactive hyperplasia |
result of ill-fitting dentures, bad denture hygiene or wearing dentures 24 hours a day; associated with Candida albicans; presents as multiple, asymptomatic erythematous papillary or papular projections in epithelium with underlying well-vascularized, very inflamed, fibrous CT; inflammatory response - lymphocytes and plasma cells; tx by denture removal, improved denture hygiene, reline/refabricate denture, topical anti-fungal, surgical excision if serious (don't misdiagnose as cancer!) |
|
|
What type of reactive hyperplasia is associated with Candida albicans |
Denture-induced |
|
|
What is an epulis fissuratum |
reactive lesion that develops in response to chronic irritation or trauma; induced by ill-fitting denture; more so in females; presents as asymptomatic, unilateral or bilateral folds of hyperplastic tissue in vestibules; typically clinically diagnostic but should be biopsied because could be carcinoma; dense hyperplastic, fibrous CT with hyperparakeratotic and hyperplastic epithelium; usually treat with surgical excision, also reline/refabricate denture |
|
|
What three lesion types are induced by ill-fitting dentures |
Denture-induced reactive hyperplasia Epulis fissuratum Leaf-like denture fibroma |
|
|
What is a leaf-like denture fibroma |
reactive lesion in response to ill-fitting denture; typically a flat, pedunculated, serrated mass on hard palate underneath max denture; dense, hyperplastic, fibrous CT with hyperparakeratotic and hyperplastic epithelium; surgical excision, reline/refabricate denture |
|
|
What is a reactive osseous and chondromatous metaplasia |
cutright lesion; in response to chronic mechanical denture irritation; presents as solitary, painful, tumor-like, exophytic growth, develops on atrophied alveolar ridge (usually posterior mandibular); tx: curative excisional biopsy, recontour/graft alveolar ridge, new denture; see metaplastic cartilage and bone |
|
|
What are some of the etiologic factors that can induce generalized gingival hyperplasia |
Local factors - plaque, calculus Hormonal factors - puberty and pregnancy Medications - phenytoin, cyclosporin, nifedipine (three most common causes of this disorder) Leukemia Hereditary factors |
|
|
How does generalized gingival hyperplasia present |
begins as marginal gingivitis, followed by enlargement of interdental papillae; erythema and edema due to superimposed inflammation; dental plaque and calculus/oral hygiene plays a huge role
tx: gingivectomy, good oral hygiene program, stop meds |
|
|
What are the four main examples of tumor-like reactive growth |
Irritation fibroma Pyogenic granuloma Peripheral ossifying fibroma Peripheral giant cell granuloma |
|
|
What is the most common intraoral soft tissue growth |
Fibroma |
|
|
How do most fibromas develop |
In response to chronic irritation or trauma |
|
|
How does a fibroma usually present |
As a smooth surfaced, painless, sessile, nodular growth of varying size; similar or lighter color than surrounding mucosa; can become secondarily symptomatic; seen anywhere in oral mucosa; NO malignant potential |
|
|
How do most pyogenic granulomas develop |
reactive lesion in response to chronic irritation or trauma or altered physiologic condition; more so in females - possible hormonal influence |
|
|
How does a pyogenic granuloma present |
Polypoid, red, pedunculated mass that bleeds easily when touched; can grow rapidly and achieve large size within a few weeks; overlying mucosa ulcerated; usually see on gingiva, especially in pregnant women; periapical radiograph reveals cupping/pressure resorption of underlying alveolar bone; commonly on fingers and face; granulation tissue histology; recurrence common |
|
|
What is a variant of pyogenic granuloma that develops exclusively on the gingiva |
Epulis granulomatosum; grow out of recent extraction socket, may represent exuberant tissue response to an irritant |
|
|
What is a peripheral ossifying fibroma |
reactive lesion in response to chronic irritation or trauma; mainly in young adults/adolescents, more so in females; ONLY in gingiva, usually from interdental papilla |
|
|
How does a peripheral ossifying fibroma present |
red, sessile or pedunculated mass of varying size; usually present a number of weeks before noticed; usually ulcerated; radiopacity in radiograph; cupping/pressure resorption of underlying alveolar bone; dystrophic calcifications |
|
|
What is a peripheral giant cell granuloma |
benign, reactive lesion that develops in response to chronic irritating factors - tooth extraction, ill-fitting denture, calculus; usually 40-60 years old; more so in females; usually sessile; ONLY on gingiva/edentulous ridge; can be 2cm; ulceration secondary to trauma; cupping/pressure resorption on underlying alveolar bone |
|
|
How does a peripheral giant cell granuloma present histologically |
unencapsulated, richly cellular mass - abundant, scattered multinucleated giant cells; atrophic or ulcerated epithelium with significant intersitial hemorrhage; hemosiderin deposits; islands of metaplastic bone |

