![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
117 Cards in this Set
- Front
- Back
|
Cellular Swelling of the kidney.
Cytoplasm ranges from finely granular to frankly vauolated. |
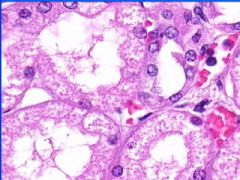
This is an example of?
|
|
|
Cellular swelling
|
Note the pattern of changes occurring in the nephron. What is happening?
|
|
|
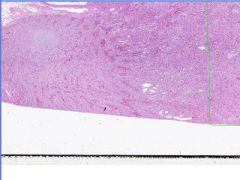
Hypertrophy of Myocardium.
|
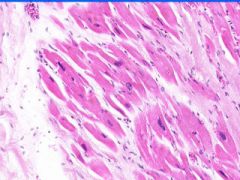
Note the range in cell and nuclear size. Where is this? What is occurring?
|
|
|
Box-car nucleus of hypertrophied myocyte. Hyertrophy of myocardium.
|
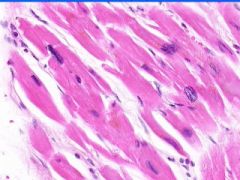
This is an example of what?
|
|
|
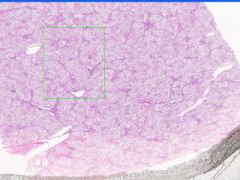
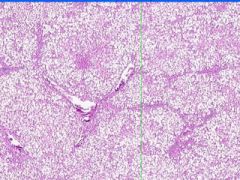
Fatty changes of the liver
|
This is an example of what?
|
|
|
Fatty changes of the liver.
In alcoholic fatty liver, lipid accumulates within the cytoplasm of hepatocytes, creating large clear vacuoles within cells. The nuclei in such cells are compressed to the periphery of the cell. Hepatic steatosis may be associated with slight hepatomegaly and elevations of serum bilirubin and alkaline phosphatase. |
This is an example of what?
|
|
|
Cell Death: Liver Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
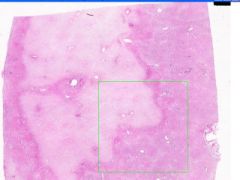
Where is this?
What do you see? Mechanism? Microscopic features? What causes it? |
|
|
Cell Death: Liver Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
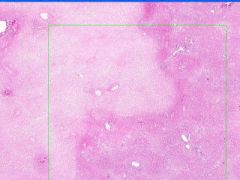
What is this?
What do you see? Mechanism? Microscopic features? What causes it? |
|
|
Cell Death: Liver Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
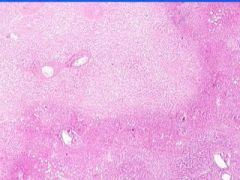
What is this?
What do you see? Mechanism? Microscopic features? What causes this? |
|
|
Cell Death: Liver Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
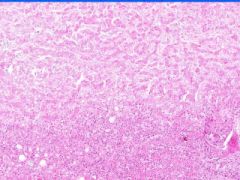
What is this?
What do you see? Mechanism? Microscopic features? What causes this? |
|
|
Cell Death: Liver Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
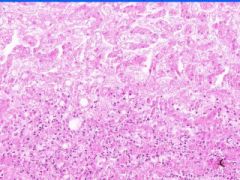
What is this?
What do you see? Mech? Micro? Causes? |
|
|
Cell Death: Liver Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
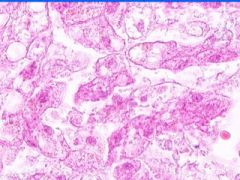
What is this?
What do you see? Mechanism? Micro features? Causes? |
|
|
Cell Death: Spleen Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
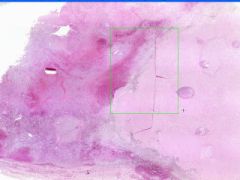
What is this?
What do you see? Mechanism? Micro features? Causes? |
|
|
Cell Death: Spleen Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
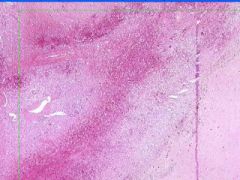
What is this?
|
|
|
Cell Death: Spleen Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
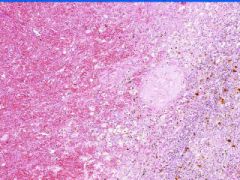
Example of:
|
|
|
Cell Death: Spleen Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
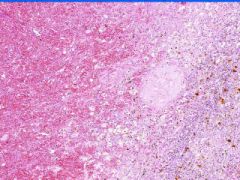
Example of:
|
|
|
Cell Death: Spleen Coagulation Necrosis
Preservation of structural outline of dead cells. Bordered by area of hemorrhage and congestion and then viable tissue. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
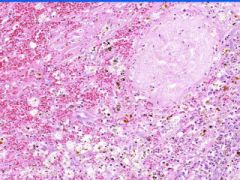
Example of:
|
|
|
Cell Death: Spleen Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
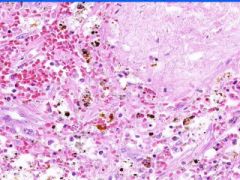
Example of:
|
|
|
Cell Death: Kidney Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
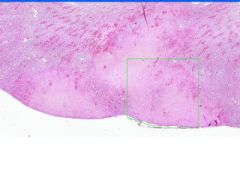
Example of:
|
|
|
Cell Death: Kidney Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
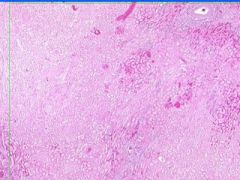
Example of:
|
|
|
Cell Death: Kidney Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
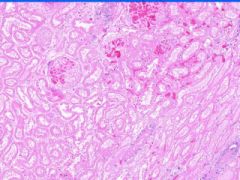
Exmaple of:
|
|
|
Cell Death: Kidney Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
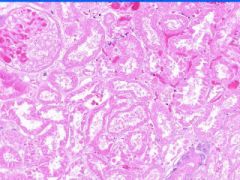
Example of:
|
|
|
Cell Death: Kidney Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
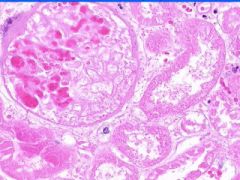
Example of:
|
|
|
Cell Death: Kidney Coagulation Necrosis
Preservation of structural outline of dead cells. Mechanism is the denaturization of enzymes and structural proteins and the inactivation of intracellular enzymes prevent dissolution. Microscopic features are outlines of cells, absent nuclei, karyolysis Infarction |
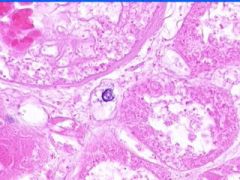
Example of:
|
|
|
Cell Death: Pancreatic Fat Necrosis in Acute Pancreatitis
Digested fat appears translucent pink. Purple staining material are calcium "soaps" of fatty acids. Activation of pancreatic lipase causes hydrolysis of TAGs. Saponification combines FAs and calcium. Pale outlines of fat cells filled with basophilic calcified areas. |
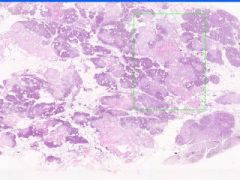
Example of:
|
|
|
Cell Death: Pancreatic Fat Necrosis in Acute Pancreatitis
Digested fat appears translucent pink. Purple staining material are calcium "soaps" of fatty acids. Activation of pancreatic lipase causes hydrolysis of TAGs. Saponification combines FAs and calcium. Pale outlines of fat cells filled with basophilic calcified areas. |
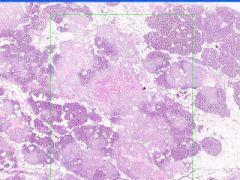
Example of:
|
|
|
Cell Death: Pancreatic Fat Necrosis in Acute Pancreatitis
Digested fat appears translucent pink. Purple staining material are calcium "soaps" of fatty acids. Activation of pancreatic lipase causes hydrolysis of TAGs. Saponification combines FAs and calcium. Pale outlines of fat cells filled with basophilic calcified areas. |
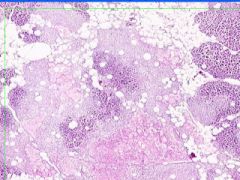
Example of:
|
|
|
Cell Death: Pancreatic Fat Necrosis in Acute Pancreatitis
Digested fat appears translucent pink. Purple staining material are calcium "soaps" of fatty acids. Activation of pancreatic lipase causes hydrolysis of TAGs. Saponification combines FAs and calcium. Pale outlines of fat cells filled with basophilic calcified areas. |
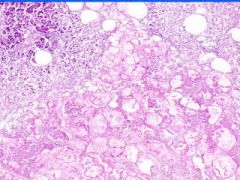
Example of:
|
|
|
Cell Death: Pancreatic Fat Necrosis in Acute Pancreatitis
Digested fat appears translucent pink. Purple staining material are calcium "soaps" of fatty acids. Activation of pancreatic lipase causes hydrolysis of TAGs. Saponification combines FAs and calcium. Pale outlines of fat cells filled with basophilic calcified areas. |
Example of:
|
|
|
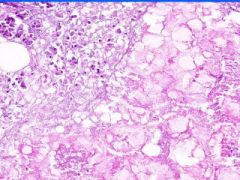
Cell Death: Pancreatic Fat Necrosis in Acute Pancreatitis
Digested fat appears translucent pink. Purple staining material are calcium "soaps" of fatty acids. Activation of pancreatic lipase causes hydrolysis of TAGs. Saponification combines FAs and calcium. Pale outlines of fat cells filled with basophilic calcified areas. |
Example of:
|
|
|
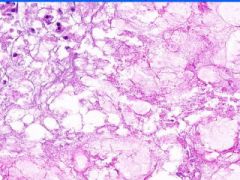
Cell Death: Pancreatic Fat Necrosis in Acute Pancreatitis
Digested fat appears translucent pink. Purple staining material are calcium "soaps" of fatty acids. Activation of pancreatic lipase causes hydrolysis of TAGs. Saponification combines FAs and calcium. Pale outlines of fat cells filled with basophilic calcified areas. |
Example of:
|
|
|
Cell Death: Caseation Necrosis of Spleen
Variant of coagulation necrosis with acellular cheese-like material. Formed by release of lipid from the cell walls of TB or histoplasma after destruction by macrophages. Acelleular material contains activated macrophages, CD4s, multinucleated giant cells. |

Example of:
|
|
|
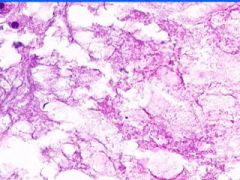
Cell Death: Caseation Necrosis of Spleen
Variant of coagulation necrosis with acellular cheese-like material. Formed by release of lipid from the cell walls of TB or histoplasma after destruction by macrophages. Acellular material contains activated macrophages, CD4s, multinucleated giant cells. |
Example of:
|
|
|
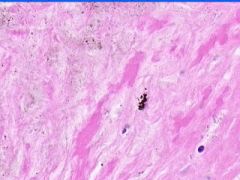
Cell Death: Caseation Necrosis of Spleen
Variant of coagulation necrosis with acellular cheese-like material. Formed by release of lipid from the cell walls of TB or histoplasma after destruction by macrophages. Acellular material contains activated macrophages, CD4s, multinucleated giant cells. |
Example of:
|
|
|
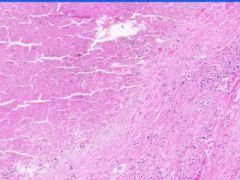
Cell Death: Caseation Necrosis of Spleen
Variant of coagulation necrosis with acellular cheese-like material. Formed by release of lipid from the cell walls of TB or histoplasma after destruction by macrophages. Acellular material contains activated macrophages, CD4s, multinucleated giant cells. |
Example of:
|
|
|
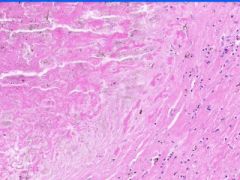
Cell Death: Caseation Necrosis of Spleen
Variant of coagulation necrosis with acellular cheese-like material. Formed by release of lipid from the cell walls of TB or histoplasma after destruction by macrophages. Acellular material contains activated macrophages, CD4s, multinucleated giant cells. |
Example of:
|
|
|
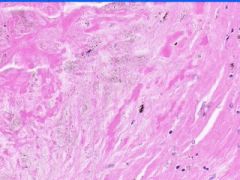
Cell Death: Caseation Necrosis of Spleen
Variant of coagulation necrosis with acellular cheese-like material. Formed by release of lipid from the cell walls of TB or histoplasma after destruction by macrophages. Acellular material contains activated macrophages, CD4s, multinucleated giant cells. |
Example of:
|
|
|
Cellular Death: Liquefaction Necrosis of Brain
Rapid hydrolysis. Microglial cells, which have phagocyized debris have a foamy, granular cytoplasm, "glitter cells". Lysosomal enzymes released by necrotic cells or neutrophils cause liquefaction. Autocatalytic effect of hydrolytic enzymes will produce a cystic space. |
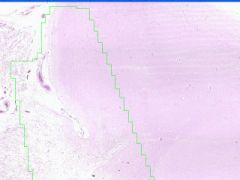
Example of:
|
|
|
Cellular Death: Liquefaction Necrosis of Brain
Rapid hydrolysis. Microglial cells, which have phagocyized debris have a foamy, granular cytoplasm, "glitter cells". Lysosomal enzymes released by necrotic cells or neutrophils cause liquefaction. Autocatalytic effect of hydrolytic enzymes will produce a cystic space. |
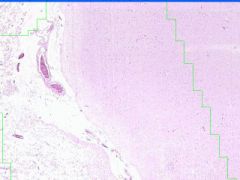
Exmaple of:
|
|
|
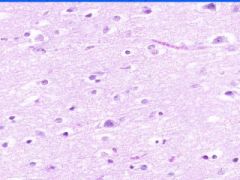
Cellular Death: Liquefaction Necrosis of Brain
Rapid hydrolysis. Microglial cells, which have phagocyized debris have a foamy, granular cytoplasm, "glitter cells". Lysosomal enzymes released by necrotic cells or neutrophils cause liquefaction. Autocatalytic effect of hydrolytic enzymes will produce a cystic space. |

Example of:
|
|
|
Cellular Death: Liquefaction Necrosis of Brain
Rapid hydrolysis. Microglial cells, which have phagocyized debris have a foamy, granular cytoplasm, "glitter cells". Lysosomal enzymes released by necrotic cells or neutrophils cause liquefaction. Autocatalytic effect of hydrolytic enzymes will produce a cystic space. |
Example of:
|
|
|
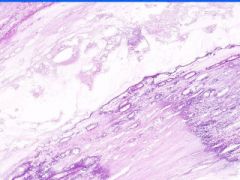
Cell Injury: Hyalinization
Lumen of arteriosclerotic vessel is reduced. The hyaline material contains foci of dystrophic calcification that stains purple and it also contains cholesterol clefts. |
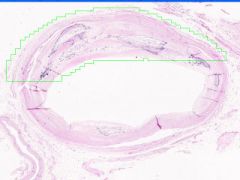
Example of:
|
|
|
Cell Injury: Hyalinization
Lumen of arteriosclerotic vessel is reduced. The hyaline material contains foci of dystrophic calcification that stains purple and it also contains cholesterol clefts. |
Example of:
|
|
|
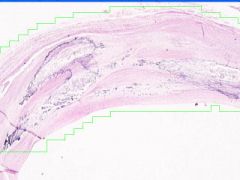
Cell Injury: Hyalinization
Lumen of arteriosclerotic vessel is reduced. The hyaline material contains foci of dystrophic calcification that stains purple and it also contains cholesterol clefts. |
Example of:
|
|
|
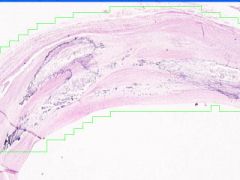
Cell Injury: Hyalinization
Lumen of arteriosclerotic vessel is reduced. The hyaline material contains foci of dystrophic calcification that stains purple and it also contains cholesterol clefts. |
Example of:
|
|
|
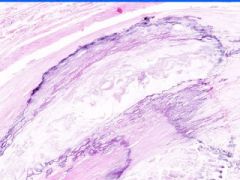
Cell Injury: Hyalinization
Lumen of arteriosclerotic vessel is reduced. The hyaline material contains foci of dystrophic calcification that stains purple and it also contains cholesterol clefts. |
Example of:
|
|
|
Cell Injury: Hyalinization
Lumen of arteriosclerotic vessel is reduced. The hyaline material contains foci of dystrophic calcification that stains purple and it also contains cholesterol clefts. |
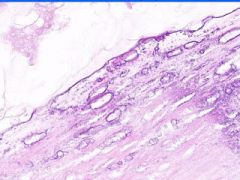
Example of:
|
|
|
Cell Injury: Hyalinization
Lumen of arteriosclerotic vessel is reduced. The hyaline material contains foci of dystrophic calcification that stains purple and it also contains cholesterol clefts. |
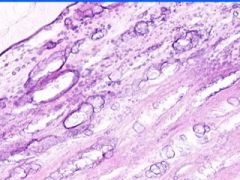
Example of:
|
|
|
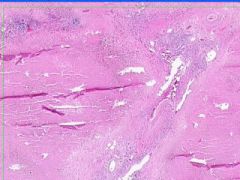
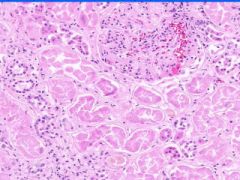
Cell Injury: Hyaline Change of Kidney
Walls of arteries (small and medium) and afferent arterioles are thickened and have amorphous pale pink structureless appearance. |
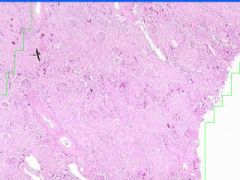
Example of:
|
|
|
Cell Injury: Hyaline Change of Kidney
Walls of arteries (small and medium) and afferent arterioles are thickened and have amorphous pale pink structureless appearance. |
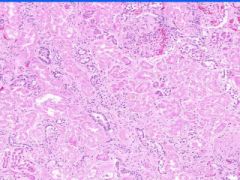
Example of:
|
|
|
Cell Injury: Hyaline Change of Kidney
Walls of arteries (small and medium) and afferent arterioles are thickened and have amorphous pale pink structureless appearance. |
Example of:
|
|
|
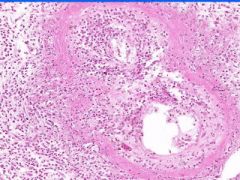
Cell Injury: Fibrinoid Necrosis of Liver
Deeply eosinophilic, smudged, fibrin-like appearance. Deposition of pink-staining proteinaceous material in damaged vessel walls due to basement membranes. Associated with Immune Vasculitis and Malignant Hypertension |
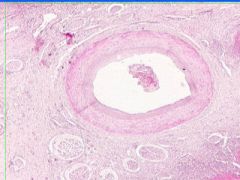
Example of:
|
|
|
Cell Injury: Fibrinoid Necrosis of Liver
Deeply eosinophilic, smudged, fibrin-like appearance. Deposition of pink-staining proteinaceous material in damaged vessel walls due to basement membranes. Associated with Immune Vasculitis and Malignant Hypertension |
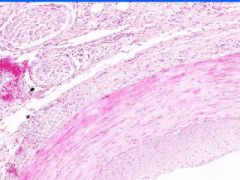
Example of:
|
|
|
Cell Injury: Fibrinoid Necrosis of Liver
Deeply eosinophilic, smudged, fibrin-like appearance. Deposition of pink-staining proteinaceous material in damaged vessel walls due to basement membranes. Associated with Immune Vasculitis and Malignant Hypertension |
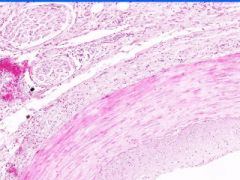
Example of:
|
|
|
Cell Injury: Fibrinoid Necrosis of Liver
Deeply eosinophilic, smudged, fibrin-like appearance. Deposition of pink-staining proteinaceous material in damaged vessel walls due to basement membranes. Associated with Immune Vasculitis and Malignant Hypertension |
Example of:
|
|
|
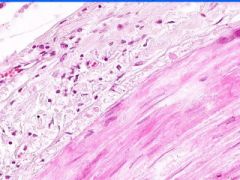
Cell Injury: Fibrinoid Necrosis of Liver
Deeply eosinophilic, smudged, fibrin-like appearance. Deposition of pink-staining proteinaceous material in damaged vessel walls due to basement membranes. Associated with Immune Vasculitis and Malignant Hypertension |
Example of:
|
|
|
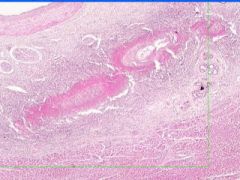
Cell Injury: Fibrinoid Necrosis of Liver
Deeply eosinophilic, smudged, fibrin-like appearance. Deposition of pink-staining proteinaceous material in damaged vessel walls due to basement membranes. Associated with Immune Vasculitis and Malignant Hypertension |
Example of:
|
|
|
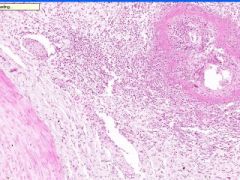
Cell Injury: Fibrinoid Necrosis of Liver
Deeply eosinophilic, smudged, fibrin-like appearance. Deposition of pink-staining proteinaceous material in damaged vessel walls due to basement membranes. Associated with Immune Vasculitis and Malignant Hypertension |
Example of:
|
|
|
Cell Injury: Fibrinoid Necrosis of Liver
Deeply eosinophilic, smudged, fibrin-like appearance. Deposition of pink-staining proteinaceous material in damaged vessel walls due to basement membranes. Associated with Immune Vasculitis and Malignant Hypertension |
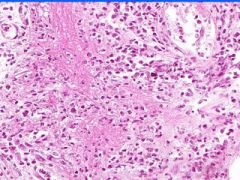
Example of:
|
|
|
Pulmonary Edema due to Starling pressure (transudate)
BV are engorged with blood. Pink-staining proteinaceous edema transited from capillaries. Macrophages and neutrophils are seen in alveoli. Too recent for "heart failure cells". Bronchioles filled with degenerated neutrophils from tracheobronchitis in addition to pulmonary edema and congestion. Bronchioles have lost their epithelium in the acute inflammation process. Increased hydrostatic pressure (L heart failure) Decreased oncotic pressure (nephrotic syndrome, cirrhosis) |
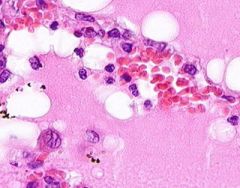
Example of:
|
|
|
Pulmonary Edema due to Starling pressure (transudate)
BV are engorged with blood. Pink-staining proteinaceous edema transited from capillaries. Macrophages and neutrophils are seen in alveoli. Too recent for "heart failure cells". Bronchioles filled with degenerated neutrophils from tracheobronchitis in addition to pulmonary edema and congestion. Bronchioles have lost their epithelium in the acute inflammation process. Increased hydrostatic pressure (L heart failure) Decreased oncotic pressure (nephrotic syndrome, cirrhosis) |
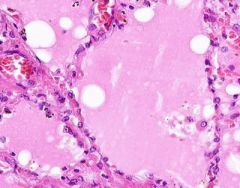
Example of:
|
|
|
Pulmonary Edema due to Starling pressure (transudate)
BV are engorged with blood. Pink-staining proteinaceous edema transited from capillaries. Macrophages and neutrophils are seen in alveoli. Too recent for "heart failure cells". Bronchioles filled with degenerated neutrophils from tracheobronchitis in addition to pulmonary edema and congestion. Bronchioles have lost their epithelium in the acute inflammation process. Increased hydrostatic pressure (L heart failure) Decreased oncotic pressure (nephrotic syndrome, cirrhosis) |
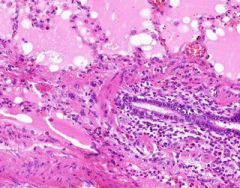
Example of:
|
|
|
Pulmonary Edema due to Starling pressure (transudate)
BV are engorged with blood. Pink-staining proteinaceous edema transited from capillaries. Macrophages and neutrophils are seen in alveoli. Too recent for "heart failure cells". Bronchioles filled with degenerated neutrophils from tracheobronchitis in addition to pulmonary edema and congestion. Bronchioles have lost their epithelium in the acute inflammation process. Increased hydrostatic pressure (L heart failure) Decreased oncotic pressure (nephrotic syndrome, cirrhosis) |
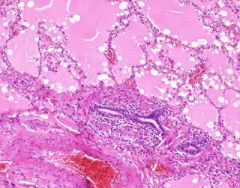
Example of:
|
|
|
Pulmonary Edema due to Starling pressure (transudate)
BV are engorged with blood. Pink-staining proteinaceous edema transited from capillaries. Macrophages and neutrophils are seen in alveoli. Too recent for "heart failure cells". Bronchioles filled with degenerated neutrophils from tracheobronchitis in addition to pulmonary edema and congestion. Bronchioles have lost their epithelium in the acute inflammation process. Increased hydrostatic pressure (L heart failure) Decreased oncotic pressure (nephrotic syndrome, cirrhosis) |
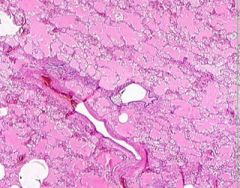
Example of:
|
|
|
Pulmonary Edema due to Starling pressure (transudate)
BV are engorged with blood. Pink-staining proteinaceous edema transited from capillaries. Macrophages and neutrophils are seen in alveoli. Too recent for "heart failure cells". Bronchioles filled with degenerated neutrophils from tracheobronchitis in addition to pulmonary edema and congestion. Bronchioles have lost their epithelium in the acute inflammation process. Increased hydrostatic pressure (L heart failure) Decreased oncotic pressure (nephrotic syndrome, cirrhosis) |
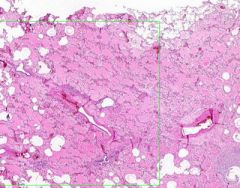
Example of:
|
|
|
Congestion of Liver
Principally in the central areas of the lobules. Sinusoids are dilated and filled with blood. Finely granular, brown, blood pigments (hemosiderin) found extracellularly and in centro-hepatic and Kupffer cells. Necrosis around central veins, due to anoxia, which may lead to infiltration of PMNLs.a |
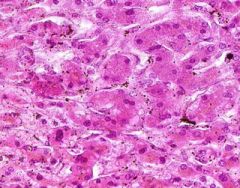
Example of:
|
|
|
Congestion of Liver
Principally in the central areas of the lobules. Sinusoids are dilated and filled with blood. Finely granular, brown, blood pigments (hemosiderin) found extracellularly and in centro-hepatic and Kupffer cells. Necrosis around central veins, due to anoxia, which may lead to infiltration of PMNLs.a |
Example of:
|
|
|
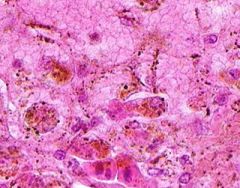
Congestion of Liver
Principally in the central areas of the lobules. Sinusoids are dilated and filled with blood. Finely granular, brown, blood pigments (hemosiderin) found extracellularly and in centro-hepatic and Kupffer cells. Necrosis around central veins, due to anoxia, which may lead to infiltration of PMNLs.a |
Example of:
|
|
|
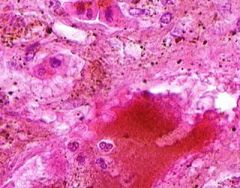
Congestion of Liver
Principally in the central areas of the lobules. Sinusoids are dilated and filled with blood. Finely granular, brown, blood pigments (hemosiderin) found extracellularly and in centro-hepatic and Kupffer cells. Necrosis around central veins, due to anoxia, which may lead to infiltration of PMNLs.a |
Example of:
|
|
|
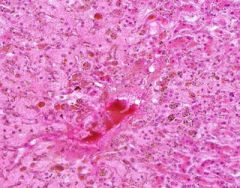
Congestion of Liver
Principally in the central areas of the lobules. Sinusoids are dilated and filled with blood. Finely granular, brown, blood pigments (hemosiderin) found extracellularly and in centro-hepatic and Kupffer cells. Necrosis around central veins, due to anoxia, which may lead to infiltration of PMNLs. |
Example of:
|
|
|
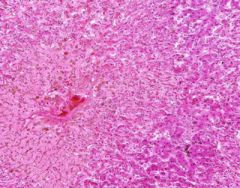
Congestion of Liver
Principally in the central areas of the lobules. Sinusoids are dilated and filled with blood. Finely granular, brown, blood pigments (hemosiderin) found extracellularly and in centro-hepatic and Kupffer cells. Necrosis around central veins, due to anoxia, which may lead to infiltration of PMNLs. |
Example of:
|
|
|
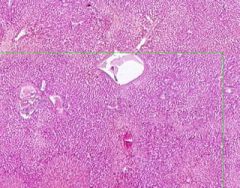
Congestion of Liver
Principally in the central areas of the lobules. Sinusoids are dilated and filled with blood. Finely granular, brown, blood pigments (hemosiderin) found extracellularly and in centro-hepatic and Kupffer cells. Necrosis around central veins, due to anoxia, which may lead to infiltration of PMNLs. |
Example of:
|
|
|
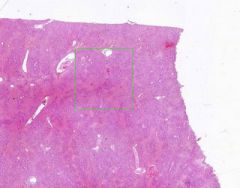
Edema of lung without congestion.
Alveoli are uniformly filled by homogenous, pale-staining, pink edema fluid. Alveolar capillaries show no distention or congestion. Macrophages laden with black coal dust pigment (anthracosis). |
Example of:
|
|
|
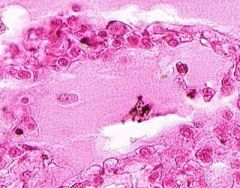
Edema of lung without congestion.
Alveoli are uniformly filled by homogenous, pale-staining, pink edema fluid. Alveolar capillaries show no distention or congestion. Macrophages laden with black coal dust pigment (anthracosis). |
Example of:
|
|
|
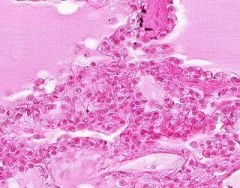
Edema of lung without congestion.
Alveoli are uniformly filled by homogenous, pale-staining, pink edema fluid. Alveolar capillaries show no distention or congestion. Macrophages laden with black coal dust pigment (anthracosis). |
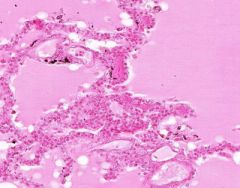
Example of:
|
|
|
Edema of lung without congestion.
Alveoli are uniformly filled by homogenous, pale-staining, pink edema fluid. Alveolar capillaries show no distention or congestion. Macrophages laden with black coal dust pigment (anthracosis). |
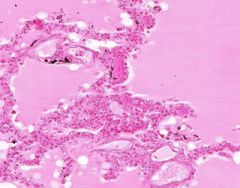
Example of:
|
|
|
Edema of lung without congestion.
Alveoli are uniformly filled by homogenous, pale-staining, pink edema fluid. Alveolar capillaries show no distention or congestion. Macrophages laden with black coal dust pigment (anthracosis). |
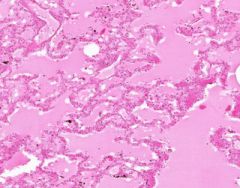
Example of:
|
|
|
Edema of lung without congestion.
Alveoli are uniformly filled by homogenous, pale-staining, pink edema fluid. Alveolar capillaries show no distention or congestion. Macrophages laden with black coal dust pigment (anthracosis). |
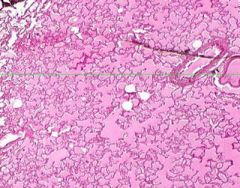
Example of:
|
|
|
Edema of lung without congestion.
Alveoli are uniformly filled by homogenous, pale-staining, pink edema fluid. Alveolar capillaries show no distention or congestion. Macrophages laden with black coal dust pigment (anthracosis). |
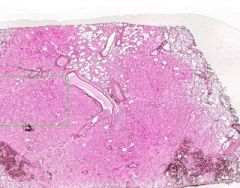
Example of:
|
|
|
Inflammatory Edema: Acute Bronchopneumonia
Alveolar septa are widened and alveolar spaces contain inflammatory cells, including neutrophils and fluid. Fibrillar material is seen in some spaces, from cleavage of plasma protein fibrinogen. Dark blue dot-like structures are present within alveoli and admixed with the inflammatory exudate. These are bacteria. |
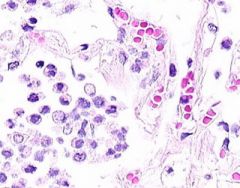
Example of:
|
|
|
Inflammatory Edema: Acute Bronchopneumonia
Alveolar septa are widened and alveolar spaces contain inflammatory cells, including neutrophils and fluid. Fibrillar material is seen in some spaces, from cleavage of plasma protein fibrinogen. Dark blue dot-like structures are present within alveoli and admixed with the inflammatory exudate. These are bacteria. |
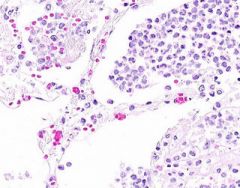
Example of:
|
|
|
Inflammatory Edema: Acute Bronchopneumonia
Alveolar septa are widened and alveolar spaces contain inflammatory cells, including neutrophils and fluid. Fibrillar material is seen in some spaces, from cleavage of plasma protein fibrinogen. Dark blue dot-like structures are present within alveoli and admixed with the inflammatory exudate. These are bacteria. |
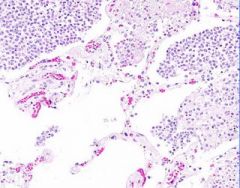
Example of:
|
|
|
Inflammatory Edema: Acute Bronchopneumonia
Alveolar septa are widened and alveolar spaces contain inflammatory cells, including neutrophils and fluid. Fibrillar material is seen in some spaces, from cleavage of plasma protein fibrinogen. Dark blue dot-like structures are present within alveoli and admixed with the inflammatory exudate. These are bacteria. |
Example of:
|
|
|
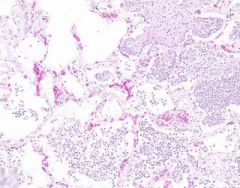
Inflammatory Edema: Acute Bronchopneumonia
Normal areas of lung show relatively empty alveoli, in contrast to areas where alveolar septa are widened and alveolar spaces contain inflammatory cells, including neutrophils and fluid. Fibrillar material is seen in some spaces, from cleavage of plasma protein fibrinogen. Dark blue dot-like structures are present within alveoli and admixed with the inflammatory exudate. These are bacteria. |
Example of:
|
|
|
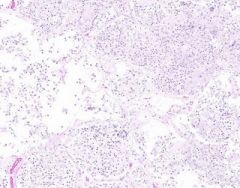
Inflammatory Edema: Acute Bronchopneumonia
Alveolar septa are widened and alveolar spaces contain inflammatory cells, including neutrophils and fluid. Fibrillar material is seen in some spaces, from cleavage of plasma protein fibrinogen. Dark blue dot-like structures are present within alveoli and admixed with the inflammatory exudate. These are bacteria. |
Example of:
|
|
|
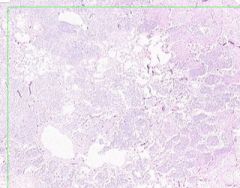
Inflammatory Edema: Acute Bronchopneumonia
Some areas appear relatively normal. Adjacent to the bronchiole is an area that appears almost solid. Alveolar septa are widened and alveolar spaces contain inflammatory cells, including neutrophils and fluid. |
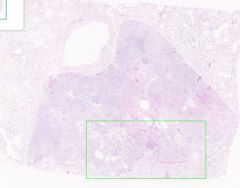
Example of:
|
|
|
Congestion of Spleen
Red pulp is congested. Sinusoids are dilated. Degenerating erythrocytes are present. Lymphoid follicles are small. Trabeculae are prominant, and hyalinized arterioles are present within them. Hyalinization is present in the arterioles of the Malpighian bodies (white pulp, lymph follicles). Hyalin lies in the intima about the lumen of arterioles. |
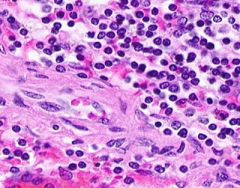
Example of:
|
|
|
Congestion of Spleen
Red pulp is congested. Sinusoids are dilated. Degenerating erythrocytes are present. Lymphoid follicles are small. Trabeculae are prominant, and hyalinized arterioles are present within them. Hyalinization is present in the arterioles of the Malpighian bodies (white pulp, lymph follicles). Hyalin lies in the intima about the lumen of arterioles. |
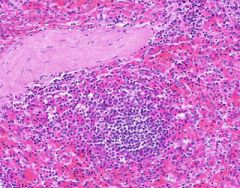
Example of:
|
|
|
Congestion of Spleen
Red pulp is congested. Sinusoids are dilated. Degenerating erythrocytes are present. Lymphoid follicles are small. Trabeculae are prominant, and hyalinized arterioles are present within them. Hyalinization is present in the arterioles of the Malpighian bodies (white pulp, lymph follicles). Hyalin lies in the intima about the lumen of arterioles. |
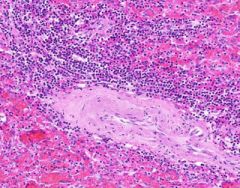
Example of:
|
|
|
Congestion of Spleen
Red pulp is congested. Sinusoids are dilated. Degenerating erythrocytes are present. Lymphoid follicles are small. Trabeculae are prominant, and hyalinized arterioles are present within them. Hyalinization is present in the arterioles of the Malpighian bodies (white pulp, lymph follicles). Hyalin lies in the intima about the lumen of arterioles. |
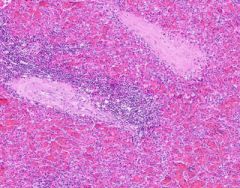
Example of:
|
|
|
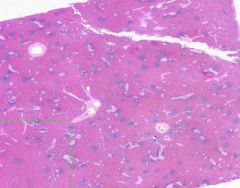
Congestion of Spleen
Red pulp is congested. Sinusoids are dilated. Degenerating erythrocytes are present. Lymphoid follicles are small. Trabeculae are prominant, and hyalinized arterioles are present within them. Hyalinization is present in the arterioles of the Malpighian bodies (white pulp, lymph follicles). Hyalin lies in the intima about the lumen of arterioles. |
Example of:
|
|
|
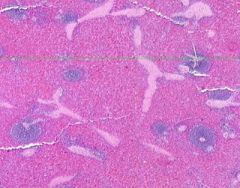
Congestion of Spleen
Red pulp is congested. Sinusoids are dilated. Degenerating erythrocytes are present. Lymphoid follicles are small. Trabeculae are prominant, and hyalinized arterioles are present within them. Hyalinization is present in the arterioles of the Malpighian bodies (white pulp, lymph follicles). Hyalin lies in the intima about the lumen of arterioles. |
Example of:
|
|
|
Post-mortem clot
Chicken-fat layer- fibrin with enmeshed leukocytes. Currant-jelly layer- erythrocytes, some leukocytes and fibrin. |

Example of:
|
|
|
Post-mortem clot
Chicken-fat layer- fibrin with enmeshed leukocytes. Currant-jelly layer- erythrocytes, some leukocytes and fibrin. |

Example of:
|
|
|
Artery has old organized and recanalized thrombus in its lumen. Collagenous tissue containing moderate number of fibrocytes and endothelial-lined vascular channels. The intima, into which the thrombus blends, shows hyaline thickening. The internal elastic lamina is serrated and reduplicated in areas. Media has calcification, as streaks and aggregates of dark blue and purple. Near the zone of calcification there appears to be bone spicules.
|
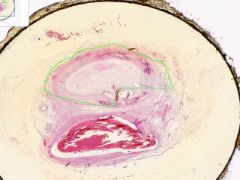
Example of:
|
|
|
Artery has old organized and recanalized thrombus in its lumen. Collagenous tissue containing moderate number of fibrocytes and endothelial-lined vascular channels. The intima, into which the thrombus blends, shows hyaline thickening. The internal elastic lamina is serrated and reduplicated in areas. Media has calcification, as streaks and aggregates of dark blue and purple. Near the zone of calcification there appears to be bone spicules.
|
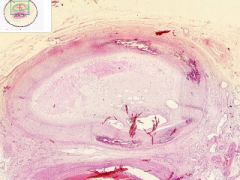
Example of:
|
|
|
Artery has old organized and recanalized thrombus in its lumen. Collagenous tissue containing moderate number of fibrocytes and endothelial-lined vascular channels. The intima, into which the thrombus blends, shows hyaline thickening. The internal elastic lamina is serrated and reduplicated in areas. Media has calcification, as streaks and aggregates of dark blue and purple. Near the zone of calcification there appears to be bone spicules.
|
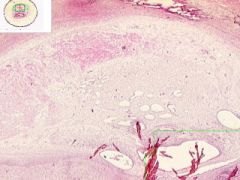
Example of:
|
|
|
Artery has old organized and recanalized thrombus in its lumen. Collagenous tissue containing moderate number of fibrocytes and endothelial-lined vascular channels. The intima, into which the thrombus blends, shows hyaline thickening. The internal elastic lamina is serrated and reduplicated in areas. Media has calcification, as streaks and aggregates of dark blue and purple. Near the zone of calcification there appears to be bone spicules.
|
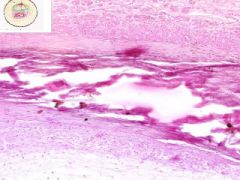
Example of:
|
|
|
Antemortem clot
Clear bands of fibrosis and leukocytes alternating with a layer of RBCs, are "lines of Zahn". |
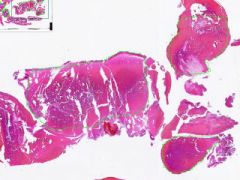
Example of:
|
|
|
Antemortem clot
Clear bands of fibrosis and leukocytes alternating with a layer of RBCs, are "lines of Zahn". |
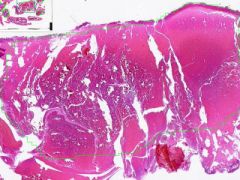
Example of:
|
|
|
Antemortem clot
Clear bands of fibrosis and leukocytes alternating with a layer of RBCs, are "lines of Zahn". |
Example of:
|
|
|
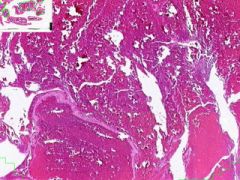
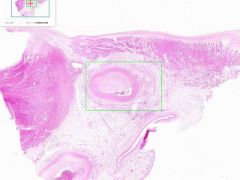
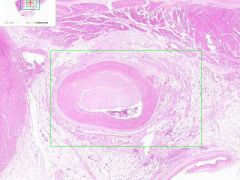
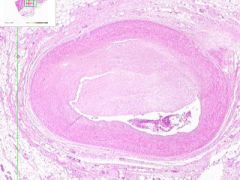
Coronary artery atheroma
Lumen is narrowed. Irregular thickening is due to fibrointimal plaque characteristic of atherosclerosis. |
Example of:
|
|
|
Coronary artery atheroma
Lumen is narrowed. Irregular thickening is due to fibrointimal plaque characteristic of atherosclerosis. |
Example of:
|
|
|
Coronary artery atheroma
Lumen is narrowed. Irregular thickening is due to fibrointimal plaque characteristic of atherosclerosis. |
Example of:
|
|
|
Coronary artery atheroma
Lumen is narrowed. Irregular thickening is due to fibrointimal plaque characteristic of atherosclerosis. |
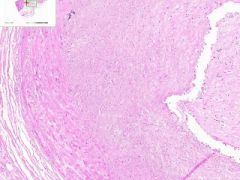
Example of:
|
|
|
Myocardial infarct
Myocytes are undergone coagulative necrosis. Outlines of myocytes are retained. Deeply eosinophilic cytoplasm. Loss of nuclei. Interstitial infiltrate of neutrophils. Necrotic myocyutes are disintegrating and are surrounded by dying neutrophils. |
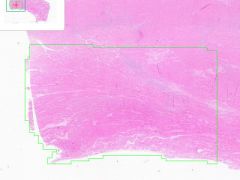
Example of:
|
|
|
Myocardial infarct
Myocytes are undergone coagulative necrosis. Outlines of myocytes are retained. Deeply eosinophilic cytoplasm. Loss of nuclei. Interstitial infiltrate of neutrophils. Necrotic myocyutes are disintegrating and are surrounded by dying neutrophils. |

Example of:
|
|
|
Myocardial infarct
Myocytes are undergone coagulative necrosis. Outlines of myocytes are retained. Deeply eosinophilic cytoplasm. Loss of nuclei. Interstitial infiltrate of neutrophils. Necrotic myocyutes are disintegrating and are surrounded by dying neutrophils. |
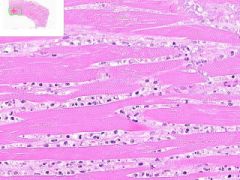
Example of:
|
|
|
Renal Infarct
Inside infarct in coagulative necrosis with loss of cellular detail. Chromatin debris and degenerated PMNLs are present in infarct. Edges of infarct have zone of inflammation and hyperemia. Infiltration of PLMNs in interstitial tissue. Away from infarct the proximal convoluted tubular epithelium shows cloudy swelling, probably due to post mortem autolysis. |
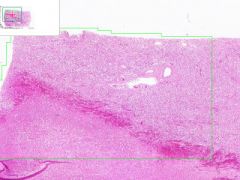
Example of:
|
|
|
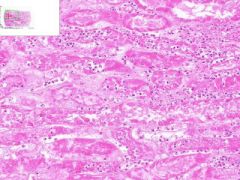
Renal Infarct
Inside infarct in coagulative necrosis with loss of cellular detail. Chromatin debris and degenerated PMNLs are present in infarct. Edges of infarct have zone of inflammation and hyperemia. Infiltration of PLMNs in interstitial tissue. Away from infarct the proximal convoluted tubular epithelium shows cloudy swelling, probably due to post mortem autolysis. |
Example of:
|
|
|
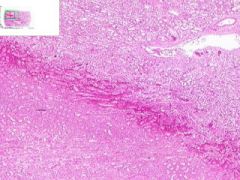
Renal Infarct
Inside infarct in coagulative necrosis with loss of cellular detail. Chromatin debris and degenerated PMNLs are present in infarct. Edges of infarct have zone of inflammation and hyperemia. Infiltration of PLMNs in interstitial tissue. Away from infarct the proximal convoluted tubular epithelium shows cloudy swelling, probably due to post mortem autolysis. |
Example of:
|
|
|
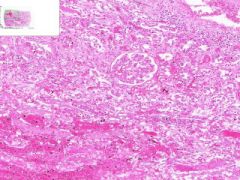
Renal Infarct
Inside infarct in coagulative necrosis with loss of cellular detail. Chromatin debris and degenerated PMNLs are present in infarct. Edges of infarct have zone of inflammation and hyperemia. Infiltration of PLMNs in interstitial tissue. Away from infarct the proximal convoluted tubular epithelium shows cloudy swelling, probably due to post mortem autolysis. |
Example of:
|
|
|
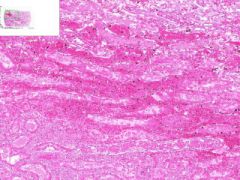
Renal Infarct
Inside infarct in coagulative necrosis with loss of cellular detail. Chromatin debris and degenerated PMNLs are present in infarct. Edges of infarct have zone of inflammation and hyperemia. Infiltration of PLMNs in interstitial tissue. Away from infarct the proximal convoluted tubular epithelium shows cloudy swelling, probably due to post mortem autolysis. |
Example of:
|
|
|
Pulmonary Infarct
Hemorrhagic Infarct Distinguished from a simple hemorrhage by the destruction of alveolar walls. The infarct extends to the pleura on one side. Brownish-black pigment is hemosiderin, from breakdown of hemoglobin in degenerated erythrocytes. The segmented nuclei of degenerated neutrophils are scattered about the infarct. |
Example of:
|
|
|
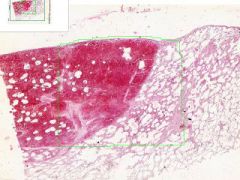
Pulmonary Infarct
Hemorrhagic Infarct Distinguished from a simple hemorrhage by the destruction of alveolar walls. The infarct extends to the pleura on one side. Brownish-black pigment is hemosiderin, from breakdown of hemoglobin in degenerated erythrocytes. The segmented nuclei of degenerated neutrophils are scattered about the infarct. |
Example of:
|
|
|
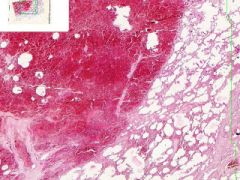
Pulmonary Infarct
Hemorrhagic Infarct Distinguished from a simple hemorrhage by the destruction of alveolar walls. The infarct extends to the pleura on one side. Brownish-black pigment is hemosiderin, from breakdown of hemoglobin in degenerated erythrocytes. The segmented nuclei of degenerated neutrophils are scattered about the infarct. |

Example of:
|
|
|
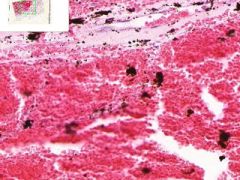
Pulmonary Infarct
Hemorrhagic Infarct Distinguished from a simple hemorrhage by the destruction of alveolar walls. The infarct extends to the pleura on one side. Brownish-black pigment is hemosiderin, from breakdown of hemoglobin in degenerated erythrocytes. The segmented nuclei of degenerated neutrophils are scattered about the infarct. |
Example of:
|

