![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
205 Cards in this Set
- Front
- Back
|
Define Adenoma
|
Benign tumor derived from or forming glands
|
|
|
Define Cystadenoma
|
Benign cystic masses arise from product of neoplastic glandular cells.
Think closed cavity or sac lined by epithelium |
|
|
Define Papilloma
|
Benign finger-like projections from epithelium
|
|
|
Define Polyp
|
Abnormal growth pattern projects above skin or mucosal surface; may be neoplastic or nonneoplastic (inflammatory)
|
|
|
What are the types of polyps?
|
sessile - flat
pedunculated - peduncle on a stalk; think tee-ball papillary - finger-like projections |
|
|
Name the benign tumors in the following locations:
Bone Fibrous tissue Cartilage Smooth Muscle Skeletal Muscle Fat Blood vessels Lymph vessels Epithelium |
Bone - osteoma
Fibrous tissue - fibroma Cartilage - chondroma Smooth Muscle - leiomyoma Skeletal Muscle - rhabdomyoma Fat - lipoma Blood vessels - hemangioma; angioma Lymph vessels - lymphangioma Epithelium - adenoma; cystadenoma; papilloma |
|
|
What is a carcinoma
|
malignant epithelial tumor
endodermal of ectodermal origin |
|
|
What is a squamous cell carcinoma
|
malignant tumor of squamous epithelium
|
|
|
What is an adenocarcinoma
|
malignant tumor of glandular, duct, or columnar cell origin
|
|
|
What is a sarcoma
|
"fleshy malignant tumor"
Non-epithelial malignant tumor in/on organs. |
|
|
Name the two neoplasms of neuroectodermal cells (melanocytes). which is benign? which is malignant?
How do you tell the difference? |
Nevus - benign (mole)
Melanoma - Malignant Nevus is usually brown and fairly circular. usually do not grow or change over time. Melanomas are usually black and do not have clear circular border. Grow rapidly |
|
|
What two tumors are derived from the placenta?
|
Hyditaform mole - benign
Choriocarcoma - malignant |
|
|
What is a seminoma?
|
germ cell tumor of testes
Called dysgerminoma on ovaries. |
|
|
What is a pleomorphic adenoma?
|
mixed tumor. More than one origin cell.
most common neoplasm of the salivary and lacrimal glands. Usually benign |
|
|
What is the most common benign breast tumor?
|
fibroma
|
|
|
What is the most common kidney tumor of children?
|
nephroblastoma
AKA Wilm's tumor malignant |
|
|
What is a renal anlage?
|
embryonic precursor of Wilm's tumor
|
|
|
Define teratoma
|
"monstrous" tumor originating from totipotential cells and germ cells from gonads. Have several different types of tissues inside.
|
|
|
Differentiate between benign and malignant teratoma
|
benign, aka mature teratoma, are dermoid cysts with several types of tissue inside (hair, skin, bone, etc)
malignant, aka immature teratoma, resembles malformed fetus as cells attempt to differentiate and develop embryo |
|
|
Which of the following neoplasms are benign? Which are malignant?
Hepatoma Melanoma Lymphoma Multiple myeloma Mesothelioma Glioma Seminoma |
All malignant even though their names sound benign.
|
|
|
What are the two types of hematopoetic neoplasms?
|
Lymphoma and Leukemia
|
|
|
Define the following tumors?
Are they benign or malignant? Meningioma Neuroma Glioma |
Meningioma - Neoplasm of meninges. Benign but can still kill due to location
Neuroma - benign tumor of nerve origin Glioma - malignant tumor of brain |
|
|
Differentiate between heterotopia, choristoma and hamartoma
|
Heterotopia - normal tissue at abnormal site
Choristoma - cyst or mass of mutiple tissues aggregating in abnormal site. Think: Abnormal tissue in abnormal site. Hamartoma - focal malformation composed of abnormal mixture of tissues native to site. Think: Right tissue. Rite site. Wrong configuration. |
|
|
What do you call a benign tumor of mesenchyme with a mucoid appearance
|
myxoma
|
|
|
Where is the most common myxoma?
|
Heart. Benign tumor that can kill due to location.
|
|
|
What are the four phases of tumor progression?
|
1) Transformation
2) Growth of the transformed cells 3) Local Invasion 4) Distant Metastases |
|
|
What is transformation
|
a malignant change in a target cell
|
|
|
What is differentiation?
|
extent to which neoplastic cells resemble comparable normal cells
|
|
|
What grade would you give a well differentiated tumor?
|
Grade 1
|
|
|
Define anaplasia
|
Lack of differentiation.
|
|
|
What are the morphologic features of anaplasia?
|
1. pleomorphism - variation in size/shape
2. Hyperchromatic nuclei - increased staining 3. Increased nucler/cytoplasmic ratio 4. increased mitotic activity; abnormal mitoses 5. loss of orientation/polarity of cells 6. other: tumor giant cells; necrosis |
|
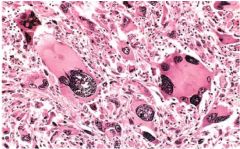
What morphologic features of anaplasia are present here?
|
Pleomorphism
loss of orientation hyperchromatism tumor giant cells |
|
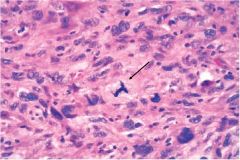
What is going on in this picture?
|
Abnormal mitosis
|
|
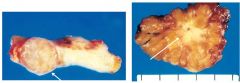
Which breast tumor is malignant? How can you tell?
|
One on right is malignant.
One on left is still encapsulated. One on right is not. |
|
|
what two malignancies do not metastisize
|
gliomas of CNS
basal cell carcinomas of skin |
|
|
What are three pathways of metastisis?
|
Seeding - invasion of surrounding cavity
Lymphatic - tumor invades lymph system. Usually carcinomas Blood borne - tumor invades blood vessels. Usually sarcomas. |
|
|
Where do blood born metasteses usually go?
|
Lung or liver
|
|
|
What is staging and how is it determined?
What is the TNM system? |
Predictor of outcome and course of cancer progression. Derived from statistical studies. Varis with each organ. Used to determine need for further therapy.
T = tumor size/depth N = nodal involvement M = blood born metasteses |
|
|
Stage the following cancers
1. Tis, N0, M0 2. T2, N1, M0 3. T4, N1, M1 4. T1, N0, M1 |
1. Stage I
2. Stage II (small size with nodal invovlement but no blood borne metastisis) 3. Stage IV (large, and has matastisized via node and blood) 4. Stage IV (any blood born metastises = Stage IV, regardless of T or N score) |
|
|
What are the most common forms of cancer in men and women?
|
1. Prostate (men); Breast (women)
2. Lungs 3. Colon/rectum |
|
|
What are the most common cause of cancer deaths in men and women?
|
1. Lungs
2. Prostate (men); Breat (women) 3. Colon/rectum |
|
|
What cancers are associated with alcohol abuse?
|
oropharynx, larynx, esophagus
Hepatocellular 2nd to cirrhosis. Rectal cancers in beer drinkers Increased risk of breast cancer |
|
|
What cancers are associated with smoking?
|
Lip, mouth/oropharynx, larynx, lungs (90% of lung cancer deaths are smoking related), esophagus, pancreas, urinary tract (renal cell carcinoma, transitional carcinoma of kidney, ureters and bladder), carcinoma of uterine cervix
In other words, don't smoke, you idiot! |
|
|
Which cancers are associted with the following occupational carcinogens?
Arsenic Asbestos Benzene Beryllium Ethylene oxide Naphthylamines Radon Vinyl Chloride |
Arsenic - lung, skin, angiosarcoma
Asbestos - lung; mesothelium Benzene - leukemia; lymphoma Beryllium - lung Ethylene oxide - leukemia Naphthylamines - bladder Radon - lung Vinyl Chloride - angiosarcoma |
|
|
what two malignancies do not metastisize
|
gliomas of CNS
basal cell carcinomas of skin |
|
|
What are three pathways of metastisis?
|
Seeding - invasion of surrounding cavity
Lymphatic - tumor invades lymph system. Usually carcinomas Blood borne - tumor invades blood vessels. Usually sarcomas. |
|
|
Where do blood born metasteses usually go?
|
Lung or liver
|
|
|
What is staging and how is it determined?
What is the TNM system? |
Predictor of outcome and course of cancer progression. Derived from statistical studies. Varis with each organ. Used to determine need for further therapy.
T = tumor size/depth N = nodal involvement M = blood born metasteses |
|
|
Stage the following cancers
1. Tis, N0, M0 2. T2, N1, M0 3. T4, N1, M1 4. T1, N0, M1 |
1. Stage I
2. Stage II (small size with nodal invovlement but no blood borne metastisis) 3. Stage IV (large, and has matastisized via node and blood) 4. Stage IV (any blood born metastises = Stage IV, regardless of T or N score) |
|
|
Which occupational carcinogens are associated with the following cancers?
Lung Skin angiosarcoma Mesothelioma Leukemia Lymphoma Bladder |
Lung - arsenic, asbestos, beryllium, radon
Skin - arsenic angiosarcoma - arsenic; vinyl chloride Mesothelioma - asbestos Leukemia - benzene, ethylene oxide Lymphoma - benzene Bladder - naphthylamides |
|
|
Your patient has primary lung cancer. Which of the following is the least likely carcinogen responsible?
a. tobacco smoke b. asbestos c. radon gas d. UV radiation e. aspergillum |
d. UV
If the sun's shining on your lungs you got bigger problems than cancer. |
|
|
How does inflammation contribute to cancer predisposition
|
1. cytokines stimulate cell growth
2. generation of ROS |
|
|
When do most familial cancers tend to occur?
|
While a larger percentage of young people with cancer have familial causes, the largest age group to acquire familial cancer is still >50 yo
|
|
|
What type of genes are most associated with familial cancers
|
tumor suppressor genes
(hence the larger percentage of early onset cancers being familial) |
|
|
Describe Knudson's Two Hit hypothesis
|
Need two bad alleles for tumor suppressor gene to stop working.
Familial cancer pt is born with one bad allele already. Increased risk of developing cancer since they only have to mutate one allele. |
|
|
According to Knudson's Two Hit hypothesis, what four things are patients with inherited mutation of retinoblastoma more likely to get
|
‐ more likely to get cancer
‐ more likely to get cancer at a younger age ‐ more likely to have bilateral cancers ‐ more likely to get second cancers of a different type |
|
|
Your 35 year old breast cancer patient has a mother and an uncle with history of breast cancer. You determine she likely has familial breast cancer. What genes are most likely invovled?
Based on this diagnosis, what other cancers is she at higher risk for? |
BRCA-1
BRCA-2 (particularly associated with male breast cancer) she is at higher risk for second cancer in the other breast and ovarian cancer. |
|
|
What is it called when person with Autosomal Dominant familial cancer gene loses or experiences mutation of the second allele
|
Loss of Heterozygosity
|
|
|
What are the features of Famial adenomatous polyps of the colon and what gene is correlated with it?
|
- develop multiple adenomas of the colon
- has 100% risk for colon cancer - Gene = APC |
|
|
What are the features of Multiple endocrine neoplasia (MEN 2B) and what gene is associated with it?
|
- Marfanoid habitus with ganglioneuromas of tongue
- Gene = RET |
|
|
What familial cancer syndrome is associated with the NF1 gene and what are the features?
|
Neurofibromatosis 1
- Multiple benign neurofibromas, each at risk for transformation, i.e. neurofibrosarcoma. - They have Café au lait spots and lisch nodules in the iris |
|
|
Which is not an autosomal recessive syndromes of defective DNA repair?
A. Xeroderma pigmentosa B. Neurofibromatosis 1 C. Ataxia telangectasia D. Bloom syndrome E. Fanconis anemia |
B. Neurofibromatosis 1
Is is Autosomal Dominant |
|
|
What happens to someone who inherits two nonfunctional NER genes?
What clinical feature will tip you off about this disease? |
Xeroderma pigmentosa
Gets skin cancer in childhood. (Very rare) 2000x more likley to get skin cancer due to inability to repair UV damage. |
|
|
What gene is implicated in Ataxia telangectasia?
How many copies are defective? |
ATM gene
both copies are disfunctional. Autosomal Recessive condition. |
|
|
Explain the Initiator/Promotor concept in relation to cancer using cigarette smoke and alcohol as your carcinogenic agents.
|
Initiator: Benzopyrene in cigarette smoke mutates p53 in cells of esophagus.
Promotor: Alcohol irritates the mutated cells promoting them to reproduce at a higher rate (proliferation od mutation). Note: Cigarette smoke is also a promotor due to irratation. so smoking and drinking is a double whammy. |
|
|
What do the following have in common?
Benzopyrene Vinyl chloride Aflatoxin B1 |
All are potent indirect acting chemical carcinogens capable of initiating genetic mutation
|
|
|
What cancer is associated with Aflotoxin B1?
|
hepatocellular carcinoma
|
|
|
What cancer is associated with Vinyl Chloride?
|
angiosarcoma of the liver
|
|
|
What cancer is associated with benzopyrene?
|
Lung cancer in tobacco smokers
|
|
|
What neoplasms are associated with HTLV-1?
|
T-cell leukemia and lymphoma
|
|
|
describe the oncogenesis of HPV caused cancer
|
1. The HPV genome is integrated into the nuclear DNA
2. The E2 viral repressor becomes lost 3. Loss of repressor promotes overexpression of E6 and E7. 4. E6 inhibits P53 and degrades BAX as well as ativating telomerase. This all results in inhibition of apoptosis. 5. E7 inhibits RB and thus promotes progression of the cell cycle. |
|
|
List the malignancies associated with Epstein Barr virus infection and their characteristics.
What factors are responsible for oncogeneic effects. |
Malignancies and characteristics
1. Aids related or B-cell lymphoma= Pt has aids 2. Burkits lymphoma= PT in africa with facial swelling 3. Hodgkins lymphoma= Enlarged Lymphnode 4. Nasopharyngeal carcinoma= Tumor in the throat, viral genome is in tumor Factors responsible 1. LMP-1- ultimately ends up activating bcl-2 and imortalizing b cells 2. VIL-10- prevents activation of cytotoxic t cells. |
|
|
What viruses are associated with hepatocellular carcinoma? How do they cause cancer? Where is this malignancy common and why?
|
Virus = Hepatitis B (DNA virus) and C (RNA virus)
How: by creating innflammation / cirrhosis and the regenerating cells undergo DNA damage resulting in cancer. Where is it common: in Africa and Asia Why: because their food is contaminated with aspergillus (Initiator) and they have a high rate of Hepatitis B (promotor). Aflotoxin from aspergillus initiates the cells by mutating P53, this combined with the promotion of cell growth due to hepatitis inflammation results in cancer. |
|
|
What two viruses work synergistically to cause Kaposi sarcoma?
|
Human Herpes 8 and HIV
|
|
|
What malignancies are associated with Helicobacter pylori?
|
Adenocarcinoma of the stomach and Gastric Malt lymphoma
|
|
|
List the effects of tumors on the host
|
1-Cachexia (wasting)
2-Local effects= impingment on adjacent structures 3-Metastases with distant regional effects 4-Functional activity such as hormone synthesis 5-Paraneoplastic syndrome |
|
|
Compare and contrast local effects of tumor with metastases and with paraneoplastic syndromes
|
1. Local effects are local to the tumor and are caused directly by the tumor.
2. Metastases requires the tumor or parts of the tumor to travel to a different region. 3. Paraneoplastic effects are located away from the tumor and are caused by products of the tumor, but do not require the tumor to metastasize. |
|
|
Which malignancies are associated with the following serum markers?
1. CEA 2. Alpha-fetoprotein 3. B-HCG 4. PSA 5. VMA, HVA, NSE 6. CA-15-3 7. CA-125 |
1. CEA- colon cancer
2. Alpha-fetoprotein- liver and testicular cancer 3. B-HCG- choriocarcinoma 4. PSA-prostate cancer 5. VMA, HVA, NSE- neuroblastoma 6. CA-15-3 = Breast cancer 7. CA-125= Ovarian Cancer. |
|
|
What are the growth factor oncogenes? What product do they create? What cancers are associated with them?
|
1. SIS creates PDGF-Beta and is associated with Glioblastoma
2. HST creates Fibroblast Growth Factor and is associated with Kaposi Sarcoma |
|
|
Which of the following is NOT a Growth Factor Receptor oncogene?
A. erbB B. kit C. fms D. ret E. ras |
E. ras
RAS is a GTP binding protein |
|
|
What product does ERB-B1 create? What cancers are associated with them? What treatments do they respond to?
|
ERB B1 makes EGFR and is associated with Head, Neck, and lung Carcinomas.
Gene can be mutated or overexpressed. If mutated, treat with monoclonal antibodies. If overexpressed treat with tyrosine kinase |
|
|
What product does ERB-B2 create? What cancers are associated with them? What treatments do they respond to?
|
ERB B2 creates Her-2 neu and is associated with Breast Cancers.
Amplified in ~25% breast cancers and is associated with poor prognosis Treatment: tumors positive for Her-2 respond to monoclonal antibody Trastuzumab |
|
|
What is the most commonly mutated oncogene and what cancer is it most often associated with?
|
RAS is mutated in up to 20% of all human tumors.
It is most often mutated in adenocarcinomas of the pancreas and colon. |
|
|
What is RAS's mechanism of mutation?
|
Point mutation in response to chemical injury
|
|
|
What is the function of oncogenic ABL? How does it get activated and what neoplasm is most associated with its activation?
|
ABL is a nonreceptor tyrosine kinase protooncogene (is normally dampened)
Activation: When ABL (on chromosome 9) fuses with BRC (on chromosome 22) > cABl fusion gene is produced and dampening is lost >leads to signal transduction and cancer development. - t(9:22) Associated tumor: chronic myelogenous leukemia |
|
|
What cancers are most associated with MYC oncogene activation
|
- Burkitt lymphoma (B cell lymphoma) - MYC constitutively activated by t(8:14)
- Neuroblastomas- N-MYC amplified |
|
|
What oncogene cell cycle regulators are commonly dysfunctional in cancers?
|
Cyclin D/CDK4
|
|
|
Where do the most common cell cycle oncogenes act?
|
G1/S
|
|
|
What are the forms of dysregulation for oncogenes?
|
amplified, mutated, or activated by translocation
|
|
|
What is a Gatekeeper Gene?
|
A tumor supressor gene.
Regulates cell growth and halts cell proliferation. They are also involved in cell differentiation. Tumor suppressor genes include: RB and p53 |
|
|
What is Loss of heterozygosity and what are some examples of familial cancers related to this process
|
Loss of heterozygosity (LOH) Person born with 1 defective/mutated copy of a gene. Heterozygous individuals are normal. Occurs in familial cancer syndromes. LOH is associated with cancer
Examples o RB: familial retinoblastoma o WT1 -> Wilm’s tumor (nephroblastoma) o VHL -> von Hippel Lindau (clear cell renal carcinoma) |
|
|
What in the world is CpG island methylation and why does it matter?
|
cytosine-phosphodiester-guanine islands
- Inactivates 2nd X chromosome - Genomic imprinting - Stabilizes coding DNA sequences - Contributes to neoplasia - Gene silencing of tumor suppressors, pro-aptosis, DNA repair, and anti-angiogenesis • Rb, VHL, BRCA-1; mismatch repair genes |
|
|
If you see a number with a "p" in front of it, what is it likely related to?
|
dysfunction of inhibitors of CDK
• P21 induced by p53 and blocks actions of several cyclin/CDK complexes • P27 responds to TGF-B • p16 Inhibits RB - associated with familial melanoma, HPV |
|
|
What RB dysfunction is going to be involved in cancer?
|
HYPERphosphorylated RB will remain inactive and will not inhibit transcription factor E2F to shut off S phase.
In other words, cell keeps replicating |
|
|
How does a functioning/activated p53 suppress cancer?
|
Senses DNA damage and then will Activate transcription of genes that
‐arrest cell cycle (quiescence) ‐permanent cell arrest (senescence) ‐cause apoptosis |
|
|
What ways does mutated p53 promote neoplasia?
|
‐ activation of proinflammatory cytokines
‐ function as oncogene: stimulation of genes of cell proliferation ‐ blocks ATM protection against DNA breaks ‐ confers resistance to chemotherapy |
|
|
What mutates p53?
|
tobacco (benzopyrene), UV light, aflatoxin
|
|
|
What renders p53 nonfunctional?
|
oncogenic DNA viruses: HPV, Hepatitis B, (EBV maybe)
|
|
|
What is LiFraumeni Syndrome
|
An inherited germ line heterozygous mutation of p53 that can increase a person's risk of developing cancer by 25x. Results in many types of cancer such as breast, brain tumors, sarcomas, leukemia and adrenal cortical tumors
|
|
|
What is cellular senescence? What genes confer this property?
|
Senescence: loss of cell’s ability to complete mitosis due to irreversible arrest of cell cycle
• Protective response in cells in which oncogenes have been activated • Characteristic feature of benign tumors • Cells remain viable • Major critical arbiters : p53 and Rb |
|
|
What are the gene product and functions for APC? What sporadic and familial neoplasm is associated with this gene?
|
APC regulates Beta-catebin
B-catebin binds to E-cadherin to maintain cell to cell cohesion APC degrades B-catenin Sporadic neoplasm: pre-cancerous colon polyps, colon cancers, hepatic cancers Familial: Familial adenomatous polyposis |
|
|
What is the gene product function and related neoplasms of E-cadherins
|
Product/Function: Cell-cohesion
Neoplasms: visceral cancers and breast cancer |
|
|
What is the gene product function and related neoplasms of NF-1
|
Product/Function: Codes for neurofibromin, a GTPase activating protein (GAP) that Inactives RAS
Neoplasm: sporadic and familial neurofibromas |
|
|
What is the gene product function and related neoplasms of NF-2
|
Product/Function: Codes for merlin-neurofibromin 2; Homologous to RBC cytoskeletalprotein
Neoplasm: schwannomas, meningiomas |
|
|
What are the functions, familial and sporadic neoplasms most associated with VHL?
|
Functions: VHL protein is part of a ubiquitin ligase complex. Lack of VHL activity prevents ubiquitination and degradation of HIF1-α and produces increased levels of VEGF & PDGF. So angiogenesis is increased when VHL is lacking (mutated).
Familial Neoplasms: Von Hippel Lindau syndrome due to germline mutation of VHL gene: -> Hereditary renal cell carcinoma; Pheochromocytoma (adrenal medulla tumor); Hemangioblastomas of CNS Sporadic Neoplasms: Both VHL gene alleles inactive (mutation; promoter hypermethylation) is the most common form of sporadic renal cell carcinoma. |
|
|
What are the functions and neoplasms related to PTEN?
|
Functions of PTEN (Phosphate and tensin homologue):
- Potent tumor suppressor gene. Puts the brakes on pro-survival/pro-growth pathway PI3K/AKT - Second most frequent gene mutation in cancers - Dephosphorylates proteins and lipids - Impacts multiple signaling pathways, including p53 & RAS Neoplasms: - Cowden syndrome: familial mutation. Benign skin appendage hamartomas; increased risk for cancers, especially breast - Sporadic monoallelic loss: breast, colon, prostate, lung and brain tumors - Homozygous mutations: endometrial |
|
|
What are the functions and neoplasms most related to WT-1
|
Functions:
- located on chromosome 11p13. (not sure why this was highlighted but it was) - It has tumor suppressor activity: growth arrest - Oncogenic activity: •Transcription activator of genes involved in renal and gonadal development. •RNA processing •Anti-apoptotic activity Neoplasms related to WT-1 -familial Wilm’s tumor: nephroblastoma and pediatric renal cancer -It is overexpressed in most common adult malignancies |
|
|
What cancer is BCL-2 over expression
|
B-cell follicular lymphoma
|
|
|
What are the apoptotic effects of p53?
|
p53 mutation = no BAX
BAX initates apoptosis |
|
|
What are the apoptotic effects of PTEN dysregulation
|
PTEN impacts p53 signaling pathway; so problems with PTEN = problems with p53
|
|
|
What inherited and sporadic malignancy is associated with defective mismatch repair?
|
Inherited: Hereditary nonpolyposis colon cancer syndrome (HNPCC)
Sporadic: colon cancer |
|
|
What are the most common mismatch repair genes and what defect accumulates in the genome?
|
Genes: MLH-1; MSH-2
Defect: microsatellites - tandem repeats of up to 6 nucleotides |
|
|
What inherited disease is associated with defective nucleotide excision repair?
what cancers are associated with this defect? |
Xeroderma Pigmentosum
Skin cancer |
|
|
What genes are involved in DNA repair by homologous recombination? What are the cancer predispositions associated with these?
|
Genes: BRCA 1 and BRCA 2, ATM
Cancer predispositions - Ataxia telangiectasia (ATM) - develop leukemia; lymphoma - Fanconi anemia - risk for leukemia, squamous cell carcinoma and hepatoma - BRCA 1 and BRCA 2 => familial breast cancer; ovarian |
|
|
What is the role of telomeres and telomerase in neoplasms?
|
In normal cells, Short telomeres activate P53 dependent checkpoints to trigger arrest (senescence) or apoptosis
Telomerase is reactivated in 90% of all cancers preserving replication functions |
|
|
What is the significance of angiogenesis to neoplasia?
|
- Tumors cannot grow beyond 1-2 mm in diameter or thickness unless vascularized
- Pro- and antiangiogenesis factors are produced by tumor; its matrix and inflammatory cells - Angiogenic switch occurs when proangiogenesis factors predominate |
|
|
What factors are proangiogenic? anti-angiogenic?
|
Proangiogenic factors:
• VEGF (vascular endothelial growth factor) • bFGF (Basic fibroblastic growth factor) Antiangiogenic factors - Factors produced or induced by tumor cells - Thrombospondin-1: induced by p53 - Agents produced in response to tumor by proteolytic cleavage of ECM (collagen, plasminogen, tranthyretin) •Angiostatin •Endostatin •Vasculostatin |
|
|
Steps of invasion. What are they? What factors are involved?
|
1) Invasion of the ECM
Factors: loss of E-cadherin; reduced catenin protein (link with cytoskeleton) 2) Degradation of ECM Factors: matrix metalloproteinases (type 4 collagenase- MMP9), cathepsin D, or urokinase plaminogen activator. 3) Attachment Factors: laminin and fibronectin receptors; Autocrine motility factor 4) Migration Factors: Movement through ECM facilitated by proteases secreted by tumor and associated host cells (macrophages, leukocytes) ; Invadopodia: cell projections containing actin, integrins; MMPs, other enzmes |
|
|
What determines metastatic sites?
|
Natural drainage explains some, but not all sites. Predictable for each tumor type.
•Prostate -> to lumbar vertebrae •Bronchogenic carcinoma -> to adrenals, brain •Neuroblastomas -> to liver, bone •Breast -> to bone, liver, lung Metastatic site tropism •Endothelial cells of various organs express different ligands for adhesion molecules. •Chemokines of target tissues may attract cancer cells (most cancer cells have specific chemokine receptors). •Target organs may also release chemoattractants (IGF’s I and II). •Target tissue may be nonpermissive: skeletal muscle, spleen, heart |
|
|
What are possible outcomes of metastases?
|
- Many circulating cancer cells may never develop gross metastases
- Dormancy: years/decades of survival of micrometastases w/o clinical disease • Breast, prostate, melanoma - Tumor cell products: cytokines, grow factors plus products of ECM make site habitable |
|
|
Characterize interactions of malignant cells with their stromal environment.
|
- Cross-talk btw ECM and tumor cells provides paracrine growth signals
- Inflammatory cells may promote cancer survival and progression - Fibroblasts; stroma may drive genetic changes in the tumor - Leukocytes facilitate invasion of cancer cells by production of enzymes and expression of adhesion molecules. |
|
|
What is the dominant metabolic pathway of cancer cells (the Warburg effect) and what is the clinical application?
|
Warburg effect
- Occurs when cancer cells shift their glucose metabolism away from mitochondrial directed to that of aerobic glycolysis - This may provide an advantage in hypoxic microenvironment - Mutations in many genes shown to shift metabolism (PTEN, RAS, p53, MYC) - Metabolic shift increases supply of building blocks for cell division Clinical application - The glucose-hunger of tumors is used to visualize them using PET scanning. - Patients are injected with 18F-fluorodeoxyglucose which is preferentially taken up by cancer cells and dividing cells (bone marrow cells, CNS). |
|
|
Summarize the molecular multistep basis of cancer.
|
- Each cancer results from accumulation of multiple mutations
- To date, experiments show that no single oncogene can fully transform a cell • All cancers analyzed demonstrate activation of several oncogenes and loss of 2 or more tumor suppressor genes - Mutations appear to be incremental, over time - Mutations associated with phenotypic changes |
|
|
Name four things a frozen section may be used for?
|
1. Presence or Absence of Malignancy
2. Presence or Absence of Inflammation/Organisms 3. Whether Surgical margins are free of neoplasm (basal cells) 4. Whether Diagnostic tissue is present |
|
|
What is an Intermediate Filament?
What are the five intermediate filaments and their cells of origin |
Tumor cells often contain Intermediate Filaments Characteristic of their Cell of Orgin
1. Keratins - Carcinomas, Mesotheliomas 2. Desmin - Muscle Tumors: Smooth and Striated 3. Vimentin - Mesenchymal Turmors, Some Carcinomas 4. Glial Filaments - Gilomatous Tumors 5. Neurofilaments - Neuronal Tumors |
|
|
What two things can flow cytometry measure?
|
1. Membrane Antigens
2. DNA content of Tumor Cells Surface antigens identification is used to classify leukemia’s and lymphomas |
|
|
DNA content or ploidy can be divided into what two subsets?
|
1. Diploid Tumors: Normal Diploid DNA value (good)
2. Aneuploid Tumors: DNA content other than Diploid (bad) |
|
|
CD 1,3,4,5,8 are all associated with.....
|
T cells
|
|
|
CD 10,19,20,21,23 are all associated with?
|
B cells
|
|
|
CD 11,13,14,15 are all associated with?
|
Monocyte/Macrophage
|
|
|
What cells are associated with CD16 & CD56
|
NK Cells
|
|
|
CD34 is associated with what type of cell?
|
Stem cell
|
|
|
What CD is present on all leukocytes?
|
CD45
|
|
|
What are the main uses for FISH?
|
Detection of specific DNA or RNA sequences in tissue sections or cell preps using labeled complementary nuclei acid sequence or probe
- Detection of Viral Infections |
|
|
What advantage does FISH have over standard karyotyping?
|
- Can detect Productive and Latent Infections
> Can segregate subtypes - Analysis the chromosomes of nondividing cells > DNA probes recognize specific sequences |
|
|
What does a DNA microarray analysis measure?
|
- Measures the expression levels of several thousand genes simultaneously
- Gives the molecular profile for each tissue analyzed > Can subcategorize phenotypically similar tumors (done b/c different survival rates b/w subcategories) > May show novel gene targets for the development for new drugs |
|
|
What six areas are genomic approaches having an impact on?
|
1. Tissue Classification
2. Prognostic Markers 3. Predictive indicators of drug response 4. The development of new drug therapies 5. Strategies for monitoring disease 6. Management of susceptibility to cancer |
|
|
What two features are helpful to divide blood vessels into benign and malignant categories?
|
1. Degree of well formed Vascular Channels present
2. Extent and regularity of the Endothelial Cell Proliferation > Malignant: Solidly cellular w/anaplasia, mitosis, usually No Well Organized Vessels > Benign: Vascular channels filled w/blood, lining is a monolayer of Normal Endothelial Cells, No Atypia |
|
|
Summarize the molecular multistep basis of cancer.
|
- Each cancer results from accumulation of multiple mutations
- To date, experiments show that no single oncogene can fully transform a cell • All cancers analyzed demonstrate activation of several oncogenes and loss of 2 or more tumor suppressor genes - Mutations appear to be incremental, over time - Mutations associated with phenotypic changes |
|
|
What are the three categories of hemangiomas?
|
1. Capillary Hemangioma
2. Cavernous Hemangionoma 3. Pyogenic granuloma |
|
|
Capillary hemangiomas are found predominantly in what locations?
|
Skin, Subcutaneous Tissue and Mucous Membranes
|
|
|
What is the natural course of the strawberry or juvenile type of capillary hemangioma?
|
Found in Newborns, Grow Rapidly for a few months then it fades at 1 to 3 years.
80% are gone by age 7 |
|
|
What are the gross and microscopic characteristics of a pyogenic granuloma? 1/3 develope after what?
|
- Peduncular red nodules on the skin, oral mucosa grows rapidly and bleeds easily
- 1/3 Develop after a Trauma - Proliferating Capillaries w/Edema and acute and chronic infection |
|
|
Where do glomus tumors occur?
|
Distal Portions of the Digits, under the fingernails
|
|
|
What are Glomus Tumors composed of?
|
> Glomus Bodies are neuromyoarterial receptor that is sensitive to temperature
> Two components of a glomus tumor 1- Branching vascular channels 2- Aggregates, nests and masses of specialized glomus cells. |
|
|
What distinctive clinical symptom do Glomus Tumors have?
|
Exquisitely Painful
|
|
|
What is the common name for a nevus flammeus?
|
ordinary birthmark.
Most fade. |
|
|
What is a port-wine stain and what is the clinical significance?
|
A nevus flammeus that grows with the child and developes a thickened surface, they do not fade.
Significance= Lesion is in a trigeminal nerve distribution and can be seen in sturge-weber syndrome. |
|
|
What causes bacillary angiomatosis?
|
Infectious Disease (Gram-negative Bacilli of Bartonella Genus)
|
|
|
Who gets bacillary angiomatosis?
Is it a true tumor? How do you treat it? |
Immunocompromised patients
No, it is a Non-Neoplastic proliferation of vessels in skin, lymph nodes and visceral organs Macrolide Antibiotics (Erythromycin) |
|
|
What are the two intermediate grade blood vessel tumors?
|
1. Hemangioendothelioma - Vascular neoplasms showing histologic features and clinical behavior
intermediate b/w benign hemangiomas and anaplastic angiosarcoma 2. Epitheloid Hemangioendothelioma - Occurs around veins in soft tissues of adults - Well-defined vascular channels hard to find, turmor cells plump, cuboidal = epithelial like |
|
|
What are the four types of Kaposi sarcoma?
|
1. Chronic – Classic, European Form
- Older men, Eastern Europe, Mediterranean, not associated with HIV - Usuall asymptomatic and remain localized 2. Transplant-Associated - Patients taking long term immunosuppression - Lesions may regress when immunosuppression therapy is lessened but there is a risk of organ rejection 3. AIDS Associated (she said know this one) - Most prevelant malignancy in AIDS pts. 4. Lymphadenopathic – African Kaposi - South African Bantu children, not associated with HIV - Very Aggressive - Most common tumor in Central Africa |
|
|
What is considered the underlying infectious cause of Karposi Sarcoma?
|
95% of the lesions have Human Herpesvirus 8 (KS associated herpesvirus KSHV)
|
|
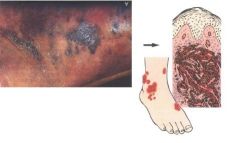
What is pictured here?
|
Late Stage Karposi Sarcoma
|
|
|
What are the microscopic hallmarks of KS?
|
Spindle Shaped Stromal Cells with Irregular, Angulated, Slit-Like spaces filled with red cells (this is what she said in class, and to know the late stage pics)
|
|
|
What are the two malignant blood vessel tumors?
|
1. Angiosarcoma (hemangiosarcoma)
- Malignant endothelial neoplasms (differentiated or anaplastic) - Local invasion, distant spread, 5 year survival is 30% - Can be induced by radiation , foreign material introduced into the body 2. Hemangiopericytoma - Derived from Pericytes - Most common in lower extremity and retroperitoneum. - numerous branching vascular channels and sunusoidal spaces surrounded by nests and masses of spindle shaped cells |
|
|
What three carcinogens are hepatic angiosarcomas associated with?
|
1. Arsenic
2. Thorotrast 3. Polyvinyl Chloride (PVC) |
|
|
What does the term lymphangiosarcoma refer to?
|
Rare malignant tumor which occurs in long-standing cases of primary or secondary lymphedema. It involves either the upper or lower lymphademateous extremities but most common in the upper extremities
|
|
|
What are the two types of lymphangiomas?
|
1. Simple lymphangioma - masses of small lymphatic channels, subcutaneous head/neck
-Differentiated from capillary by lack of blood cells 2 Cavernous lymphangioma/Cystic Hygroma - cavernous lymphatic spaces in children in the neck. |
|
|
What syndrome are lymphangioma associated with?
|
Turner Syndrome
|
|
|
Describe the most common neoplasm of childhood. Recognize appearance and know behavior
|
hemangioma. They are present at birth and size will initally increase as the child grows, but most will regress by school age. Morphology-lobules of capillaries, unencapsulated
|
|
|
When are benign hemangiomas deemed medical emergencies?
|
When they impinge upon the eye and may cause blindness. They also develop to obstruct an airway
|
|
|
Describe the features Sturge-Weber syndrome
|
Port wine stains with intertrigeminal distribution
Vascular anomolies extend into eye; meninges; risk for glaucoma |
|
|
What is lymphangioma?
|
Tumor of lymph vessels characterized by cystic or cavernous spaces. May be a neoplasm or hamartoma. Complications develop due to pressure of tumor growth
|
|
|
What is a hygroma?
|
lymphatic lesion that contain large cyst like cavitites filled with watery fluid. Benign but can be disfiguring.
|
|
|
Where are teratomas usually located in young children and what is the usual behavior?
|
Most common location is the sacrococcygeal. Other location include the mediastinum; retroperitoneum; head and neck.
75% are mature, a.k.a. benign. |
|
|
How do pediatric malignancies differ from those in adults?
|
Pediatric malignancies have a frequent relationship of abnormal development (teratogenesis) to tumor induction (oncogenesis) There is more of underlying gentic cause to pediatric malignancies. Fetal and neonatal tumors tend to spontaneously regress, cytodifferentiate (mature to benign type). Choldren who survive their malignancies are more likely to develop a second malignancy
|
|
|
What is the common histology seen in seen in pediatric malignancies?
|
Small round blue cell tumors (SRBCT) are seen in most of the primitive tumors of childhood.
Tumors that have SRBCT: -PNET -Malignant lymphoma -poorly differentiated neruoblastoma, rhabdomyosaroma -occasional Wilm's tumor |
|
|
What is the most common location, derivation, and the behavior of neuroblastoma?
|
adrenal medulla. Neuroblastoma also found in paravertebral locales in abdomen and posterior mediastinum. They are derived from primordial neural crest of the sympathetic gangion. Microscopic lesions tend to remain silent and spontaneously regress.
|
|
|
What are the symptoms and morphology associated with pediatric neuroblastoma?
|
symptoms:
• Abdominal mass •Fever, weight loss • May present as metastatic disease > “Blueberry muffin” appearance of cutaneous metastases- deep blue nodules • Nerve impingement: bowel, bladder dysfunction Morphology. -small round blue cell tumors -homer wright pseudorosettes: tumor cells concentric around space filled with eosinophilic neuropil |
|
|
How is neuroblastoma staged? What is the significance of stage?
|
-stage I-tumor localized; completely excised ipsilateral disease without nodal metastasis
stageII-nothing given here stage III-tumor extends across the midline; unresectable Stage IV-distant metastasis-most common spots are bone and liver Stage IV (special)- localized primary tumor with extensive metastatic disease to liver, skin, bone marrow (limited to an infant less than a year old) |
|
|
What are the serum tumor markers for neuroblastoma?
|
-90% will produce catecholamines
-elevated blood levels of catecholamines -elevated urine levels of metabolites- VMA and HVA |
|
|
Compare and contrast good prognostic markers with poor prognostic markers for neuroblastoma?
|
Good prognosis: hyper-diploid (near triploid), Trk-A
Poor prognosis: diploid (near diploid); N-myc oncogene; 17q+, 1p, hemizygous 11q; Trk-B |
|
|
What are the two names of the most common renal tumor of childhood? Is it benign or malignant?
|
Wilm's Tumor a.k.a. Nephroblastoma
Malignant |
|
|
What are the genes related to gentic predisposition for Wilms tumor?
|
tumor supressor gene, transcription factor for normal renal, ginadal development. Chromosome 11 involved
|
|
|
What are the genes related to genetic
predisposition for Wilm’s tumor? What are the three genetic diseases with increased risk for Wilm’s tumor. |
1. WAGR : WT1 gene
2. Denys-Drash : WT1 gene 3. Beckwith-Wiedemann syndrome : WT2 |
|
|
WT-1 gene
|
• Tumor suppressor gene
• Transcription factor for normal renal, gonadal development • Located on chromosome 11 |
|
|
What's WAGR?
|
W. A. G. R. =W ilms, A niridia (absent irises),
G enital malformation, mental R etardation - Chromosome 11 p deletion - Deleted region includes PAX6 gene for irises - Risk for Wilm’s tumor, 30% |
|
|
What's Denys-Drash
|
male pseudohermaphroditism
and nephropathy, renal failure - Missense mutation in WT1 gene - high risk of Wilms tumor |
|
|
What's Beckwith-Wiedeman syndrome
|
• Enlargement of organs; macroglossia
• Hemihypertrophy • Adrenal cortical cytomegaly and other defects • WT-2 locus of multiple genes involved: genomic imprinting by methylation of promoter genes • Insulin like growth factor-2 overexpressed • Risk for Wilms tumor and multiple other tumors |
|
|
What is the microscopic morphology of Wilms tumor?
|
• Nephrogenic rests/ Nephroblastosis
- Putative precursor lesion of Wilms tumor - Found in 100% of bilateral cases •Gross of Wilms tumor - Large, solitary circumscribed mass; tan to gray • TRIPHASIC microscopic morphology - Recapitulation of stages of nephrogenesis - blastema; stroma and epithelial cell types • Unfavorable histology: diffuse anaplasia |
|
|
What are the gene, the clinical features, and the behavior of retinoblastoma?
|
Gene: RB1 inactivation on chromosome 13
Clinical Features: • Median age of presentation: 2 years old • May be present at birth • “Cat’s eye reflex” – leukocoria; whitish hue • Poor vision, strabismus may occur • Treatment includes: enucleation (1eye may be spared if bilateral) followed by radiation and chemotherapy • Prognosis with therapy: usually very good • Familial retinoblastoma at risk for 2nd cancers Behavior: • Starts as a retinal nodule with satellite seeding of eye • Extension beyond eye is usually via optic nerve; subarachnoid (beneath membranes surrounding optic nerve) • May penetrate sclera; grow into orbit; brain • Metastasizes to bone marrow, CNS • Fatal without treatment of other organs |
|
|
Review the types of tumor antigens. Know the specific examples given
|
a. Tumor-specific antigens (TSA) – molecules that are unique to cancer cells (BCR-ABL)
b. Tumor-associated antigens (TAA) – molecules shared by normal cells and cancers of same type; expressed differently on tumor cells: i. prostatic acid phosphate ii. oncofetal angitgens – α-fetoprotein; CEA iii. cancer-testis antigens: MAGE family iv. Differentiation antigens: CD20 in B cell lymphoma v. Overexpressed antigens: Her2/neu vi. Altered cell surface glycolipids; glycoproteins c. Mutated genes and their products – p53, RAS d. Antigen products of oncogenic viruses – HPV E7 |
|
|
What are the antitumor effector mechanisms that may be involved in tumor immunity. Which is most effective?
|
a. Principal mechanism of antitumor immune activity – cytotoxic T lymphocytes (CTLs): CD8+ cells
b. Natural killer cells (NK) destroy without prior sensitization; clinical significance unclear c. Macrophages: cell killing by reactive oxygen species d. Antibodies against tumor antigens |
|
|
What is the significance of antitumor antibodies?
|
a. Little evidence for effective humoral immunity in humans
b. Antibodies are commonly formed – may cause paraneoplastic syndromes, may be detected by blood tests (possible blood test for early dx of CA?) c. Therapeutic antibodies have been developed to kill tumor cells i. Anti-Her2/neu ii. Anti-EGFR |
|
|
What is the status of the immune system by the time cancer is diagnosed? What are the mediators of this?
|
a. Tumors induce immunstimulatory molecules and immunosuppressive molecules
b. At time of dx, balance is switched to immunosuppression = i. switch from Th1 to Th2 response ii. immunosuppressive cytokines: TGFb, IL10 iii. immunosuppressive immune cells: Tregs, macrophages, and PMNs iv. disrupted cell signaling: loss of class I MHC |
|
|
What are the functions of TGF-Beta in cancer?
|
a. Tumor suppressor gene
b. Produced by regulatory T cells and fibroblasts in the stroma of invasive carcinoma c. Effects related to invasion include: increased angiogenesis, increased deposit of ECM, and immunosuppression |
|
|
Describe the clinical presentation and behavior of cardiac myxoma. What is its morphology?
|
a. Benign; uncommon
b. 90% in atria; L>R by 4:1 Clinical presentation – ball valve obstruction (heart failure), fragmentation with systemic embolization, systemic inflammatory reaction (fever due to IL6 from tumor) Morphology – sessile or pedunculated. Myxoid (gelatinous); mesenchymal cells. |
|
|
What is the most common neoplasm of the heart in infants?
|
Rhabdomyoma – rare, benign tumor or hamartoma
i. Occurs in infants/children – first year of life ii. May produce obstruction |
|
|
What aggressive primary malignancies occurs in the heart?
|
a. Lipomas – located anywhere within heart
b. Papillary fibroelastoma – occur on valves; incidental at autopsy c. Cardiac angiosarcoma – aggressive, metastasizing, usually fatal |
|
|
What are the three ways radiation is measured and what are the units?
|
1. Amount of radiation emitted by a source. Measured in Curies (Ci)
Ci → represents the disintegrations per second of a radioisotope 2. Radiation dose absorbed by a person. Measured in Grays.(Gy) Gy → energy absorbed per unit mass 3. Biological effect of the radiation. Measured in Sieverts (Sv) Sv → Unit equivalent dose that depends on the biological effect of radiation. Corresponds to absorbed dose (Gy) multiplied by the relative biological effectiveness of the radiation |
|
|
What is the Annual permissible radiation exposure of industry workers
|
50 mSv (milli-Sieverts)
|
|
|
What cells are most radiosensitive?
|
hematopoietic cells (especially lymphocytes), germ cells (ova and spermatogonia), GI epithelium, salivary glands, skin and endothelium
|
|
|
When is a cell most vulnerable to radiation injury
|
cells in G2, M are most sensitive to radiation injury
|
|
|
Which cells are most resistant to radiation injury?
|
bone and cartilage in adults, muscle and peripheral nerves
|
|
|
What is the pathogenesis of radiation injury
|
• Direct damage to DNA from gamma/x-rays or particulate radiations may occur
‐ High dose of radiation =>tissue necrosis • Intermediate dose: kills proliferating cells • Lower dose radiation, indirect: => O2‐derived free radicals cleaved from water •Low doses: may exhibit no histologic defect ‐ DNA damage may produce delayed effect of organ dysfunction or cancer |
|
|
What are the tissue processes involved in acute and chronic radiation injury?
|
Acute effects of radiation injury
i. Cell death ii. Endothelium is a major target to radiation injury: apoptosis, endothelial cell death and cytokine release cause “burns” iii. Damage to intestinal crypt stem cells: GI syndrome of radiation sickness Chronic/delayed effects of radiation i. Vascular injury – fibrosis of wall with obliteration, thrombosis, ectatic (dilated) vessels (telangiectasia) ii. Ischemia of organs supplied by scarred vessels iii. Fibrosis due to ischemia; loss of stem cells |
|
|
What cancers are associated with radiation exposures in Hiroshima, Chernobyl and anticipated in Fukushima? What is the latency period?
|
Carcinogenesis
i. Latent period of 10-20years ii. Accident/occupational exposures = risk for skin cancer, leukemia, lung cancer, and osteogenic sarcoma iii. Thyroid cancer increased in particle emissions: atomic bomb, nuclear energy plant accident iv. Hiroshima/Nagasaki survivors 1. Adults: acute leukemias 20x more common 2. Children: thyroid, breast, GU and GI cancers |
|
|
describe the morphologic consequences related to radiation injury in utero
|
i. Preimplantation – radiation may be lethal
ii. Organogenesis – implantation to 9wks, malformations likely iii. Fetal period- 9wks to birth, CNS dysfunction, underdeveloped reproductive organs, increased risk of leukemia, brain tumors iv. Infants – retarded bone growth and maturation. CNS, teeth, and eye development perturbed |
|
|
describe the morphologic consequences related to radiation injury in the skin
|
i. Erythema day 2-3 followed by edema, blistering, early hair loss
ii. Late or delayed: radiation dermatitis - Telangiectasia from weakened vessels - Dermal atrophy c fibrosis, hair follicles lost 3. Hyper or hypopigmentation 4. Impaired healing, increased infections |
|
|
describe the morphologic consequences related to radiation injury in Bone marrow, hematopoietic cells, and lymphoid cells
|
i. Acute: atrophy of bone marrow precursors
1. Lymphocytopenia within hours 2. Granulocytes fall at end 1st week 3. Platelets lag behind granulocytes 4. Anemia with heavy exposure after 2 weeks ii. Late or delayed 1. Hypoplasia – aplasia/aplastic anemia 2. Precancer – myelodysplasia 3. Leukemia/lymphoma |
|
|
describe the morphologic consequences related to radiation injury to the Gonads/reproductive organs
|
i. Male/female permanent sterility may occur even with low does
ii. Necrosis and atresia of germ cells result in testicular atrophy: permanent sterility 1. Ovarian germ cell; granulosa cell loss: infertility iii. Fertility rates are decreased but offspring born years later are normal |
|
|
describe the morphologic consequences related to radiation injury to the lungs
|
i. Early: pulmonary congestion/edema from endothelial damage, ARDS
ii. Late (months to years): “radiation pneumonitis” – interstitial fibrosis, primary lung cancer |
|
|
describe the morphologic consequences related to radiation injury to the GI tract
|
i. Acute injury: villar atrophy leads to malabsorption/diarrhea
1. Mucosal ulcers may occur: nausea, vomiting, diarrhea 2. Susceptible to infection 3. Fluid and electroly loss ii. Late effects from vascular injury 1. Fibrosis with strictures, obstructions 2. Ulceration |
|
|
describe the morphologic consequences related to radiation injury to the heart
|
i. Myocardial fibrosis with restrictive cardiomyopathy
ii. Fibrosis of pericardium with contrictive pericarditis iii. Accelerated atherosclerosis iv. Myocardial ischemia from vascular injury |

