![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
What image regarding Oncogenes & their protein products did Gilbert ask us to memorize?
|
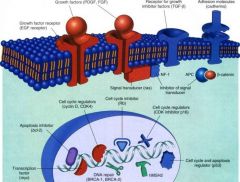
Robbins pg 294, Fig 7-31
|
|
|
What is the 2nd image regarding Growth factors & growth factor receptors that Gilbert wants us to understand - by looking at the explanation in the text?
|
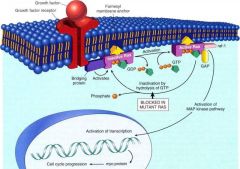
Robbins pg 297, Fig 7-32
|
|
|
What is the age range with highest cancer diagnoses?
|
55-74 years old, but NO age group is spared
|
|
|
What are some common environmental factors with high cancer indicence?
|
Sun exposure - melenoma
Alchohol abuse - GI tract CA Promiscuity/STDs- Cervical CA |
|
|
What are some hereditary factors that affect cancer?
|
Autosomal dominant syndromes (familial retinoblastoma)
Familial cancers (breast, ovarian, colon) Defective DNA repair, autosomal recessive syndroms (Xeroderma pigmentosum) |
|
|
Why do you have to differentiate between hereditary and genetic factors for cancer?
|
ALL cancer is genetic - it's a problem that has occured in DNA
NOT all cancer if hereditary (inherited from parents) |
|
|
List 4 inherited cancer cyndromes - Autosomal dominant
|
Retinoblastoma
Li-Fraumeni syndrome (various tumors Melanoma Familial adenomatous polyposis/colon cancer |
|
|
P53 - what is it?
|
It's a tumor repressor gene ("brakes" for cell growth)
Mutation of this gene is associated with 50% of cancer diagnoses because it can no longer induce apoptosis of damaged cells |
|
|
Which phrase regarding development of cancer do you need to have memorized?
|
METAPLASIA leads to DYSPLASIA, Carcinoma-in-situ and the CANCER
Memorize this! |
|
|
Acquired PRE-neoplastic disorders:
List 2 that are chronic inflammatory diseases |
Ulcerative colitis (required colectomy because it WILL become malignant)
Hepatitis |
|
|
Acquired PRE-neoplastic disorders:
List 2 NON-neoplastic disorders |
Salar keratosis (spots on hands, forehead, etc)
Leukoplakia (white placques, in oral cavity due to irritation like denture, pipe smoker) |
|
|
What's the Molecular Basis of Cancer?
(Gilbert suggests NOT reading the text book on this, too tough!) |
-Nonlethal genetic damage (ie UV radiation)
-Regulatory genes - something gets out-of-whack *Proto-oncogenes promote growth *Antioncogenes inhibit growth *Apoptosis *DNA repair gene KNOW: Cardinogenesis is a multistep process @ phenotypic & genetic level |
|
|
What are oncogenes?
|
Cancer-causing genes
Derived from protooncogenes (aka V-onc) that promote normal growth & differentiation Spontaneously arising cancers will have oncogenes in DNA Cell oncogenes (aka C-onc) can be formed from V-oncs by retroviral tranduction or by somether that alatered V-onc behavior in situ. |
|
|
What are the 5 protein products of Oncogenes? Why are these clinically significant?
|
Growth factors
Growth factor receptors Proteins in signal transduction Nuclear regulatory proteins Cell cycle regulators *Significant because if we can block any of these, we can halt cancer |
|
|
What is an example of a drug that interferes with a growth factor receptor?
|
Herceptin is a newer drug that attaches to HER2 growth stimulating receptors, blocking but not stimulating them.
HER2 receptors cause breast cancer when overexpressed. Drug has only lengthened survival time by 4 mon |
|
|
What is the "ras" gene?
|
Point mutation of the ras gene is the single most common cause of abnormality of dominant oncogenes in humans (affects Signal Transduction proteins)
|
|
|
How are oncogenes activated?
|
Either changes in structure of the gene
Or in the regulation of gene expression (Point mutations, chr rearrangements & gene ampl) |
|
|
What happens in a translocation?
|
Overexpression of protoncogenes due to chromosomal rearrangement
Ex: Philadelpia chr- a movement of a chunk of Chr 9 exchanged with a chunk of Chr 22 - characteristic of chronic myeloid leukemia |
|
|
What happens in gene amplification?
|
Overexpresson of Oncogenes from reduplication & manifold ampl of the genes's DNA seqence
May result of several hundred copies in tumor cell |
|
|
How does gene amplification affect age on cancer onset?
|
When genes are prone to amplification, each generation will have a larger number of copies at birth.
These multiply in each generation, causing offspring to have a more copies of the gene at a younger age than the previous generation. Ex. Onset of cancer for Gma=73, Mom=55, Daughter=39 |
|
|
How is age on onset related to cancer severity?
|
Younger onset usually means more agressive (possibly due to hormonal issues)
|
|
|
What are 3 tumor repressor genes that have to do with cell cycle regulation?
|
RB (retinoblastoma)
BRCA1 & BRCA2 p53 |
|
|
How does HPV cause cancer?
|
By phosphorylating (& thus activating) a cell cycle control gene, stimulating growth in the cervix & leading to cervical cancer
|
|
|
What is the role of Hypoxia in cancers?
**Gilbert says he always tests on this |
Hypoxia induces mutations in p53, preventing DNA repair & allowing proliferation of cancer cells
|
|
|
What percent of breast cancer is caused by BRCA1 or 2?
|
5-10% are due to these genes, the rest are "spontaneous" cancers - not inherited
|
|
|
what is APC and what does it lead to?
|
APC is ademomatus polyposis coli gene - (means colon polyps)
LOSS/mutation of APC is a step in evolution of colorectal cancer. A Signal Transduction regulator - APC slows down proliferation of cells |
|
|
What is neurofibromatosis?
|
"Lumps and Bumps" - tumors formed. Location determines severity. Can lead to brain tumors. Gene (NF-1) regulates signal transduction
|
|
|
Name 3 cell surfacr recptors that are associated with cancer
|
TGF-G (growth inhibitory factor, upregulates transcription of growth-inhib genes)
Cadherins - "glue" holding tissue together, when lost, favors local invasion/metastases DCC - Deleted in Colon Carcinoma |
|
|
Name 2 genes that inibit cell death
|
bcl-2
bcl-xl |
|
|
Name 3 genes that favor apoptosis
|
bax
bad bcl-xS |
|
|
How does normal p53 affect the bax gene and what is the result?
|
p53 upregulates bax to cause apoptosis.
|
|
|
What's the molecular basis of mutistep carcinogenesis?
|
*Multiple steps in initiation & promotion
*Activation of several oncogenes & loss of 2+ cancer-suppessor genes *"gatekeeper" genes directly regulate growth of tumors *"caretaker" genes affect genomic stability |
|
|
Steps of specific morphologic & molecular change in colon cancer development
|
Start with normal epithelium.
Loss/mutation of APC leads to metaplasia Loss of DNA methylation leads to early dysplasia Mutation of ras gene leads to intermed dysplasia Loss of tumor suppressor on 18q leads ot late dysplasia Loss of p53 leads to carcinoma-in-situ Which leads to cancer |
|
|
List 4 karyotypic changes found in tumors
|
Translocations
Deletions Gene amplification Whole chromosomes may be lost or gained |
|
|
What is involved in tumor growth?
|
Doubling time - growth rate
Angiogenesis - can't grow over 2mm w/out own blood supply Tumor progression & heterogeneity - invasiveness, metastatic ability, etc |
|
|
How many "doubling times" for a tumor to be detectable?
|
30 doubling times to form a 1 g tumor, the smallest detectable via CT
Just 10 more doubling times could give a 2.2 lb tumor |
|
|
Describe tumor angiogenesis
|
Newly formed endothelial cells produce growth factors such as:
VEGF - Vascular Endothelial Growth Factor bFDF - basic Fibroblast Growth Factor |
|
|
Tumor progression & heterogeneity - what does term this mean?
|
Most malignant tumors are monoclonal (all from one cell) but cells are heterogeneous (cell subpopulations have different characteristics)
|
|
|
What differentiates carcinoma in -situ from metastasis?
|
Tumor is carcinoma-in-situ UNTIL a basment membrane is breached by altered cells.
|
|
|
How does a tumor cell invade extracellular matrix?
|
1) Detachment of tumor cells from each other - attachments loosen up
2) Attach to matrix components (laminin) 3) Degradation of extracellular matrix (collagenase) 4) Migration of tumor cells through basement membrane |
|
|
Describe vascular dissemination and homing of tumor cells
|
-Tumor cells in circulation are vulnerable to immune defenses
F-ormation of platelet-tumor aggregates seem to enance tumor cell survival & implantability **It appears some organs have chemoattractants that recruit tumor cells to that organ (ie adrenals) OR that there are stimulating factors for cancer that travel to areas that already have pre-cancerous cells |
|
|
What is an initiator?
|
A carcinogenic agen that causes permanent DNA damage
Most are metabolized by cytochrome P-50 enzymes |
|
|
What is a promoter?
|
Something that induces cell growth, & thus tumors, in INITIATED cells. Does NOT cause tumors in uninitiated cells so is nontumorigenic by itself
|
|
|
Examples of initiators?
|
Chemotherapy drugs
Immunosuppressive Rx Hydrocarbons from burning (ie tobacco) Hep B birus Nitrates (preservative Asbestos, chromium, nickel, etc |
|
|
Examples of promoters?
|
Cigarette smoke
Viral infections Estrogens Bile salts High levels of dietary fat |
|
|
How does UV light effect carcinogenesis?
|
UVB causes pyrimidine dimers in DNA - thus it is an initiator
|

