![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
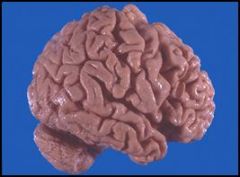
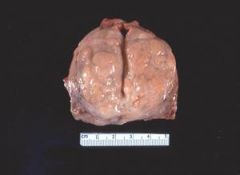
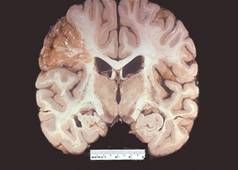
Gross image of an atrophic brain from a patient with Alzheimer’s disease:
The atrophic areas are toward the frontal pole (on the right) of the brain. Grossly, we see that the gyri are narrowed and the sulci are thus compensatorily widened. Microscopically we would see a decrease in the number of neurons. This patient has Alzheimer’s disease, which causes localized cerebral atrophy. We see this disease primarily in older patients (although there is a subset of patients who get this disease through heredity). Patients will present clinically with symptoms such as dementia, loss of cognitive function, short-term memory loss, and motor dysfunction. This is a progressive disease in which the condition of the patient deteriorates over a period of about a decade and after 10-15 years the patient is typically no longer able to recognize family members. Patients with this disease typically die of pneumonia or some other form of infection or sepsis from being bedridden. |
Describe
|
|
|
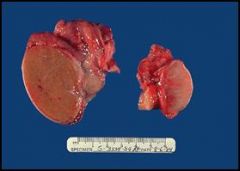
Atrophic and fibrotic testis due to a decrease in hormonal stimulation or blood flow, or cryptorchidism (in younger males):
To the left we see the cut surface of a normal (5-6 cm in greatest dimension) testis (there are portions of the spermatochord still attached). The testis on the right is smaller and atrophic. The cut surface of the atrophied testis is more white/tan than the cut surface of the normal testis, which is brownish. This is due to the replacement of the parenchyma with fibrotic tissue (collagen and adipose tissue). Causes of an atrophic testis include, a decrease in hormonal stimulation (at the gonadal or pituitary level), decrease in blood flow (atherosclerosis), or in younger males, cryptorchidism (an undescended testis that either stays in the abdominal cavity or inguinal canal). |
Describe.
|
|
|
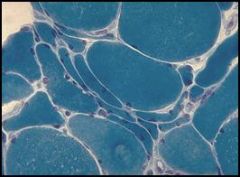
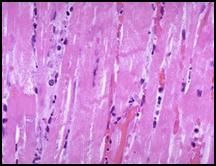
Atrophic skeletal muscle, most likely resulting from a dennervation (traumatic or surgical):
These are skeletal muscle cells. Notice that the cells in the center are much smaller. This is pathological because they should all be the same size. The muscle cells in the center have undergone atrophy caused by a dennervation, most likely the result of trauma or through an operative procedure. Note that skeletal muscle cells have multiple nuclei and as the cells atrophy, the nuclei get closer together and can mimic the appearance of giant cells. |
Describe.
|
|
|
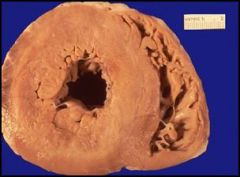
Heart with a hypertrophic left ventricle, most likely due to hypertension:
In the left ventricle of this heart, hypertension and increased pressure and demand overtime has led to the hypertrophy of the myocardial wall. Normal thickness of the LV:1.3 - 1.5 cm (DO NOT need to know!). Clinically, a patient with hypertension may have symptoms (headaches or palpitations). Complications resulting from a thickened heart include arrhythmias, and ischemia of the myocardium (increased thickening of the myocardial wall makes if difficult to supply the cardiac muscle with blood). More importantly, the heart over time will just not able to keep up and the patient will develop congestive heart failure (CHF).. Hypertrophy of the left ventricle can also be caused by valvular problems, cardiomyopathy and congenital problems. Note that the overall weight of the heart will also be increased. This is a type of cardiomegaly (an enlarged heart) due to the increase in muscle and/or ventricular cavity space. |
Describe
|
|
|
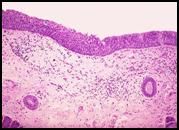
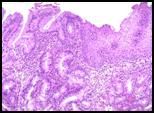
A microscopic image of BPH:
Normal Prostate: glandular component made up of epithelial cells with a space in between and the stroma and surrounding tissue that has both a smooth muscle component as well as a fibrous component.. Looking at the architecture of the glands in the specimen with BPH, we see there are just too many glands to begin with and they are growing too close to each other. The glands should be spaced apart. There often is hypertrophy of the stromal component. The symptoms of BPH include compression of the urethra and urinary symptoms. The treatment involves nothing at first; the patient has to be symptomatic to justify treatment. If the patient is symptomatic, they can be treated medically by blocking the androgen stimulation or surgically by removing part of the prostate immediately surrounding the urethra (Trans-Urethral Prostatic Resection or TURP…yes it’s backwards) to alleviate symptoms. |
Describe
|
|
|
Metaplasia of laryngeal respiratory epithelium into squamous epithelium:
In this image we see respiratory epithelium (ciliated pseudostratified columnar) to the right and non-keratinized squamous epithelium (it does not look exactly like normal squamous mucosa…but it’s trying) to the left and the lamina propria beneath the epithelial cells. This is around the area of the larynx. We have respiratory epithelium with cilia and mucus secretions to get rid of junk that should not be entering the respiratory tract. The transition is subtle, but there is a definite area of metaplasia. The chronic irritant in this case is smoking, which causes squamous metaplasia in long-time smokers. Do we care? Yes and no. Are the patients symptomatic? Not directly, but yes because. Since they have less cilia they are therefore prone to more secondary infections because they lack the ability to clear out the junk. How do we treat this? We just tell them to quit smoking. Why is it clinically significant? I’m sure everyone’s heard the term “squamous cell carcinoma” before and that’s a type of lung cancer that can follow squamous metaplasia. We also see in this image a lot of fluid and inflammatory cells in the lamina propria, but we didn’t really get into this. |
Describe.
|
|
|
Barrett’s esophagus, metaplasia of esophageal squamous mucosa into gastric-type columnar mucosa:
This is a microscopic image of the esophagus of a patient with acid reflux (of GERD). The normal lining is squamous epithelium (toward the right) there is an abrupt transition to columnar epithelium and the formation of glands (to the left). Clinically the patients have the signs and symptoms of acid reflux, heartburn and epigastric pain. They don’t have symptoms from the transition. We find out from and endoscopy that the have a Barrett’s esophagus. The metaplastic epithelium appears to mimick the foveolar epithelium of the stomach, although we find in it mucus producing glands (goblet cells) that are not present in the epithelium of the stomach but rather in the intestines. Note that there’s more than just metaplasia happening in this slide, we also see atypical cells (at least Dr. Furlong sees them…) and there’s a worry that the glandular cells will develop into cancer (not the squamous cells, but the metaplastic cells). Question: Will you point out a goblet cell? Answer: You could see them in class but I can really point any out at this magnification…they look like little white blobs in the glandular mucosa. Note that only a small percentage of people with acid reflux will get Barrett’s esophagus and an even smaller number will develop cancer. |
Describe.
|
|
|
Cardiac cells undergoing coagulative necrosis following a myocardial infarction:
In this image we see myocardial tissue, which has features of both smooth and skeletal muscle. You can see changes in the cytoplasm of the cells. There is a loss of nuclei (some of them are becoming fragmented and are falling apart, see the arrow) and some cells have no nuclei at all. There is also a breakdown of the cytoplasm (areas where it’s whiter). This is caused by ischemic damage from a heart attack (myocardial infarction). If we’re looking at acute myocardial infarction (e.g. after a few hours) we see less cell damage. This section was probably taken somewhere close to 24 hours after the ischemic event. We know this because we are beginning to see neutrophils (center of the dotted circle). As the tissue dies, inflammatory cells are attracted to the site. Neutrophils begin to invade within about 24 hours. Signs and symptoms include substernal chest pain. The infracted tissue will die and is replaced by scar tissue. |
Describe.
|
|
|
Cardiac cells undergoing coagulative necrosis following a myocardial infarction:
In this image we see myocardial tissue, which has features of both smooth and skeletal muscle. You can see changes in the cytoplasm of the cells. There is a loss of nuclei (some of them are becoming fragmented and are falling apart, see the arrow) and some cells have no nuclei at all. There is also a breakdown of the cytoplasm (areas where it’s whiter). This is caused by ischemic damage from a heart attack (myocardial infarction). If we’re looking at acute myocardial infarction (e.g. after a few hours) we see less cell damage. This section was probably taken somewhere close to 24 hours after the ischemic event. We know this because we are beginning to see neutrophils (center of the dotted circle). As the tissue dies, inflammatory cells are attracted to the site. Neutrophils begin to invade within about 24 hours. Signs and symptoms include substernal chest pain. The infracted tissue will die and is replaced by scar tissue. |
Describe.
|
|
|
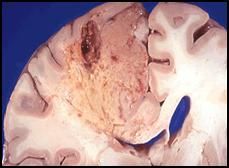
Liquefactive necrosis of the brain following a stroke:
Note that the color and feel (if we were to tough it, it would fall apart) of the tissue is different. This is and area of ischemic damage to the brain, also known as a cerebrovascular accident or stroke. In this case it was most likely the middle cerebral vessel that was occluded. The distal tissue dies, becomes gelatinous and eventually liquefies. Signs and symptoms of a stroke depend on the location, but include motor and/or sensory deficits on one side of the body. Strokes are sometimes reversible if the occlusion is rectified quickly and the patient undergoes rehabilitation. |
Describe.
|
|
|
A higher magnification image of liquefactive necrosis of the brain following a stroke:
This is most likely a more recent infarct. This area would heal by forming a hole (the tissue is not replaced by another tissue type. |
Describe
|
|
|
Liquefactive necrosis of the lung, forming an abscess in a patient with pneumonia:
In this image we are looking at the cut surface of fixed lung tissue. In the upper lobe we see a collection of puss (neutrophils) trying to get rid of an overwhelming infection (see the arrow). Normal fixed lung tissue appears brown in coloration; therefore the white/tan appearance of this tissue is abnormal. The lung appears white because the airspaces are filled with neutrophils; this is an example of pneumonia. In the areas of pneumonia, the alveolar walls are still intact (the tissue has NOT been destroyed) but the alveolar air spaces are filled with neutrophils. In the area of the abscess (upper lobe), the alveolar walls have been destroyed. |

Describe.
|
|
|
Liquefactive necrosis of the liver in the form of an abscess, most likely the result of a parasitic infection:
Recall that in normal liver tissue, the hepatocytes are arranged in cords, plates and sinusoids. In the center of this image, we see a collection of neutrophils and dead hepatocytes (darker). You can see how the body has made an attempt to wall off the infection with fibrous tissue. This walling off (the formation of an abscess) can actually make the infection more difficult to treat. This abscess of the liver could be the result of a parasitic infection. |
Describe.
|
|
|
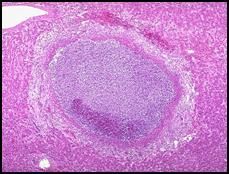
Slide 30: Caseous necrosis of a hilar lymph node in a patient with tuberculosis:
Note that this is a fresh lung preparation (although the air spaces have collapsed) and we are looking at a cut surface. At the center of the image we see a hilar lymph node involved in caseous necrosis. Note that the chunk taken out of the upper right hand corner of the lung (relative to the picture, see the arrow) is also abnormal. Dr. Furlong suspects that this (the missing chunk) was the primary site of tuberculosis infection (which usually involves the upper lobes) and that the tissue was biopsied. The infected node we are looking at now is most likely the draining lymph node from that site. The granulomatis response is an attempt to wall off the organism. |
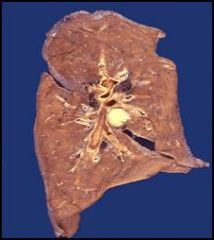
Describe.
|
|
|
Extensive casueos necrosis of the lung in a patient with tuberculosis:
Note that in immunocompromised patients the necrotic reaction can become more fulminant and disseminated (overwhelming and spreading). This is a much more extensive case of caseous necrosis (all of the whitish areas). |
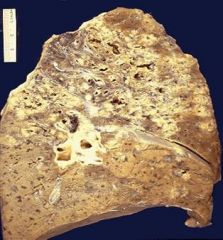
Describe.
|
|
|
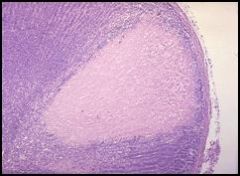
Microscopic view of a grauloma of the lung (caseous necrosis) in a patient with tuberculosis:
If we were able to see this granuloma in three dimensions, it would appear to be a ball. However, since this is only two dimensional, we have to pretend. We’re looking at a section of the granuloma center (upper right hand corner) filled with necrotic lung tissue in which the cells lost their nuclei. We can see the inflammatory response towards the periphery of the granuloma and maybe even some microbacteria. Patients with tuberculosis experience symptoms such as night sweats, a productive cough (coughing up sputum), and fever. The only way to make a definitive diagnosis of tuberculosis is with a sputum culture (which takes weeks). |
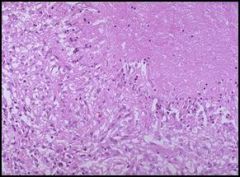
Describe.
|
|
|
“Dry” gangrenous toes of a patient with frostbite:
In this slide we see grossly the amputated toes of a patient with frostbite. Whether or not a person gets frostbite actually depends on more than just the temperature (freezing), but also the moistness of the air. In this case the tissue actually crystallizes and freezes. The gangrene is the blackish stuff and the skin actually sloughs off. |
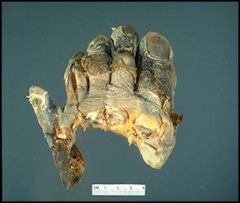
Describe.
|
|
|
“Wet” gangrene of the leg in a patient with diabetes resulting in amputation:
This is a below-the-knee amputation of a gangrenous leg from a diabetic patient. In the case of diabetics, the gangrene is a vascular issue. The capillaries and small vessels become occluded and over time the chronic ischemia leads to changes in the tissue and skin. These changes frequently begin with ulcers and then the skin starts to slough off and the tissue becomes black. Note that we may or may not make a distinction between “dry” and “wet” gangrene. We say “dry” is if there’s no overlying infection of the tissue. “Wet” gangrene frequently involves and infection that leads to enzymatic degradation of the tissue. |
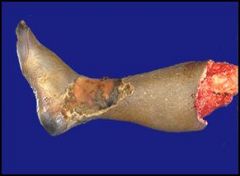
Describe.
|

