![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
74 Cards in this Set
- Front
- Back
|
Define:
INFLAMMATION |
-a host response
-comprising movement of fluid and leukocytes from the blood to the extra-vascular tissues -To localize and eliminate material perceived as unwanted |
|
|
Define:
EMIGRATION |
movement of leukocytes out of the vessels into the tissues
(EXTRAVASATION: laminar flow, stasis, rolling, adhesion, diapedesistransmigration, chemotaxis) |
|
|
Define:
DIAPEDESIS |
transmigration of cell across endothelium
final step in extravasation of leukocytes, induced by endothelial activation and expression of surface molecules |
|
|
Define:
CHEMOTAXIS |
locomotion along a chemical gradient
chemoattractants: -exogenous (target-derived), eg bacterial lipids and peptides -endogenous (host-derived), eg chemokines (C5a, LTB4, IL-8) |
|
|
Define:
FIBRINOUS INFLAMMATION |
type of ACUTE INFLAMMATION, characterized by large increase in vascular permeability, allowing fibrinogen to pass vascular barrier, fibrin is formed and deposited forming a fibrinous exudate (e.g. fibrinous pericarditis and meningitis)
|
|
|
Define:
EXUDATE vs. TRANSUDATE |
TRANSUDATE: clear, liquid, spec.grav<1.020, protein < 3.0gm/dl, few cells
EXUDATE: turbid, viscous (pus-like), spec.grav.>1.020, protein>3.0gm/dl, numerous cells (leukocytes) HALLMARK OF ACUTE INFLAMMATION |
|
|
Define:
SUPPURATIVE |
type of inflammation, neutrophils completely digest tissues and themselves, leaving a collection of puss = ABSCESS
|
|
|
Define:
PUS |
exudate
typical of ACUTE INFLAMATION protein rich fluid secondary to increased vascular permeability |
|
|
Define:
GRANULATION TISSUE |
scar tissue, if inflammation is not resolved (restored to normal), this healing tissue replacement is formed by proliferating fibroblasts and vascular endothelial cells
|
|
|
Define:
GRANULOMA |
-a FOCUS of CHRONIC INFLAMMATION consisting of:
-microscopic aggrecation of macrophages, transformed into epithelioid histocytes (sometimes giant cells) surrounded by collar of mononuclear leukocytes, principally lymphocytes and occasionally plasma cells (body's attempt to wall off undigestable material) |
|
|
ulcer
|
local defect, or excavation of the surface of an organ or tissue that is produced by the sloughing off of INFLAMMATORY NECROTIC TISSUE
can occur only when tissue necrosis and resultant inflammation exist on or near a surface ACUTE STAGE: intense PMN infiltration and vasodilation CHRONICITY: marbgins and base develop fibroblastic proliferation, scarring, & accumulation of Lymphocytes, MOs and Plasma cells |
|
|
fibrosis
|
Healing by connective tissue replacement (fibrosis). This occurs after substantial tissue destruction, when the inflammatory injury involves tissues that are incapable of regeneration, or when there is abundant fibrin exudation. When the fibrinous exudate in tissue or serous cavities (pleura, peritoneum) cannot be adequately cleared, connective tissue grows into the area of exudate, converting it into a mass of fibrous tissue-a process also called organization.
|
|
|
fibrin
|
an acute phase protein, elevated in inflammation
involved in clot formation, Processes in the coagulation cascade activate the zymogen prothrombin to the serine protease thrombin, which is responsible for converting fibrinogen into fibrin. Fibrin is then cross linked by factor XIII to form a clot. |
|
|
resolution of inflammation
|
restoration of the site of acute inflammation to normal -results when the injury is limited or short-lived or when there has been little tissue destruction and the damaged parenchymal cells can regenerate.
-involves neutralization or spontaneous decay of the chemical mediators, with subsequent return of normal vascular permeability, cessation of leukocytic infiltration, death (largely by apoptosis) of neutrophils, and finally removal of edema fluid and protein, leukocytes, foreign agents, and necrotic debris from the site -Lymphatics and phagocytes play a role |
|
|
organization following inflammation
|
Conversion of the fibrinous exudate to scar tissue
|
|
|
abscess
|
localized collection of purulent inflammatory tissue caused by suppuration buried in a tissue, an organ, or a confined space. They are produced by deep seeding of pyogenic bacteria into a tissue
-have a central region that appears as a mass of necrotic leukocytes and tissue cells. -usually a zone of preserved neutrophils around this necrotic focus -outside this region vascular dilation and parenchymal and fibroblastic proliferation occur, indicating the beginning of repair. |
|
|
pyogenic
|
"PUS-PRODUCING"
Certain bacteria (e.g., staphylococci) produce this localized suppuration and are therefore referred to as pyogenic (pus-producing) bacteria. A common example of an acute suppurative inflammation is acute appendicitis. |
|
|
pavementing, describes what part of LEUKOCYTE EXTRAVASATION
|
the endothelial wall of a bloodvessel can be virtually lined by white cells during the adhesion phase just before diapedesis
|
|
|
margination, describes what part of LEUKOCYTE EXTRAVASATION
|
accumulation of leukocytes (WBCs) in peripheral position along endothelial surface of vessel lumen
|
|
|
resolution of acute inflammation
|
-clearance if injurious stimuli
-clearance of mediators and acute inflammatory cells -replacement of injured cells -normal function |
|
|
typical triggers of ACUTE INFLAMMATION
|
-Infections and microbial toxins
-Trauma -Physical and chemical agents (burns, etc..) -Tissue Necrosis -Foreign Bodies -Immune Rxns |
|
|
typical triggers of CHRONIC INFLAMMATION
|
-Viral infections
-Chronic Infections -Persistent Injury -Autoimmune diseases |
|
|
Vascular changes that typify ACUTE INFLAMMATION
|
-VASODILATION
-INCREASED PERMEABILITY of microvasculature -INCREASED VISCOSITY OF BLOOD -STASIS |
|
|
Leukocyte subtypes involved in ACUTE INFLAMMATION
|
predominately PMN NEUTROPHILS
some macrophages, few plasma cells and eosinophils plus MORE NEUTROPHILS |
|
|
exudate is a hallmark characteristic of acute or chronic inflammation?
|
HALLMARK OF ACUTE INFLAMMATION!!!
along with PMNs! |
|
|
typical chronic inflammatory response is characterized by:
|
combinations of lymphocytes,
plasma cells and macrophages infiltrating a tissue in response to a specific stimulus |
|
|
temporal sequence of alterations in blood flow, during response to injury
|
stimulus: HISTAMINE: major inducer of vascular changes
-mediators (esp. histamine, NO) induce vasodilation of arterioles, opening capillaries (+bloodflow/hydrostatic pressure) -microvascular leakage (-oncotic pressure) -Increased Viscosity/Stasis -leukocyte accumulation, eventual transcytosis |
|
|
sequence of events in leukocyte extravasation
|
1. in lumen: margination, rolling, expression of adhesion molecules and adhesion to activated
endothelium 2. transmigration (diapedesis)across endothelium 3. migration in interstitial tissues toward a chemotactic stimulus (chemotaxis) 4. Leukocyte activation 5. Phagocytosis (recognition/attachment, engulfment, killing & degradation) 6. EC release of lysosomal enzymes by leukocytes |
|
|
Once neutrophils reach target of chemotaxis what do they do?
|
PHAGOCYTOSIS
RELEASE OF LYSOSOMAL ENZYMES |
|
|
CARDINAL SIGNS OF INFLAMMATION
4: described by CELCUS Virchow added one more |
RUBOR (redness),
TUMOR (swelling), CALOR (heat), DOLAR (pain), Functio Laesa (loss of fxn) |
|
|
carefully orchestrated process involving:
- Soluble mediators -Vascular responses -Cellular responses -Humoral responses |
Inflammation
|
|
|
3 Major events of ACUTE INFLAMMATION
|
1)Hemodynamic changes
2)+ vascular permeability 3) Extravasation of Leukocytes |
|
|
How does Inflammation
start? |
Stimuli too numerous to list:
Bacteria Dead tissue Foreign bodies Antibody-complement Trauma |
|
|
What surrounds splinter in acute inflammatory response?
|
cellulose cavity and EXUDATE = PUS
BLOOD VESSELS, dilated, surrounded by cells (GOING TO BE NEUTROPHILS) |
|
|
HISTAMINE
-source: -primary role in inflammation: |
source: MAST CELLS and BASOPHILS
primary role in inflammation: + VASCULAR LEAKAGE |
|
|
C3a and C5a are involved in what parts of he inflammatory response?
|
VASCULAR LEAKAGE
CHEMOTAXIS MACROPHAGE (opsonization) C5a more powerful chemoattractant than C3a |
|
|
Fibrin is a mediator of what inflammatory processes?
|
VASCULAR LEAKAGE
CHEMOTAXIS of NEUTROPHILS |
|
|
primary role of prostaglandins in inflammation
|
potentiate other mediators (involved in vasodilation, pain, fever)
|
|
|
SRS-A
(source:) (mediates what process?) |
produced by leukocytes, mast cells
BRONCHOCONSTRICTION |
|
|
TRIPLE RESPONSE OF LEWIS
|
vessel changes in acute inflammation
1) Increased Blood Flow (vasodilation of pre-cap arterioles induced by: -cell-derived (mast cells: histamine, platelets: serototonin) or -plasma-derived (complement) VASOACTIVE MEDIATORS 2. Increased permeability of post-cap venules (a) gaps (b) transcytosis (c) unexplained mechanisms 3) Stasis |
|
|
How does opsonization aid in recognition of target material for phagocytosis
|
opsonins (host-derived factors) bind to target
1) Leukocyte "recognizes" and binds opsonin via receptor 2) Opsonin optimizes recognition of particles as "foreign" 3) Helps overcome electrostatic repulsion (negative charges on microbes and on host leukocytes repel one another) |
|
|
OPSONINS:
cell-derived plasma-derived |
cell-derived: Fc portion of IgG binds FcgR on leukocyte
plasma-derived: 1)C3b/C3bi bind CR1, CR2, CR3 on leukocyte 2) Carbohydrate-binding proteins/collectins bind C1q receptor on leukocyte |
|
|
Phagocytosis (engulfmant of target material) involves:
|
a) pseudopodia engulf material to form PHAGOSOME
b) lysosomal granules fuse with phagosome to form PHAGOLYSOSOME |
|
|
Destruction of target material by Leukocyte
a) Oxygen dependent |
a) O2 dpdt:
(1) NADPH oxidase: --> 2O2- 2) 2O2- + 2H+ --> H2O2 + O2 3) Myeloperoxidase converts H2O2 to HOCl |
|
|
Destruction of target material by Leukocyte
a) Oxygen independent |
1) BPI (bactericidal permeability increasing protein)
2) Lysozyme 3) Lactoferrin 4) Major basic protein 5) Defensin |
|
|
most chronic inflammation reactions are what type of immune reactions?
|
humoral
cellular or BOTH |
|
|
chronic inflammation involves predominantly what cell types?
|
lymphocytes (CD4 and CD8) and macrophages
|
|
|
Examles of CHRONIC INFLAMMATION
|
1. Predominantly lymphocytes:
(a) Chronic (HASHIMOTO'S) thyroiditis (b) RA 2. GRANULOMA (a) foreign body rxn (b) hypersensitivity granuloma |
|
|
reactions involved in Chronic thyroiditis
(gross appearance) |
Type II (Ab-mediated cytotoxicity) and Type IV (DTH) reactions
HYPOTHYROIDISM, GOITER with possible compression of trachea |
|
|
histological characteristics of RA chronic inflammation
|
involves VILLOUS SYNOVITIS:
villous projections with many lymphocytes and plama cells |
|
|
histo of FOREIGN BODY REACTION granuloma
|
inert foreign material, surrounded by activates MOs, may fuse to form multinucleate giant cells
mild fibrous reaction may occur |
|
|
histo of Hypersensitivity granuloma
|
-circumscribed collection of epithelioid cells
-lymphocyes always persent -may or may not also include multinucleate giant cells, necrosis, surrounding fibrosis |
|
|
outcome of hypersensitivity granuloma
|
early granulomas may go on to resolution,
most granulomas lead to at least some fibrosis and scarring |
|
|
SCAR
|
alternate tissue laid down as part of "healing," when regeneration of original tissue is not possible due to ECM damage
|
|
|
KELOID
|
if the scar tissue grows beyond the boundaries of the original wound and does not regress, it is called a keloid.
-more common in African- Americans -mechanisms of keloid formation are unknown. |
|
|
hypertrophic scar
|
The accumulation of excessive amounts of collagen may give rise to a raised scar known as a hypertrophic scar;
|
|
|
KELOID
|
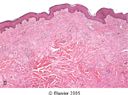
What part of sequelae of inflammation is depicted here?
|
|
|
Potential Sequelae of ACUTE Inflammation
|
*Resolution (RARE)
*Chronic Inflammation *Abscess Formation *Fibrosis (SCAR) *Effusion *Serositis (purulent or fibrinous) *Necrosis *Chronic Inflammation |
|
|
Potential Sequelae of CHRONIC Inflammation
|
*Tissue Necrosis
*Joint Destruction *Autoamputation (leprosy) *Severe tissue scarring (TB) *Amyloid Deposition *Neurological problems |
|
|
What does resolution of inflammation entail?
|
RETURN TO NORMAL:
no more calor, rubor, dolor, tumor or functio laesa (example, poison ivy vesicles, zoster lesions- BM remains INTACT!) |
|
|
why do smallpox lesions not resolve?
|
Lesions cause tissue necrosis right down through dermis, BM destroyed, tissue dissolved (resulting in SCARRING)
|
|
|
deletorious effects of scarring:
|
*Scar tissue has no function other than binding together damaged sittue elements.
*can obstruct, block, constrict normal tissue *can entrap normal tissues or structures thus diminishing or destroying their function *Over time scar tissue constricts/tightens structures *Scar tissue can bind-together structures that should remain independent (adhesions) |
|
|
volvulus
|
loop of bowel twisted off due to adhesion (estrangulated, ischemic, infarcted)
|
|
|
Histopathological definition of CHRONIC inflammation, characterized by what cell types?
|
lymphocytes, plasma cells and macrophages predominate
|
|
|
How does Staphylococcus aureus (ACUTE INFLAMMATION) become chronic in AIDS patient?
|
in this situation what should be an “acute” inflammatory process has become “chronic” because patient can’t RESOLVE (has lost even ability for neutrophils to function appropriately, not opsonizing bacteria for neutrophil engulfment
|
|
|
CHRONIC INFLAMMATION usually results in:
|
SCAR
or TISSUE DEATH |
|
|
What is found inside RA nodules?
|
necrotic tissue
|
|
|
What is found in the center of a TB GRANULOMA (of lung)?
|
CASEOUS NECROSIS
|
|
|
Fistula
|
NON anatomic tube (forms in healing response instead of scar)
|
|
|
Inflammation in RA joints stimulates formation of these on synovial surface:
|
stimulates PROLIFERATION of CELLS forming VILLI on Synovial surface
|
|
|
Amyloidosis
|
deposition of insoluble proteins diffusely in tissues, replacing normal tissues (INFLAMMATION CAN BE ELSEWHERE IN BODY)
examples: TB, leprosy |
|
|
How can chronic inflammation result in seizures or hydrocephaly?
|
inflammation of surface of brain can produce glial scars (astrocytic gliosis = eleptogenic)
|
|
|
abscess as sequelae of ACUTE INFLAMMATION involves complete
|
complete digestion of existing material by neutrophils, including neutrophils digesting themselves
|
|
|
contracture
|
develops when the normally elastic tissues are replaced by fibrous tissue. prevents normal movement.
Contractures occur primarily in the skin, underlying tissues, muscle, tendons, and joint areas. |

