![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
50 Cards in this Set
- Front
- Back
|
Acute leukemia is characterized by
|
an accumulation of primitive hematopoietic cells in the bone marrow which are termed blast cells or blasts cells, lymphoblasts in acute lymphoblastic leukemia (ALL) and myeloblasts in acute myeloid leukemia (AML).
|
|
|
Chronic leukemia is composed of more mature elements e.g.,
|
lymphocytes in chronic lymphocytic leukemia or myelocytes to segmented neutrophils in chronic myelogenous leukemia
|
|
|
. Fatigue and pallor associated with anemia due to depressed erythropoiesis are often present. Bleeding, commonly due to thrombocytopenia, is a reflection of decreased megakaryocytes in the bone marrow. Infection may be related to neutropenia as a result of depressed granulopoiesis
|
ACUTE LEUKEMIA
|
|
|
The combination of anemia, thrombocytopenia, and neutropenia is termed
|
pancytopenia.
|
|
|
Cytogenic feats of Acute lymphoblastic leukemia
Nucleus configuration Chromatin pattern Nucleoi Cytoplasm |
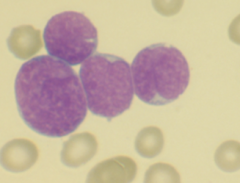
-Round to oval; may be indented
-slight chromatin clumping -Often not seen. may have 1-2 usually. inconspicuous nucleoli. -Scanty (smaller than usual), agranular |
|
|
-Cytogenic feats of Acute meyloblast leukemia
Nucleus configuration Chromatin pattern Nucleoi Cytoplasm |
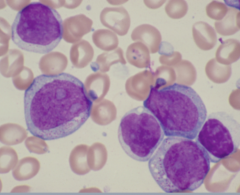
-Round to oval to polygonal, may be indented, lobular, folded
-Finely distributed chromatin -2-5 usually prominent nucleoli -Relatively abundant; may contain a few azurophilic granules and/or Auer rods. |
|
|
Unfortunately these cytologic parameters are not always definitive in the distinction between ALL and AML. Lineage of blast cells is established today via
|
flow cytometry.
|
|
|
most common malignancy of childhood in the U.S.
|
Acute lymphoblastic leukemia (ALL) occur in children below the age of 6 years
|
|
|
Atypical lymphoblast
Size Nuclear configuration Nucleoi Cytoplasm |
-large cells with 2x the diameter of RBC
-Uniform to lobulated nuclear contours -Large prominent nucleoli with a peripheral chromatin condensation -cytoplasm >20 of surface area |
|
|
Burkitt Leukemia characteristics
Size Nuclear configuration Nucleoi Cytoplasm |

-Large
-Oval to round to regular -Usually multiple prominent nucleoli -Moderately abundant cytoplasm with intense basophilia and clear sharply demarcated vacuoles |
|
|
One should note that the atypical lymphoblasts are indistinguishable from myeloblasts morphologically, and thus these cases must be differentiated from AML using
|
flow cytometry
|
|
|
ALL is diagnosed and categorized based upon
|
immunologic phenotype
The leukemic cells can often be identified as representing specific stages of normal B-cell or T-cell maturation. |
|
|
B-lineage surface antigens including CD19, CD20, CD22 detects
|
Precursor B-cell Acute Lymphoblastic Leukemia
|
|
|
B-lineage surface antigens plus
monoclonal surface IgM for |
Burkitt Leukemia
|
|
|
T-lineage surface antigens – CD7, CD5,
CD2 for |
Precursor T Acute Lymphoblastic Leukemia
|
|
|
Although most cases of Precursor T ALL can be assigned to a particular stage of thymic maturation, specific antigens normally present may be missing in individual cases. Early thymocytes are characterized by
|
the presence of CD7 on the cell surface. This is an important marker because it is present on the cells of virtually all Precursor T ALL and is absent in B-lineage ALL.
|
|
|
CD7, CD2, CD5 markers are associated with Precursor T Acute Lymphoblastic Leukemia in whats stage of T cell maturation?
|
Early thymocyte
|
|
|
CD1, CD4, CD8 (CD7, CD2, CD5)
markers are associated with Precursor T Acute Lymphoblastic Leukemia in whats stage of T cell maturation? |
Common thymocyte
|
|
|
markers are associated with Precursor T Acute Lymphoblastic Leukemia in whats stage of T cell maturation? CD3, CD4 or CD8 (CD7, CD2, CD5)
|
Mature thymocyte
|
|
|
The incidence of Precursor B Acute Lymphoblastic leukemia is greater in
|
childhood. Incidence decreases as we age.
|
|
|
Precursor T Acute Lymphoblastic leukemia increases with
|
Age
|
|
|
Burkitt lymphona incidence is greater in
|
Infants and adults
|
|
|
PROGNOSIS of the dififerent types of acute leukemia
|
Precursor B Acute Lymphoblastic Leukemia – Best
Precursor T Acute Lymphoblastic Leukemia – Intermediate Burkitt Leukemia - Worst |
|
|
CYTOGENETICS
Bad prognosis for ALL |
t(9;22) Philadelphia Chromosome
t(8;14) Burkitt Leukemia t(4;11) Lymphoblastic Leukemia in Infants Hypodiploid karyotype <44 chromosomes |
|
|
CYTOGENETICS Good prognosis
for ALL |
Hyperdiploid Karyotype >50 chromosomes
Simultaneous trisomy 4, 10, 17 |
|
|
Of all the Precursor B ALL risk groups, only the very high risk groups has a low survival. Some of the criteria for high risk group is
|
BCR/ABL fusion or less than 44 chromosomes
|
|
|
Standard high risk for precursor B ALL some criteria are
|
WBC>50,000
Age 10 or more CNS or testicular disease |
|
|
Low risk groups for precursor B ALL criteria are
|
-triple trisonomy 4, 10, 17
-TEL/AML1 FUSION -NO CNS or testicular disease |
|
|
WBC~12500
Male ~4 yrs Hyperdiploid>50 chromosomes |
Precursor B ALL
|
|
|
WBC~63,000
Male~9 yo Mediastinal mass Lymphadenopathy |
Precursor T ALL. The typical patient with Precursor T Acute Lymphoblastic Leukemia is an adolescent male with a somewhat lymphomatous presentation (mediastinal mass and/or peripheral lymphadenopathy) and a high WBC
|
|
|
WBC~30,000
Male~8yo Lymphadenopathy |
Burkitt Leukemia. Burkitt Leukemia patients are often older children with lymphadenopathy and CNS involvement.
|
|
|
If a patient relapses while on therapy or during the first year after coming off therapy, the possibility of long term survival is
|
is markedly reduced unless successful bone marrow transplantation can be performed
|
|
|
Who is affected more by CHRONIC LYMPHOCYTIC LEUKEMIA
|
Mostly males older than 60
|
|
|
CHRONIC LYMPHOCYTIC LEUKEMIA (CLL) is characterized by
|
an accumulation mature-appearing lymphocytes in the peripheral blood, bone marrow, and lymph nodes.
-Mild anemia -Normal platelet count -Lymphadenopathy and splenomegaly -pts are asymptomatic |
|
|
What are the values of CLL?
|
An absolute lymphocytosis of >4,000 lymphocytes per uL
WBC=20,000 and 100,000. |
|
|
CLL prognosis
|
prognosis is fairly good with a median survival of 7 years, a staging scheme has been developed to assess the extent of disease which correlates with survival.
|
|
|
CLL stage A survival
|
5 yrs 79%. Stage B has greater survival chance than C
|
|
|
Most deaths due to CLL are due to
|
Infection. Bone marrow function can gradually become compromised as it is progressively infiltrated by the small lymphocytes. This is not only related to neutropenia but also to hypogammaglobulinemia observed in about 50% of CLL patients.
|
|
|
Immunophenotype of CLL
|
-demonstrate a B-cell immunophenotype: IgM on the cell surface often accompanied by IgD
-CD19, CD20, and CD23 -CD5 in all CLL pts |
|
|
most frequent single chromosomal abnormality and is seen in about 50% of CLL.
|
Deletion of 13q14.3
|
|
|
. CLL can be separated into two groups based on mutational status of the immunoglobulin genes.
|
-Those lacking somatic mutations of the variable regions of their immunoglobulin genes appear to originate from naïve B-cells and have a less favorable prognosis than
-those with somatically mutated genes which appear to originate from antigen-stimulated memory B-cells. |
|
|
The mutation status of the immunoglobulin genes is commonly assessed using a surrogate marker such as
|
CD38. Expression of CD38 correlates, although not perfectly, with a lack of mutations of the immunoglobulin genes and thus a less favorable prognosis.
|
|
|
what is the Richter syndrome.
|
the lymph nodes show conversion from an infiltration by small lymphocytes to large cell lymphoma. Rapid progression to death is the typical course after transformation
|
|
|
Is blast crisis observed in CLL?
|
No because CLL does not convert into acute leukemia
|
|
|
Hairy cell leukemia (HCL
|
Males in their 60s
chronic leukemia characterized by an insidious onset, massive splenomegaly without lymphadenopathy, pancytopenia, dry bone marrow aspiration, and the presence of abnormal circulating mononuclear cells. |
|
|
The neoplastic cells are termed hairy cells because
|
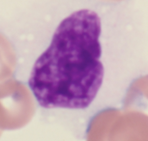
of numerous hair-like cytoplasmic projections around the periphery of the cytoplasm. abundant cytoplasm which is pale blue and appears delicate. Because only low numbers of leukemic cells are often present in the peripheral blood, the diagnosis is usually made on the bone marrow biopsy
|
|
|
The The pancytopenia caused by hairy cell leukemia relates to
|
marrow infiltration as well as hypersplenism due to the massive splenomegaly
|
|
|
The splenomegaly in hairy cell leukemia is due to
|
expansion of the red pulp by a diffuse infiltration of hairy cells similar in appearance to the bone marrow.
|
|
|
The classic cytochemical test used to confirm the presence of hairy cells is
|
the tartrate resistant acid phosphatase (TRAP) reaction.
|
|
|
Hairy cell immunophenotype
|
IgG and IgA . many cases of HCL appear to be arrested in maturation at the point of immunoglobulin heavy chain switching with multiple heavy chain isotypes including IgG, IgA, IgM, and/or IgD on the cell surface
|

