![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
101 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Past questions were based on following topics |
1 Ageing 2 Organizing geriatric services 3 Clinical assessment of older people 4 Rehabilitation 5 Falls and funny turns 6 Drugs 7 Neurology 8 Stroke 9 Psychiatry 10 Cardiovascular 11 Chest medicine 12 Gastroenterology 13 Renal medicine 14 Homeostasis 15 Endocrinology 16 Haematology 17 Musculoskeletal system 18 Pressure injuries 19 Genitourinary medicine 20 Incontinence 21 Ears 22 Eyes 23 Skin 24 Infection and immunity 25 Malignancy 26 Death and dying 27 Ethics 28 Finances 29 Peri-operative medicine |
|
|
|
Ageing
|
2 |
|
|
|
2 Organizing geriatric services |
1 |
|
|
|
3 Clinical assessment of older people
|
1 |
|
|
|
4 Rehabilitation |
1 |
|
|
|
Falls and funny turns Falls and fallers▪︎Assessment following a fall▪︎Interventions to prevent falls▪︎Syncope and presyncope▪︎HOW TO Distinguish syncope and seizures▪︎Balance and disequilibrium▪︎Dizziness▪︎HOW TO Manage multifactorial dizziness—clinical example▪︎Drop attacks▪︎Orthostatic (postural) hypotension▪︎Situational hypotension▪︎HOW TO Measure postural blood pressure▪︎Carotid sinus syndrome▪︎HOW TO Perform carotid sinus massage▪︎Falls services |
1 falls causes 2 falls management -support 3 |
|
|
|
Falls and fallers► A fall is often a symptom of an underlying serious problem and is not a partof normal ageing.A fall is an event that results in a person non-intentionally coming to rest at alower level (usually the floor) with or without loss of consciousness. Falls arecommon and important, affecting one-third of older people living in their ownhomes each year. They result in fear, injury, dependency, institutionalization, and death. Many can be prevented and their consequences minimized. |
Factors influencing fall frequency ▪︎Intrinsic factors. Maintaining balance and avoiding a fall is a complex,demanding multisystem skill. It requires 1.muscle strength (power:weight ratio), 2.stable but flexible joints, 3.multiple sensory modalities (e.g. proprioception,vision, hearing), and a 4.functional peripheral and central nervous system. 5.Higher-level cognitive function permits risk assessment, giving insight into the danger that a planned activity may pose ▪︎Extrinsic factors. These include environmental factors, e.g. lighting, obstacles,the presence of grab rails, and the height of steps and furniture, as well as the softness and grip of the floor
Magnitude of ‘stressor’. All people have the susceptibility to fall, and thelikelihood of a fall depends on how close to a ‘fall threshold’ a person sits. Older people, especially with disease, sit closer to the threshold and are more easily and more often pushed over it by stressors. ▪︎ These can be internal (e.g.transient dizziness due to orthostatic hypotension) or ▪︎external (e.g. a gust ofwind or a nudge in a crowded shop); they may be minor or major (no one can avoid ‘falling’ during syncope) If insight is preserved, the older person can, to some extent, reduce risk by limiting hazardous behaviours and minimizing stressors (e.g. walking only inside, avoiding stairs or uneven surfaces, using walking aids, or asking for supervision).
▪︎Factors influencing fall severity In older people, the adverse consequences of falling are greater, due to: Multiple system impairments which lead to less effective saving mechanisms. Falls are more frightening and injury rates per fall are higher Osteoporosis and ↑ fracture rates 2° injury due to post-fall immobility, including pressure sores, burns,dehydration, and hypostatic pneumonia. Half of older people cannot get upagain after a fall Psychological adverse effects, including loss of confidence ▪︎Falls are almost always multifactorial. Think:‘Why today?’ Often because the fall is a manifestation of acute or subacuteillness, e.g. sepsis, dehydration, or drug adverse effect‘ Why this person?’ Usually because of a combination of intrinsic and extrinsic factors that ↑ vulnerability to stressors. Banned terms The terms simple fall and mechanical fall are used commonly, but they are facile, imprecise, and unhelpful. ‘Simple’ usually refers to the approach adopted by the assessing doctor.For every fall, identify the intrinsic factors, extrinsic factors, and acute stressors that have led to it.Within each of these categories, think how their influence on the likelihood of future falls can be reduced. |
Assessment following a fall Think of fall(s) if a patient presents: ~Having ‘tripped’ With a fracture or non-fracture injury ~Having been found on the floor ~With 2° consequences of falling (e.g. hypothermia, pneumonia) Patients who present having fallen are often mislabelled as having ‘collapsed’,discouraging the necessary search for multiple causal factors. ▪︎Practise opportunistic screening—ask all older people who attend 1° or 2°care whether they have fallen recently. History Obtain a corroborative history, if possible. May often need to use very specific,detailed, and directed questions. In many cases, a careful history differentiates between falls due to: ▪︎Frailty and unsteadiness ▪︎Syncope or near-syncope ▪︎Acute neurological problems (e.g. seizures, vertebrobasilar insufficiency(VBI))
Gather information about: ▪︎Fall circumstances (e.g. timing, physical environment) ▪︎Symptoms before and after the fall ▪︎Clarification of symptoms, e.g. ‘dizzy’ may be vertigo or presyncope ▪︎Drugs, including alcohol ▪︎Previous falls, fractures, and syncope (‘faints’), even as a young adult ▪︎Previous ‘near-misses’ ▪︎Comorbidity (cardiac, stroke, Parkinson’s disease, seizures, cognitive impairment, diabetes, incontinence) ▪︎Functional performance (difficulties bathing, dressing, toileting) ▪︎Drugs associated with falls ~Falls may be caused by any drug that either is directly psychoactive or may lead to systemic hypotension and cerebral hypoperfusion. ~Polypharmacy (>4 drugs,any type) is an independent risk factor. ~The most common drug causes are •Benzodiazepines and other hypnotics •Antidepressants (tricyclics and selective serotonin reuptake inhibitors (SSRIs) •Antipsychotics •Opiates •Diuretics •Antihypertensives, especially ACE inhibitors and α-blockers •Antiarrhythmics •Anticonvulsants •Skeletal muscle relaxants, e.g. baclofen, tizanidine •Hypoglycaemics, especially long-acting oral drugs and insulin
Examination This can sometimes be focused if the history is highly suggestive of a particular pathology; ~perform at least a brief screening examination of each system. ~Functional. ▪︎Ask the patient to stand from a chair, walk, turn around, walkback, and sit back down (‘get up and go’ test). ▪︎Assess gait, use of walking aids, and hazard appreciation ▪︎Cardiovascular. ~ Always check lying and standing BP. ~Check pulse rate and rhythm. ~Listen for murmurs (especially of aortic stenosis) ▪︎Musculoskeletal. ~Assess foot wear (stability and grip). ~Remove footwear and examine the feet. ~ Examine the major joints for deformity, instability, or stiffness ▪︎Neurological. ~To identify stroke, peripheral neuropathy, Parkinson’s disease,vestibular disease, myelopathy, cerebellar degeneration, and cognitive impairment VisionTests
The following are considered routine: ECG FBC, B12, folate, U, C+E, glycosylated Hb (HbA1c), calcium, phosphate, TFT Vitamin D deficiency is common in older adults, and evidence suggests that replacing may reduce falls/harm from falls If a specific cause is suspected, then test for it, e.g.:24h ECG in a patient with frequent near-syncope and a resting ECG suggesting conducting system disease Echocardiogram in a patient with systolic murmur and other features suggesting aortic stenosis (e.g. slow-rising pulse, left ventricular hypertrophy(LVH) on ECG) Head-up tilt table testing (HUTT) in patients with unexplained syncope, normal resting ECG, and no structural heart disease However, all tests have false positive rates, and even a ‘true positive’ finding may have no bearing on the patient’s presentation. For example, a patient falling due to osteoarthritis and physical frailty will not benefit from an echocardiogram that reveals asymptomatic mild aortic stenosis. ► Use tests selectively, based on your judgement (following careful historyand examination) of the likely factors contributing to falls. |
|
|
Balance and disequilibrium Balancing is a complex activity, involving many systems. Input There must be awareness of the position of the body in space, which comes from:Peripheral input—information about body position comes from peripheral nerves (proprioception) and mechanoreceptors in the joints. This information is relayed via the posterior column of the spinal cord to the central nervous system (CNS) Eyes—provide visual cues as to position Ears—provide input at several levels. The otolithic organs (utricle and saccule) provide information about static head position. The semicircular canals inform about head movement. Auditory cues localize a person with reference to the environment Assimilation Information is gathered and assessed in the brainstem and cerebellum. Output Messages are then relayed to the eyes, to allow a steady gaze during head movements (the vestibulo-ocular reflex), and to the cortex and the cord to control postural (anti gravity) muscles. When all this functions well, balance is effortless. A defect(s) in any one contributing system can cause balance problems or disequilibrium: Peripheral nerves—neuropathy is more common. Specifically, it is believed that there is a significant age-related loss of proprioceptive function Eyes—age-related changes ↓ visual acuity. Disease (cataracts, glaucoma, etc.)is more common Ears—age-related changes ↓ hearing and lead to reduced vestibular function. The older vestibular system is more vulnerable to damage from drugs, trauma,infection, and ischaemia Joint receptors—degenerative joint disease (arthritis) is more common in older people CNS—age-related changes can slow processing. Disease processes(ischaemia, hypertensive damage, dementia, etc.) are more common with age Postural muscles—sarcopenia (a syndrome of reduced muscle mass with weakness) due to inactivity, disease, medication (e.g. steroids), or intrinsic ageing In the older person, one or more of these defects will occur commonly. In addition, skeletal changes may alter the centre of gravity, and cardiovascular changes may lead to arrhythmias or postural change in BP, exacerbated furtherby medications. An approach to disequilibrium ▪︎Aetiology is usually multifactorial ▪︎Consider each system separately, and optimize its function ▪︎Look at provoking factors (medication, cardiovascular conditions,environmental hazards, etc.) and minimize them ▪︎Work on prevention:Alter the environment (e.g. improve lighting) Develop safer ways to mobilize, and ↑ strength, stamina, and balance Small adjustments to multiple problems can make a big difference, e.g. when appropriate, combine cataract extraction, a walking aid, vascular 2°prevention, a second stair rail, brighter lighting, and a course of PT ► If falls persist, despite simple (but multiple) interventions, refer to a falls clinic. |
Dizziness A brain that has insufficient information to be confident of where it is in space generates a sensation of dizziness. This can be due to reduced sensory inputs or impairment of their integration. Dizziness is common, occurring in up to 30% of older people. However, the term dizziness can be used by patients and doctors to mean many different things, including: Movement (spinning) of the patient or the room—vertigo Light-headedness—syncope and presyncope Mixed—a combination of these sensations Other, e.g. malaise, general weakness, headache Distinguishing these is the first step in management, as it will indicate possible causal conditions. This relies largely on the history. Discriminatory questionsinclude: ‘Please try to describe exactly what you feel when you are dizzy’‘Does the room spin, as if you are on a roundabout?’ (vertigo) ‘Do you feel light-headed, as if you are about to faint?’ (presyncope)‘ Does it occur when you are lying down?’ (if so, presyncope is unlikely)‘ Does it come on when you move your head?’ (vertigo more likely)‘ Does it come and go?’ (chronic, constant symptoms are more likely to bemixed or psychiatric in origin) Causes The individual conditions most commonly diagnosed when a patient complains of dizziness are: Benign paroxysmal positional vertigo (BPPV) Labyrinthitis Posterior circulation stroke Orthostatic hypotension Carotid sinus hypersensitivity VBI Cervical spondylosis Anxiety and depression In reality, much dizziness is multifactorial, with dysfunction in several systems.This means that precise diagnosis is more difficult (and often not done) and treatment is more complex. ► Making small improvements to each contributing problem can add up to abig overall improvement (perhaps making the difference between independent living or institutional care). |
Drop attacks This term refers to unexplained falls with no prodrome, no (or very brief) loss ofconsciousness, and rapid recovery. The proportion of falls due to ‘drop attack’ ↑with age. There are several causes, including: Cardiac arrhythmia Carotid sinus syndrome (CSS) Orthostatic hypotension Vasovagal syndrome VBI Weak legs (e.g. cauda equina syndrome) The first four causes listed usually lead to syncope or presyncope, with identifiable prior symptoms (e.g. dizziness, pallor); those episodes would not be termed ‘drop attacks’. However, such prior symptoms are not universal and maynot be recollected, leading to a ‘drop attack’ presentation. In most cases, following appropriate assessment, the cause(s) can be identified and effective treatment(s) begun. ► Making a diagnosis of ‘drop attack’ alone is not satisfactory; assess more completely and, where possible, determine the likely underlying cause(s) |
|
|
Interventions to prevent falls The complexity of treatment reflects the complexity of aetiology: Older people who fall more often have remediable medical causes Do not expect to make only one diagnosis or intervention—making minor changes to multiple factors is more powerful Tailor the intervention to the patient. Assess for relevant risk factors and workto modify each one ▪︎A multidisciplinary approach is key Reducing fall frequency ▪︎Drug review. Try to reduce the overall number of medications. ~For each drug,weigh the benefits of continuing with the benefits of reduction or stopping.Stop if risk is greater than benefit. ~Reduce if benefit is likely from the drug class, but the dose is excessive for that patient. ~ Taper to a stop if withdrawal effect likely, e.g. benzodiazepine Treatment of orthostatic hypotension ▪︎Strength and balance training. In the frail older person, by a PT, exercise classes, or disciplines such as t’ai chi ▪︎Walking aids. Provide an appropriate aid and teach the patient how to use it ▪︎Environmental assessment and modification (a simple checklist can help the family minimize risk; in some cases, a more detailed assessment by an OT isbeneficial) ▪︎Vision. Ensure glasses are appropriate (avoid vari- or bifocal lenses) ▪︎Reducing stressors. This involves decision-making by the patient or carers.The cognitively able patient can judge risk/benefit and usually modifies risk appropriately, e.g. limiting walking to indoors, using a walking aid properly and reliably, and asking for help if a task (e.g. getting dressed) is particularly demanding. However:Risk can never be abolished Enforced relative immobility has a cost to health Patient choice is paramount. Most will have clear views about risk and how much lifestyle should change ▪︎Institutionalization does not usually reduce risk Preventing adverse consequences of falls Despite risk reduction, falls may remain likely. In this case, consider: ▪︎Osteoporosis detection and treatment ▪︎Teaching patients how to get up. Usually by a PT ▪︎Alarms, e.g. pullcords in each room or a pendant alarm (worn around theneck/wrist). Often these alert a distant call centre, which summons more local help (home warden, relative, or ambulance) ▪︎Supervision. Continual visits to the home (by carers, neighbours, family,and/or voluntary agencies) reduce the duration of a ‘lie’ post-fall ▪︎Change of accommodation. This sometimes reduces risk but is not a panacea.A move from home to a care home may provide a more suitable physical environment, but it will be unfamiliar and staff cannot provide continuous supervision. Preventing falls in hospital Falls in hospital are common, a product of admitting acutely unwell older people with chronic comorbidity into an unfamiliar environment. Multifactorial interventions (such as FallSafe) have the best chance of reducing falls: Treat infection, dehydration, and delirium actively Stop incriminated drugs and avoid starting them Provide good-quality footwear and an accessible walking aid Provide good lighting and a bedside commode for those with urinary or faecal urgency or frequency Keep a call bell close to hand Care for the highest-risk patients in a bay under continuous staff supervision
Interventions that are rarely effective and may be harmful ▪︎Bedrails (cotsides). Injury risk is substantial limbs snag on unprotected metal bars and patients clamber over the rails, falling even greater distances onto the floor below ▪︎Restraints. These ↑ the risk of physical injury, including fractures, pressure sores, and death. Also ↑ agitation ▪︎Hip protectors are impact-absorptive pads stitched into undergarments. Evidence of efficacy is limited to care home residents and they cause more falls in community-dwelling trials. |
Syncope and presyncope ▪︎Syncope is a sudden, transient loss of consciousness due to reduced cerebral perfusion. The patient is unresponsive with a loss of postural control (i.e. slumpsor falls). ▪︎Presyncope is a feeling of light-headedness that would lead to syncope if corrective measures were not taken (usually sitting or lying down). These conditions:Are a major cause of morbidity (occurring in a quarter of institutionalized older people), recurrent in one-third. ▪︎Risk of syncope ↑ with advancing age and in the presence of cardiovascular disease ▪︎Accounts for up to 5% of hospital attendances and many serious injuries (e.g.hip fracture) ▪︎Cause considerable anxiety and can cause social isolation as sufferers limit activities in fear of further episodes. Causes These are many. Older people with ↓ physiological reserve are more susceptible to most. They can be subdivided as follows: ▪︎Peripheral factors. Hypotension may be caused by an upright posture, eating,straining, or coughing, and may be exacerbated by low circulating volume(dehydration), hypotensive drugs, or intercurrent sepsis. •Orthostatic hypotension is the most common cause of syncope •Vasovagal syncope (‘simple faint’). Common in young and old people. Vagal stimulation (pain, fright, emotion, etc.) leads to hypotension and syncope. Usually, an autonomic prodrome (pale, clammy, light-headed) is followed by nausea or abdominal pain, then syncope. Benign, with no implications for driving. Diagnose with caution in older people with vascular disease where other causes are more common •Carotid sinus hypersensitivity syndrome •Pump problem. MI or ischaemia, arrhythmia (tachy- or bradycardia, e.g.ventricular tachycardia (VT), supraventricular tachycardia (SVT), fast atrial fibrillation (AF), complete heart block, etc.) •Outflow obstruction, e.g. aortic stenosis. PE is also a type of out flow obstruction. The main differential is seizure disorder where loss of consciousness is due to altered electrical activity in the brain . Stroke and TIA very rarely cause syncope, as they cause a focal, not global,deficit. Brainstem ischaemia is the rare exception.► A significant proportion of patients referred to specialist clinics for assessment of ‘syncope’ or ‘blackout’ are found not to have lost consciousness,but to have had a fall 2° to gait or balance abnormalities. |
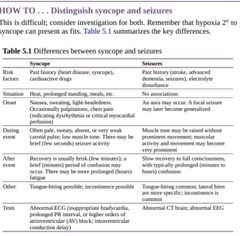
History The history often yields the diagnosis, but accuracy can be difficult to achieve the patient often remembers little. Witness accounts are valuable and should be sought. Ensure that the following points are covered: ︎Situation—was the patient ▪︎standing (orthostatic hypotension), ▪︎exercising(ischaemia or arrhythmia), ▪︎sitting, or lying down (likely seizure), ▪︎eating(postprandial hypotension), ▪︎on the toilet (defecation or micturition syncope), ▪︎coughing (cough syncope), or ▪︎in pain or frightened (vasovagal syncope)? Prodrome—was there any warning? ▪︎Palpitations suggest arrhythmia; ▪︎sweating with palpitations suggests vasovagal syndrome; ▪︎chest pain suggests ischaemia; ▪︎light-headedness suggests any cause of hypotension. ▪︎Gustatory or olfactory aura suggests seizures. However, associations are not absolute, e.g.arrhythmias often do not cause palpitations Was there loss of consciousness?—there is much terminology (fall, blackout,‘funny turn’, collapse, etc.), and different patients mean different things by each term. ☆Syncope has occurred if there is loss of consciousness with loss of awareness due to cerebral hypoperfusion; however, many (~30%) patients will have amnesia for the loss of consciousness and simply describe a fall Description of attack—ideally from an eye witness. ☆Was the patient deathly pale and clammy (likely systemic and cerebral hypoperfusion)? ☆Were there ictal features (tongue-biting, incontinence, twitching)? ☆Prolonged loss of consciousness makes syncope unlikely. A brain deprived of oxygen from any cause is susceptible to seizure; a fit does not necessarily indicate that a seizure disorder is the 1° problem. Assess carefully before initiating anticonvulsant therapy Recovery period—ideally reported by an eyewitness. □Rapid recovery often indicates a cardiac cause. □Prolonged drowsiness and confusion often follow aseizure Examination Full general examination is required. Ensure that the pulse is examined,murmurs sought, and a postural BP obtained.
Investigation Bloods—check for anaemia, sepsis, renal disease, myocardial ischaemia ECG—for all older patients with loss of consciousness or presyncope. Look specifically at PR interval, QT interval, trifascicular block (prolonged PR,right bundle branch block (RBBB), and left anterior fascicular block),ischaemic changes, and LVH Other tests depend on clinical suspicion, e.g. tilt test if symptoms sound orthostatic, but diagnosis is proving difficult (lying and standing BPs will usually suffice; tilt testing is a very labour-intensive test and should not be requested routinely); brain scan and electroencephalogram (EEG) if seizures suspected; prolonged ambulatory ECG monitoring or an implantable looprecorder if looking for arrhythmias
Treatment ◇Treat the cause ◇Often not found or multifactorial, so treat all reversible factors ◇Review medication (e.g. diuretics, vasodilators, cholinesterase inhibitors,tricyclic antidepressants) ◇Education about prevention and measures to abort an attack if there is a prodrome. ◇Advise against swimming or bathing alone, and inform abou tdriving restrictions. (Varies from no restriction to a 6-month ban, depending on the type of syncope; see details ► Transient loss of consciousness (TLOC) may be caused by syncope orseizure. |
|

Orthostatic (postural) hypotension •Orthostatic hypotension is common. •About 20% of community-dwelling and 50% of institutionalized older people are affected. •An important, treatable cause of dizziness, syncope, near-syncope, immobility,falls, and fracture. Less frequently leads to visual disruption, lethargy, neckache, or backache •Often most marked after meals, exercise, at night, and in a warm environment,and abruptly precipitated by ↑ intrathoracic pressure (cough, defecation, or micturition) •Often episodic (coincidence of precipitants) and covert (ask direct questions;walk or stand the patient and look for it). •May occur several minutes after standing
Diagnosis Thresholds are arbitrary. ▪︎A fall in BP of ≥20mmHg systolic or 10mmHg diastolic on standing from supine is said to be significant. ▪︎Severity of symptoms often does not correlate well with objective BP change. Causes ▪︎Drugs (including vasodilators, diuretics, negative inotropes or chronotropes(e.g. β-blockers, calcium channel blockers), antidepressants, antipsychotics,opiates, levodopa, alcohol) ▪︎Chronic hypertension (↓ baroreflex sensitivity and LV compliance) ▪︎Volume depletion (dehydration, acute haemorrhage) ▪︎Sepsis (vasodilation) ▪︎Autonomic failure (pure, diabetes, Parkinson’s disease, etc.) ▪︎Prolonged bed rest ▪︎Adrenal insufficiency ▪︎Raised intrathoracic pressure (bowel or bladder evacuation, cough)
Treatment ▪︎Treat the cause. Stop, reduce, or substitute drugs incrementally ▪︎Reduce consequences of falls (e.g. pendant alarms) ▪︎Modify behaviour—stand slowly and stepwise; lie down at prodrome ▪︎If still salt- or water-deplete, supplement with:Sodium (liberal salting at table or sodium chloride (NaCl) tablets) Water (oral or intravenous fluids) ▪︎Consider starting drugs if non-drug measures fail: ~Fludrocortisone (0.1–0.2mg/day) ~α-agonists, e.g. midodrine (2.5mg three times daily (tds), titrated to a maximum of 40mg/day); unlicensed in the UK; contraindicated in vascular disease ~Desmopressin 5–20 micrograms nocte, intranasal (used rarely in older patients as causes electrolyte imbalances) ▪︎In all cases, monitor electrolytes and for heart failure and supine hypertension. Caution if supine BP rises >180mmHg systolic. Dependent oedema alone is not a reason to stop treatment ▪︎The following may help: ~Full-length compression stockings ~Head-up tilt to bed (↓ nocturnal natriuresis) ~Caffeine (strong coffee with meals) or non-steroidal anti-inflammatorydrugs (NSAIDs) with extreme caution (→ fluid retention) ~Erythropoietin or octreotide |
Situational hypotension Postprandial hypotension Significant when associated with symptoms and fall in BP ≥20mmHg within 75min of meals. A modest fall is normal (and usually asymptomatic) in older people Often more severe and symptomatic in hypertensive people with orthostatic hypotension or autonomic failure Measure BP before meals and at 30min and 60min after meal. Symptoms and causes overlap with orthostatic hypotension
Treatment ▪︎Avoid hypotensive drugs and alcohol with meals ▪︎Lie down or sit after meals ▪︎Reduce osmotic load of meals (small frequent meals, low simple carbohydrates, high fibre/water content) ▪︎Caffeine, fludrocortisone, NSAIDs, and octreotide are used rarely Others Collapse at initiation or during defecation or micturition are commonly due to hypotension, and a similar management approach should be adopted Hypotension in response to cough can also occur—direct management towards reducing cough and optimizing BP. |

Carotid sinus syndrome ▪︎CSS is episodic, symptomatic bradycardia and/or hypotension due to ahypersensitive carotid baroreceptor reflex, resulting in syncope or near-syncope. ▪︎It is an important and potentially treatable cause of falls. ▪︎CSS is common in older patients and rarely occurs under 50 years. Series report a prevalence of 2% in healthy older people, and up to 35% of fallers >80 years. It is a condition that has been identified recently, and not all physicians are convinced that we fully understand the normal responses of older people to carotid sinus massage or the significance of the spectrum of abnormal results. ▪︎Normally, in response to ↑ arterial BP, baroreceptors in the carotid sinus act via the sympathetic nervous system to slow and weaken the pulse, lowering the BP. •This reflex typically blunts with age, but in CSS, it is exaggerated, probably centrally. •This hypersensitivity is associated with ↑ age, atheroma, and the use of drugs that affect the sinoatrial node (e.g. β-blockers, digoxin, and calcium channel blockers).
Typical triggers ▪︎Neck turning (looking up or around) ▪︎Tight collars ▪︎Straining (including cough, micturition, and defecation) ▪︎Prolonged standing Often, however, no trigger is identified. Subtypes ▪︎Cardioinhibitory (sinus pause of >3s) ▪︎Vasodepressor (BP fall of >50mmHg) ▪︎Mixed (both sinus pause and BP fall)
Diagnosis The diagnosis is made when all three of the following factors are present: 1》Unexplained attributable symptoms 2》A sinus pause of >3s and/or systolic BP fall of >50mmHg in response to 5s of carotid sinus massage. 3》Symptoms are reproduced by carotid sinus massage
CSS is often associated with other disorders (vasovagal syndrome and orthostatic hypotension), probably due to shared pathogenesis (autonomicdysfunction). This makes management more challenging.
Treatment Stop aggravating drugs where possible Pure cardioinhibitory carotid sinus hypersensitivity responds well to AV sequential pacing, resolving symptoms in up to 80% Vasodepressor-related symptoms are harder to treat (pathogenesis is less well understood) but may respond to ↑ circulating volume with fludrocortisone ormidodrine (not licensed), as for orthostatic hypotension. |
|
|
Falls services The assessment and 2° prevention of falls are multifactorial processes requiring a systematic approach. This is frequently appropriate in 1° and 2° care settings and can be delivered effectively by any trained member of the MDT. Morecomplex cases may benefit from assessment within a specialist setting such as a geriatric or falls clinic. Referral criteria Falls are so common that health services would be swamped if all who had fallen were referred. Instead, refer those with more sinister features suggesting a likelihood of recurrent falls, injury, or an underlying remediable cause. Referralcriteria might include: Recurrent (≥2) falls Loss of consciousness, syncope, or near-syncope Injury, especially fracture or facial injury (the latter suggesting poor saving mechanisms or loss of consciousness) Polypharmacy (≥4 drugs) Sources of patients include: ED (assess most people with non-operatively managed fractures) Acute orthopaedic units (hip and other operatively managed fractures) GP or community nurse Medical wards Self-presenting. Some services advertise directly, via posters and other media Service structure The team structure, diagnostic approach, and delivery of care vary enormously.Falls clinics are often led and delivered by non-physician health professionals such as experienced nurses, OTs, and PTs. A systematic review of possible contributing factors is essential and will usually include: Cardiovascular examination (including postural BP) Neurological examination Cognitive assessment Gait assessment Frailty assessment Environmental assessment Medication review Routine bloods (including FBC, U, C+E, haematinics, vitamin D) Screening for modifiable medical factors should be a routine part of allassessments, with referral to a medical specialist (e.g. GPSI or geriatrician) if such factors are identified. |

|

|
|
|
Drugs ▪︎Pharmacology in older patients ▪︎Prescribing ‘rules’ ▪︎Taking a drug history ▪︎HOW TO . . . Improve concordance/adherence ▪︎Drug sensitivity ▪︎Adverse drug reactions ▪︎ACE inhibitors HOW TO . . . Start ACE inhibitors ▪︎Analgesia HOW TO . . . Manage pain in older patients ▪︎Steroids ▪︎Warfarin HOW TO . . . Initiate warfarin ▪︎Direct oral anticoagulants ▪︎Proton pump inhibitors ▪︎HOW TO . . . Manage drug-induced skin rashes ▪︎Herbal medicines ▪︎Breaking the rules |
1 warfarin drug interactions Action increased by 2Skin manifestations of drug reactions |
|
|
|
Pharmacology in older patients Perhaps the most common intervention performed by physicians is to write aprescription. Older patients will have more conditions requiring medication;polypharmacy is common.
In the developed world:The over 65s typically make up around 14% of the population yet consume 40% of the drug budget 66% of the over 65s and 87% of the over 75s are on regular medication 34% of the over 75s are on three or more drugs Care home patients are on an average of eight medications Good prescribing habits are essential for any medical practitioner, but especially for the geriatrician.
Administration challenges include ▪︎Packaging may make tablets hard to access—childproof bottles and tablets in blister packets can be impossible to open with arthritic hands or poor vision ▪︎Labels may be too small to read with failing vision ▪︎Tablets may be large and difficult to swallow (e.g. co-amoxiclav) or have an unpleasant taste (e.g. potassium supplements) ▪︎Liquid formulations can be useful, but accurate dosage becomes harder(especially where manual dexterity is compromised) ▪︎Any tablet needs around 60mL of water to wash it down and prevent adherence to the oesophageal mucosa large volume for a frail older person. ▪︎Some tablets (e.g. bisphosphonates) require even larger volumes ▪︎Multiple tablets, with different instructions (e.g. before/after food) are easily muddled up or taken in a suboptimal way ▪︎Some routes (e.g. topical to back) may be impossible without assistance ▪︎Absorption Many factors are different in older patients (↑ gastric pH, delayed gastric emptying, reduced intestinal motility and blood flow, etc.) Despite this, absorption of drugs is largely unchanged with age—exceptions include iron and calcium, which are absorbed more slowly ▪︎Distribution Some older people have a very low lean body mass, so if the therapeutic index for a drug is narrow (e.g. digoxin), the dose should be adjusted ▪︎There is often an ↑ proportion of fat, compared with water. ~This reduces the volume of distribution for water-soluble drugs, giving a higher initial concentration (e.g. digoxin). ~It also leads to accumulation of fat-soluble drugs,prolonging elimination and effect (e.g. diazepam) ~There is reduced plasma protein binding of drugs with age, which ↑ the free fraction of protein-bound drugs such as warfarin and furosemide.
~Hepatic metabolism Specific hepatic metabolic pathways (e.g. conjugation) are unaffected by age ~Reducing hepatic mass and blood flow can impact on overall function which slows metabolism of drugs (e.g. theophylline, paracetamol, diazepam,nifedipine) ~Drugs that undergo extensive first-pass metabolism (e.g. propranolol, nitrates)are the most affected by the reduced hepatic function ~Many factors interact with liver metabolism (e.g. nutritional state, acute illness, smoking, other medications, etc.)
~Renal excretion ~Renal function declines with age,which has a profound impact on the handling of drugs that are predominantly handled renally. Drugs, or drugs with active metabolites, that are mainly excreted in the urine include digoxin, gentamicin, lithium, furosemide, and tetracyclines. ~Where there is a narrow therapeutic index (e.g. digoxin, aminoglycosides),then dose adjustment for renal impairment is required ~Impaired renal function is exacerbated by dehydration and urinary sepsis both common in older patients. |
Prescribing ‘rules ■. Is it indicated? 2.Treatment of new symptom Some symptoms trigger a reflex prescription (e.g. constipation—laxatives;dizziness—prochlorperazine). Before starting a medication, consider: What is the diagnosis? (e.g. dizziness due to postural drop) Can something be stopped? (e.g. opioid analgesia causing constipation) Are there any non-drug measures? (e.g. ↑ fibre for constipation) Optimizing disease management For example: a diagnosis of cardiac failure should trigger consideration of loop diuretics, spironolactone, ACE inhibitors, and β-blockers. Ensure the diagnosis is secure before committing the patient to multiple drugs 3.Do not deny older patients disease-modifying treatments simply to avoid polypharmacy 4.Do not deny treatment because of potential side effects—while these may impact on functional ability or cause significant morbidity (e.g. low BP with β-blockade in cardiac failure) and need to be discontinued, this should usually be after a trial of treatment with careful monitoring. 5.Conversely, do not start treatment to improve mortality from a disease if the patient has limited life span for other reasons. 6.Preventative medication For example: BP and cholesterol lowering. Limited evidence base in older patients—be guided by biological fitness 7.Ensure the patient understands the rationale for treatment
■Are there any contraindications?▪︎Review past medical history (drug–disease interactions common) ▪︎Contraindications often relative, so a trial of treatment may be indicated, but warn the patient, document risk, and review impact (e.g. ACE inhibitors when there is renal impairment)
■Are there any likely interactions?Review the medication list Computer prescribing assists with drug–drug interactions, automatically flagging up potential problems ■ What is the best drug? •Choose the broad category of drug (e.g. which antihypertensive) by considering which will work best in this patient according to local and national guidelines,which is least likely to cause side effects (e.g. calcium channel blockers may worsen cardiac failure), and if there is any potential for dual action (e.g. a patient with angina could have a β-blocker for both angina and BP control). •Within each category of medication, there are many choices:Develop a personal portfolio of drugs with which you are very familiar •Guidelines and formularies will often dictate choices within hospital •Cost should be a consideration •Pharmaceutical companies will try to convince you of the benefits of a new brand. Unless this is a novel class of drug, it is likely that existing brands have a greater proven safety record with similar benefit. • Older patients have greater potential to suffer harm from new drugs and are unlikely to have been included in clinical trials. Time will tell if there are real advantages—in general, stick to what you know ► Never be the first (or last) of your peers to use a new drug. ■ What dose should be started? •‘Start low and go slow’In most cases, benefit is seen with drug initiation, further increments of benefit occurring with dose optimization (e.g. ACE inhibitors for cardiac failure where 1.25mg ramipril is better than 10mg with a postural drop) •However, do not undertreat—use enough to achieve the therapeutic goal (e.g.for angina prophylaxis, a β-blocker dose should be adequate to induce a mild bradycardia) ■. How will the impact be assessed?•Schedule follow-up, looking for:Efficacy of the drug (e.g. has bradykinesia improved with a dopamine agonist?). •Medication for less objective conditions (e.g. pain, cognition)requires careful questioning of the patient and family/carers •Any adverse events—reported by the patient spontaneously, elicited by direct questioning (e.g. headache with dipyridamole) or by checking blood tests where necessary (e.g. thyroid function on amiodarone) •Any capacity to ↑ the dose to improve the effect (e.g. ACE inhibitors in cardiac failure) ■ What is the time frame? •Many older patients remain on medication for a long time; 88% of all prescriptions in the over 65s are repeats; 60% of prescriptions are active for over 2 years, 30% over 5 years, and 6% over 10 years This may be appropriate (e.g. with antihypertensives) and if so, the patients hould be aware of this and seek an ongoing supply from the GP •Some drugs should never be prescribed long-term (e.g. prochlorperazine,night sedation) •Medication should be regularly reviewed and discontinued if ineffective or no longer indicated, e.g. some psychotropic medications (e.g. lithium, depot antipsychotics) were intended for long-term use at initiation, but the patient may have had no psychiatric symptoms for years (or even decades). They can contribute to falls, and cautious withdrawal may be indicated. An evidence-based comprehensive approach to medication review (e.g.STOPP START v2 criteria) can be useful. |
Taking a drug history 1]An accurate drug history includes the name, dose, timing, route, duration, and indication for all medication. Studies have suggested that patients will report their drug history accurately around half of the time, and this figure falls with ↑age. Reasons for problems arising ▪︎Inadequate information to the patient at the time of prescribing ▪︎Multiple medications ▪︎Multiple changes if side effects develop ▪︎Use of both generic and brand names ▪︎Variable doses over time (e.g. dopa agonists, ACE inhibitors) ▪︎Cognitive and visual impairment ▪︎Over-the-counter drugs ▪︎Useful sources of information The patient’s actual drugs—they will often bring them along in a bag to outpatients or when admitted.Many seasoned patients will carry a list of their current medication—written either by them or a healthcare professional.Computer-generated printouts of current medications from the GP.Increasingly available via shared electronic networksDosette® and Nomad® systems will incorporate information about the medication they containm A telephone call to the GP surgery will yield a list of active prescriptions (but not over-the-counter medication) Family members will often know about medication, especially if they help administer them Medical notes will often contain a list of medication at the last hospital attendance These can be extremely useful but have limitations. A prescription issued does not mean that it was necessarily dispensed or that the medication is being taken correctly and consistently. Previously prescribed medications may still be taken and patients may occasionally use another patient’s medication (e.g. a spouse). Good habits Every time a patient is seen (in clinic, day hospital, admission, etc.), take time. •Old drugs to be discarded (if necessary, retain them and return to pharmacy) •Concordance to be estimated (by looking at the date of dispensing and the number of tablets left) •Clarification of doses, timings, and rationale for treatment. •In a less-pressured setting (e.g. DH), it is useful to generate a list for the patient to carry with them. •Education of the patient and family where needed (e.g. reason for taking) |
|
|
Drug sensitivity •Altered sensitivity Many older patients will have altered sensitivity to some drugs, e.g.:Receptor responses may vary with age. ~Alterations in the function of the cellular sodium/potassium pumps may account for the ↑ sensitivity to digoxin seen in older people. ~↓ β-adrenoceptor sensitivity means that older patients mount less of a tachycardia when given agonists (e.g. salbutamol) and may become less bradycardic with β-blockers. ~Altered coagulation factor synthesis with age leads to an ↑ sensitivity to the effects of warfarin. ~The ageing CNS shows ↑ susceptibility to the effects of many centrally acting drugs (e.g. hypnotics, sedatives, antidepressants, opioid analgesia,antiparkinsonian drugs, and antipsychotics) •Adverse reactions Certain adverse reactions are more likely in older people, because of this altered sensitivity: Baroreceptor responses are less sensitive, making symptomatic hypotension more likely with antihypertensives. Thirst responses are blunted, making hypovolaemia due to diuretics more common. Thermoregulation is blunted, making hypothermia more likely with prolonged sedation. |
Drugs that may require dose adjustment in older patients ▪︎Despite the variations in drug handling, most drugs have a wide therapeutic index, and there is no clinical impact. Only drugs with a narrow therapeutic index or where older patients may show very marked ↑ sensitivity may require dose alteration: ACE inhibitors Aminoglycosides (dose determined by weight, and reduced if impaired renal function) Diazepam (start with 2mg dose) Digoxin (low-body-weight older patients rarely require >62.5 micrograms maintenance dose) Opiates (start with 1.25–2.5mg morphine to assess impact on CNS) Oral hypoglycaemics (↑ sensitivity to hypoglycaemia with ↓ awareness—avoid long-acting preparations such as glibenclamide, and start with lower doses of shorter-acting drugs, e.g. gliclazide 40mg) Warfarin (load more cautiously) |
Adverse drug reactions More common and complex with ↑ age—up to three times more frequent in theover 80s. Drug reactions account for considerable morbidity, mortality, and hospital admissions (1 in 25 hospital admissions may result from avoidable drug adverse reactions). Older people are not a homogeneous group, and many will tolerate medications as well as younger ones, but a number of factors contribute to the ↑frequency: •Altered drug handling and sensitivity occur with age, made worse by poor appetite, nutrition, and fluid intake. •Frailty and multiple diseases make drug–disease interactions more common,e.g.: ~Anticholinergics may precipitate urine retention in a patient with prostatic hypertrophy ~Benzodiazepines may precipitate delirium in a patient with dementia. These relationships become even more complex when the large numbers of drugs that are prescribed for multiple conditions interact with the diseases, as well as with each other, e.g. an osteoporotic patient is prescribed a bisphosphonate, then sustains a vertebral crush fracture and is given a non-steroidal which exacerbates gastric irritation and causes a gastrointestinal bleed. •Errors in drug taking make adverse reactions more likely. Mistakes ↑ with: □↑ age↑ numbers of prescribed drugs (20% of patients taking three drugs will make errors, rising to 95% when ten or more drugs are taken) □Cognitive impairment □Living alone Strategies to minimize adverse drug reactions □Consider possible drug–drug and drug–disease interactions when ever a new drug is started. Some drugs are associated with high rates of drug–drug interactions, e.g.warfarin, amiodarone, SSRIs, antifungals, digoxin, phenytoin, and erythromycin. □For every new problem, consider if an existing medication could be the cause. ~Try to avoid the so-called prescribing cascade where side effects are treated with a new prescription, rather than discontinuing the offending drug. □If multiple medications are possible culprits, then stop one at a time and watch for improvement. □Optimize concordance/adherence □Use extreme caution at times of care transfer medicines reconciliation is important. |
|

ACE inhibitors Common indications include ▪︎BP control, ▪︎vascular risk reduction, ▪︎heart failure,and ▪︎diabetic nephropathy. Cautions Renal disease ▪︎Use ACE inhibitors with extreme caution if there is a known history of renal artery stenosis, as renal failure can be precipitated. If the clinical suspicion of this is high (renal bruit, uncontrolled hypertension that is unexplained), then consider investigating for renal asymmetry with an ultrasound before starting treatment. Renal impairment per se is not a reason to withhold ACE inhibitors (indeed they are effective treatment for some types), although the dose may need to be reduced. Monitor renal function before and after treatment. Sudden deterioration may indicate renal artery stenosis, and the ACE inhibitor should be stopped pending investigation. If a patient becomes unwell (dehydrated, septic, etc.), they may need temporary withdrawal of the ACE inhibitor—provide your patient with ‘sickday rules’ Hypotension. ▪︎Older patients are more prone to postural hypotension. Check BP lying and standing, and ask about postural symptoms (e.g. light-headedness). The risk of hypotension is greater with volume depleted patients, e.g. those on high-dose diuretics, on renal dialysis, dehydrated from intercurrent illness, or in severe cardiac failure. □Correct dehydration before initiation, where possible. ACE inhibitor-induced hypotension is common in patients with severe aortic stenosis and, although beneficial in this condition, should be started slowly‘Start low and go slow.’ Monitor carefully •Cough Many ACE inhibitors cause a persistent dry cough. Always warn the patient about this, as it can cause considerable distress. Fore warned is forearmed, and many patients will be prepared to accept this side effect if the ACE inhibitor is the best choice for them. Changing to an angiotensin receptor blocker (ARB) removes the cough in most cases. •Hyperkalaemia There is a risk of hyperkalaemia when ACE inhibitors are used with potassium-sparing diuretics, e.g. spironolactone.Be aware, and monitor electrolytes. Most tolerate a potassium level of up to5.5mmol/L. The tendency to hyperkalaemia can be useful in patients who are also on potassium-losing diuretics (e.g. furosemide), as the two may balance each other out overall hypokalaemia is more common in patients with heart failure. |

Analgesia Older patients are more likely to suffer chronic pain than younger ones, owing tothe ↑ frequency of conditions such as osteoarthritis, osteoporosis, etc.Pain management is more challenging, and a standard ‘pain ladder’ approachis not always useful because of the altered sensitivity of the older patient tocertain classes of analgesic medication.Non-steroidal anti-inflammatory drugsIncludes aspirin (especially at analgesic doses).Potential problemsFluid retention causing worsening hypertension, cardiac failure, and ankleswellingRenal toxicity—risk of acute tubular necrosis (ATN), exacerbated byintercurrent infection or dehydrationPeptic ulceration and gastrointestinal bleeding—there is an ↑ risk with ↑ age,and the bleeds tend to be more significant► The number of older patients requiring hospitalization because of NSAID-induced deterioration in renal or cardiac function actually exceeds the numberwith gastrointestinal bleeds.Age itself is probably not an independent risk factor for most complications ofNSAID treatment, but factors such as comorbidities, co-medications,hydration, nutritional status, and frailty are linked to an ↑ risk, all of which aremore common with advancing ageGuidance for use in older patientsNSAIDs should be used with extreme caution in older patients and avoidedaltogether in the very frailShould be given for a short period onlyUse low-dose moderate potency NSAIDs (e.g. ibuprofen 0.6g/day)Avoid using two NSAIDs together (this includes low-dose aspirin)Consider co-prescription of a gastric-protective agent (e.g. omeprazole) forthe duration of the therapyAvoid using ACE inhibitors and NSAIDs together—they have opposingeffects on fluid handling and are likely to cause renal toxicity in combinationOpioid analgesia•••••Wide range of drugs sharing many common features, but with qualitative andquantitative differencesPotential problems include constipation, nausea and vomiting, anorexia,confusion, drowsiness, and respiratory depressionGuidance for use in older patientsMost of these are dose-dependent, and careful up-titration will obtain the rightbalance of analgesic effect and adverse effectsConstipation is common (worse in older people) but can be managed withgood bowel careMost adverse effects are reversible once the medication is reduced ordiscontinued |
•••••••••SteroidsOral steroids (usually prednisolone) are given for many conditions in olderpatients, commonly COPD exacerbation, PMR, rheumatoid arthritis, and colitis.Treatment may be long-term. Although the benefits of treatment usuallyoutweigh the risks, awareness of these can minimize harm. Discuss likely sideeffects with patients and carers.CautionsOsteoporosis—this is most marked in the early stages of treatment. Olderpeople will have diminishing bone reserves anyhow, and all steroid-treatedolder patients should be offered bone protection at the outset, unless thecourse is certain to be very short (e.g. <2 weeks). This should consist of dailycalcium and vitamin D, along with a bisphosphonate (weekly preparations,e.g. alendronate 70mg, improve concordance)Steroids can precipitate impaired glucose tolerance or frank diabetes. Monitorsugar levels periodically (e.g. weekly capillary blood sugar or urinalysis) in allsteroid users. Steroids worsen control in known diabetics, necessitating morefrequent monitoringHypertension may develop because of the mineralocorticoid effect ofprednisolone, and this should be checked for regularlySkin changes occur and are particularly noticeable in older patients with lessresilient skin. Purpura, bruising, thinning, and ↑ fragility are commonMuscle weakness occurs with prolonged use, predominantly proximal indistribution. This leads to problems rising from chairs, climbing stairs, etc.and may be the final straw for a frail older person with limited physicalreserve (see ‘Muscle symptoms’, pp. 480–481)There is an ↑ susceptibility to infections on steroids, and the presentation maybe less acute, making diagnosis more difficult. Candidiasis (oral and genital)is particularly common and should be treated promptlyHigh doses (as used in the treatment of giant cell arteritis (GCA)) can causeacute confusion and sleep disturbance, and older people are particularlyprone. Give steroids in the morning, if possibleCataracts may develop with long-term steroid use. If vision declines, look forcataracts with an ophthalmoscope and consider specialist referralPeritonitis may be masked by steroid use—the signs being less evidentclinically. Have a higher index of suspicion of occult perforation in a steroid-treated older patient with abdominal pain. There is also an association•••••between steroid use and peptic ulceration, particularly in hospitalized patientsAdrenocortical suppression means that the stress response will be diminishedin chronic steroid users. If such a patient becomes acutely unwell (e.g. septic),the exogenous steroid dose will need to be temporarily ↑ (e.g. double the usualoral dose, replace with intramuscular (im) hydrocortisone if unable to take bymouth). Counsel the patient with ‘sick day rules’. Suppression can continuefor months after stopping chronic steroids; have a low threshold for ‘covering’acute illnessStopping treatmentMany patients are on fairly low doses of steroids for a long period. It can bedifficult to completely tail the dose, as steroid withdrawal effects (fevers,myalgia, etc.) can often be mistaken for disease recurrence, and this often needsto be done very slowly (perhaps reducing by as little as 1mg a month). There isno such thing as a ‘safe’ dose of steroid so, for every patient you see on steroids,ask the following:Can the dose be reduced?Could a steroid-sparing agent (e.g. azathioprine) be used instead?Is the patient taking adequate bone protection?What is the BP and blood glucose? |
|
Warfarin Common indications range from ■absolute (PE, DVT, artificial heart valve replacement) to ■relative (stroke prophylaxis in AF). •Increasingly direct oral anticoagulants (DOACs) are being used instead, but warfarin remains in common use.
Cautions □Risk is ↑ if the patient is unable to take medications reliably, so it is not suitable without supervision for cognitively impaired patients or those who self-neglect. If there is an absolute indication, then consider supervised therapy (by spouse, family, or carers via a dispensing system) or (rarely) a course of low-molecular-weight heparin instead. □Risk is higher if there is a high probability of trauma, e.g. recurrent falls □Excess alcohol consumption is associated with poor concordance and falls. Liver enzymes are induced, making control of anticoagulation more difficult. Highly variable intake is especially problematic. □Comorbidity may ↑ sensitivity to warfarin (e.g. abnormal liver function,congestive cardiac failure) and should be screened for GPs will often be good judges of risk—consider discussing borderline cases. Side effects •Bleeding is the major adverse event, ranging from an ↑ tendency to bruise to major life-threatening bleeds. •The most significant include intracerebral haemorrhage and gastrointestinal blood loss. Warfarin does not cause gastric irritation but may accelerate blood loss from pre-existing bleeding sources. •Ask carefully about history of non-steroidal use (including aspirin) and gastrointestinal symptoms (upper and lower). If any are present, then quantify the risk with further testing—FBC and iron studies might indicate occult blood loss. If warfarin is not essential, then a full gastrointestinal work-up may be appropriate before starting in fitter patients. •Nose bleeds are common in older patients and may become more significant on warfarin. Often due to friable nasal vessels that are amenable to treatment by ENT surgeons, so reducing the risk of epistaxis on warfarin.
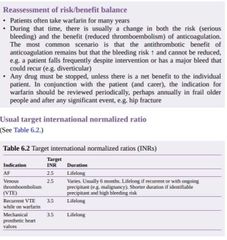
What to do when the INR is too high Always look for the reason why the INR became elevated, and correct this factor. If there is no sign of bleeding, then stop warfarin and monitor the INR as it falls. If the INR >8 but there is no bleeding, a small dose of vitamin K (0.5–2.5mg)can be given to partially reverse the INR. ■Do not give vitamin K routinely, as control of anticoagulation will be made more difficult for weeks afterwards. ■If there is bleeding, then warfarin needs reversing with vitamin K and fresh frozen plasma. ■For life-threatening bleeds (e.g. intracerebral haemorrhage), prothrombin complex concentrate can be used for rapid reversal. ► If a patient bleeds at target INR, always consider the possibility of an underlying serious disease, e.g. bladder or gastrointestinal malignancy. |

|
Direct oral anticoagulants This class of drugs (DOACs) is also known as novel/new oral anticoagulants(NOACs) and is increasingly being used in place of warfarin as evidence of non-inferiority emerges.Compared to warfarin, the main advantages are •Immediate onset of action •No need for INR monitoring •Reduced rates of bleeding (especially intracerebral) •No food or alcohol interactions The main disadvantages include •Harder to reverse—although short duration of action means that the drug clears from the system quickly, and reversal agents are in development •Dose needs adjustment for renal impairment •Some agents require twice-daily dosing •Short duration of action means that any missed doses render the patient unanticoagulated •Less familiar medications, and prescribers should note numerous drug interactions with commonly used drugs (e.g. tramadol, clarithromycin) •Limited long-term outcome data, particularly in the older patient •Not licensed for metallic heart valve thromboprophylaxis Higher cost ▪︎When to use?Patients who have been stable on warfarin should not be changed. Patients in whom it is unacceptable/very difficult to monitor INR, or who have an unstable INR, would be good candidates for a DOAC. When initiating anticoagulation, discuss options with patients and be guidedby local policy ► If a patient is not a candidate for warfarin, it is unlikely they will be ‘safe’ on a DOAC, and careful risk/benefit analysis is always needed (e.g. a frequent faller will be no safer on a DOAC than on warfarin). |
|
|
Proton pump inhibitors •PPIs (e.g. omeprazole, lansoprazole) are very effective in reducing gastric acid secretion and therefore in treating peptic ulcers and gastro-oesophageal reflux disease (GORD). They are perceived as very safe drugs but are not without problems. The combination of effectiveness and safety has led to them being one of the most commonly prescribed drug classes. •However, PPIs are often prescribed without an appropriate indication, or are initiated appropriately but not discontinued after a treatment course. Overall, over 50% of PPI use is unnecessary.
Side effects Common side effects are Headache Nausea Diarrhoea Constipation Infrequent idiosyncratic reactions include acute interstitial nephritis, erythema multiforme, pancreatitis, and microscopic colitis Hyponatraemia and hypomagnesaemia can occur—consider checking in non-specific presentations. ▪︎Hydrochloric acid aids protein digestion and absorption of vitamin B12 and calcium and is active against pathogens. •PPI use is associated with: □Community and hospital incidence of CDAD and ↑ likelihood of recurrence □Enteric bacterial infections (e.g. Salmonella, Campylobacter) □Community- and hospital-acquired pneumonia □Long-term use is associated with higher rates of hip fracture, possibly caused by altered calciumabsorption ■Interactions There are a few important interactions: •The effects of phenytoin and warfarin are enhanced •The effects of clopidogrel are reduced •Plasma concentrations of digoxin are ↑ slightly
Appropriate prescribing •Always specify the indication for treatment and its intended duration In GORD, always first address non-drug factors (obesity, alcohol), and consider the use of less potent acid suppression (e.g. H2 antagonists such as ranitidine) •Transient dyspepsia or occasional heartburn are inadequate indications for long-term PPI treatment. In 1° care, review the need for the drug periodically. In 2° care, be careful not to extend an initial appropriate PPI prescription for prophylaxis against gastrointestinal bleeding. When antibiotics are prescribed in hospital, suspend the PPI prescription to reduce the risk of CDAD. In (confirmed or possible) CDAD, stop PPIs. |
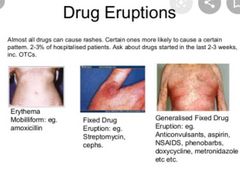
HOW TO . . . Manage drug-induced skin rashes Common side effect in older patients—thought to be due to altered immune function. Rarely life-threatening but cause considerable distress. Make the diagnosis Variable in appearance, but most commonly toxic erythema—symmetrical, erythematous, itchy rash, trunk > extremities, lesions may be measles-like or urticarial, or resemble erythema multiforme ▪︎Certain drugs may produce predictable eruptions: □Acneiform rash with lithium □Bullous lesions with furosemide □Target lesions with penicillins and phenytoin □Psoriasis-like rash with β-blockers □Urticaria with penicillin, opiates, and aspirin □Fixed drug eruption (round, purple plaques recurring in the same spot)with paracetamol, laxatives, sulfonamides, and tetracyclines □Toxic epidermal necrolysis is a rare, serious reaction to drugs such as non-steroidals, allopurinol, and phenytoin. The skin appears scalded, and large areas of the epidermis may shear off, causing problems with fluid and electrolyte balance, thermoregulation, and infection
▪︎Take a careful drug history to elicit a temporal relationship to medication administration, e.g. within 3 days of starting a new drug (may be as long as 3 weeks) or becoming worse every morning after a regular drug is given. ▪︎Stop the drug ▪︎Stop multiple medications one at a time (stop drugs started closest to the onset of the rash first), and watch for clinical improvement. ▪︎May get slightly worse before improving ▪︎Usually clears within 2 weeks ▪︎Advise the patient to avoid the drug in the future. ▪︎Soothe the skin Emollients, cooling agents (e.g. calamine), and weak topical steroids may help. ▪︎Oral anti histamines are often given, with variable success. ▪︎Sedating antihistamines (e.g. hydroxyzine) may help sleep. ▪︎Treat the complications •More likely if extensive and prolonged Risks include: Hypothermia Hypovolaemia 2° infection ► Consider dermatology referral if not improving after 2 weeks off the suspected drug. |
Breaking the rules A great deal of prescribing in geriatric practice relies on individually tailored assessment and pragmatic decision-making, as inter-individual variation ↑ with age. In addition, trials often exclude older subjects, making the evidence base an extrapolation. While what is described in the preceding pages is appropriate for many, there are times when ‘rules must be broken’ in the best interests of the individual patient. •This requires careful consideration of risks and benefits; the patient should usually be reviewed to assess the impact of the decision. ▪︎Polypharmacy can cause problems but is sometimes appropriate—depriving patients of beneficial treatments because they are old, or already on multiple other medications, can also be wrong. In a recent study of medication changes during a geriatric admission, the total number of drugs was the same at admission and discharge, but they had often been changed. • In other words, there was active evaluation of medications going on the goal being not just to limit the number of drugs, but also to optimize and individually tailor treatment. •Where side effects are very likely, but the drug is definitely indicated, then it may be appropriate to co-prescribe something to treat the expected adverse effect, e.g.: Steroids and bisphosphonates Opiates and laxatives Furosemide and a potassium-sparing diuretic (or an ACE inhibitor) Non-steroidals and a gastric protection agent While certain disease–drug interactions are very likely and should be avoided,others may be an acceptable risk. For example: ▪︎β-blockers:Are to be used with caution with asthma, yet they have such a good impacton cardiovascular risk reduction that these cautions are not absolute. Often‘asthma’ is, in fact, COPD with little β-receptor reactivity, so cautious β-blockade initiated in hospital while monitoring the lung function may be appropriate. ▪︎In patients with peripheral vascular disease, they can cause a small reduction in walking distance, but this risk is usually outweighed by the reduction in the risk of cardiac death. ▪︎Fludrocortisone (for postural hypotension) will worsen supine hypertension and cause ankle swelling. But if postural symptoms are severe, then it may be appropriate to accept hypertension and the associated risk •Amlodipine may worsen ankle swelling in a patient with chronic venous insufficiency, but if this is the best way of controlling hypertension, it may be appropriate to accept a cosmetic problem. |
|
|
Neurology ▪︎The ageing brain and nervous system ▪︎Tremor ▪︎Neuropathic pain/neuralgia ▪︎HOW TO . . . Treat neuralgia ▪︎Parkinson’s disease: presentation ▪︎Parkinson’s disease: management ▪︎HOW TO . . . Manage a patient with Parkinson’s disease who cannot take oral medication ▪︎HOW TO . . . Treat challenging symptoms in Parkinson’s disease ▪︎Diseases masquerading as Parkinson’s disease ▪︎Epilepsy ▪︎Epilepsy: drug treatment ▪︎Neuroleptic malignant syndrome ▪︎Motor neuron disease ▪︎Peripheral neuropathies ▪︎Subdural haematoma ▪︎Sleep and insomnia ▪︎HOW TO . . . Use benzodiazepines for insomnia ▪︎Other sleep disorders |
1Essential tremor Clinical features Management 2 PARKINSON'S poor prognostic features |
|
|
The ageing brain and nervous system As in other systems, intrinsic ageing (occurs in all) is often hard to distinguish from extrinsic ageing mechanisms (caused by disease processes). Histological changes in the brain include: Each neuron has fewer connecting arms (dendrites) Around 20% of brain volume and weight are lost by the age of 85 There is deposition of pigment (lipofuscin) in the cells and oxidative damage in mitochondria. The presence of senile plaques and neurofibrillary tangles ↑ with age, but they are not diagnostic of dementia. |
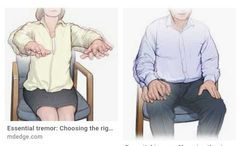
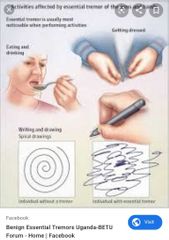
Tremor Tremor is more common with ↑ age. It can be disabling and/or socially embarrassing. It is important to try to make a diagnosis, as treatment is available in some cases. Examine the patient first at rest and distracted (relaxed with arms supported on the lap, count backwards from 10), then with outstretched hands, and finally during movement (pointing or picking up a small object). Tremors fall roughly into three categories: Rest tremor—disappears on movement and is exaggerated by movement of the contralateral side of the body. Most common cause—Parkinson’s disease. It is usually associated with ↑ tone Postural tremor—present in outstretched limbs, may continue during action but disappears at rest. Most common cause—benign essential tremor Action tremor—exaggerated with movement. When the tremor is maximal at extreme point of movement, it is called an intention tremor. Most common cause—cerebellar dysfunction Benign essential tremor The classic postural tremor of old age, worse on action (e.g. static at rest but spills tea from tea cup), may have head nodding (titubation) or jaw/vocal tremor, legs rarely affected. May be asymmetrical About half of the cases have a family history (autosomal dominant) Presents in middle age, occasionally earlier, and worsens gradually. Often more socially embarrassing than physically impairing. Improved by alcohol, gabapentin, primidone, and β-blockers, but these are often unacceptable treatments in the long term. Worth considering β-blockers as the first choice in treatment with coexistent hypertension. Weighted wristbands can reduce tremor and improve function. Cerebellar dysfunction The typical intention tremor is associated with ataxia. Acute onset is usually vascular in older patients. Subacute presentations occur with tumours (including paraneoplastic syndrome), abscesses, hydrocephalus, drugs (e.g. anticonvulsants) hypothyroidism, or toxins Chronic progressive course is seen with: ▪︎Alcoholism (due to thiamine deficiency always give thiamine 100mg once daily (od) orally (po) or iv preparation if in doubt; it might bereversible) ▪︎Anticonvulsant (e.g. phenytoin—may be irreversible if severe, more common with high plasma levels but can occur with long-term use at therapeutic levels) Paraneoplastic syndromes (anti-cerebellar antibodies can be found, e.g.anti-Yo and anti-Hu found in cancer of the ovary and bronchus) Multiple sclerosis Idiopathic cerebellar atrophy Many cases defy specific diagnosis. Consider multisystem atrophy |
|
|
|
Neuropathic pain/neuralgia This describes pain originating from nerve damage/inflammation. It is often very severe and debilitating and seems to be more common in older people. The pain is usually sharp/stabbing and is often intermittent, being precipitated by things like movement and cold.
1.Post-herpetic neuralgia Severe burning and stabbing pain in a division of nerve previously affected by shingles. Shingles and subsequent persisting neuralgia is much more common in older patients. Pain may be triggered by touch or temperature change. May go on for years, be difficult to treat, and have major impact on quality of life. Prevent by vaccination to reduce risk/severity of shingles. If shingles develops, starting antivirals within 72h reduces neuralgia incidence.
2.Trigeminal neuralgia Severe unilateral stabbing facial pain, usually V2 and V3, rather than V1. Triggers include movement, temperature change, etc. Time course—years with relapse/remission. Depression and weight loss can result. Differential diagnoses include temporal arteritis (TA), toothache, parotitis, and temporomandibular joint arthritis ▪︎Consider neuroimaging, especially if bilateral or if there are physical signs, i.e. sensory loss or other cranial nerve abnormality suggestive of 2° trigeminal neuralgia.
Neuralgia can also occur with 3.Malignancy 4.Cord compression 5.Neuropathy |
HOW TO Treat neuralgia ○This can be very debilitating, and treatment is difficult. There is often coexistent depression, so always think of this and treat appropriately. Simple measures include □Distraction □Relaxation techniques □Allaying fears (usually about a serious underlying pathology) □Acupuncture □Heat/cold treatment □Osteopathy/massage (to reduce associated muscle spasm) □Use of TENS machines Support groups, □Medications •Topical treatments, e.g. lidocaine, capsaicin •Traditional analgesics (paracetamol, NSAIDs, opiates), although these are usually not very effective. •Anti-spasticity drugs, e.g. baclofen. Used especially in trigeminal neuralgia,they treat any muscle spasm that exacerbates the pain. ■Mainstay of treatment is the neuromodulating drugs which may give superior pain control but often have important side effects. Examples include: □Antidepressants with neuroadrenergic modulating abilities, e.g.amitriptyline, duloxetine. Start with a low dose and titrate up slowly. Eventual doses may be similar to those used in younger patients. □Anticonvulsants, e.g. •gabapentin, pregabalin (post-herpetic neuralgia) or •carbamazepine, oxcarbazepine, valproate (trigeminal neuralgia). Start witha low dose and titrate up slowly. The main side effects from these drugs are sedation and confusion, and reaching a therapeutic dose may be limited by this problem. Other options □Nerve blocks or spinal stimulation, which can usually be accessed via aspecialist pain clinic □ Surgery, e.g. nerve decompression, or treatment with heat or lasers. May provide relief but can result in scarring and numbness. |
Parkinson’s disease: •A common idiopathic disease (prevalence 150/100 000) associated with inadequate dopamine neurotransmitter in the brainstem. •There is loss of neuronsand Lewy body formation in the substantia nigra. •The clinical syndrome is distinct from Lewy body dementia but they are extremes of a clinical spectrum of disease. Presentation The clinical diagnosis of Parkinson’s disease is based on the UK Parkinson’sdisease brain bank criteria and should include: 1.Bradykinesia ~slow to initiate and carry out movements, ~expressionless face, ~fatigability of repetitive movement 2.Plus at least one of the following: ▪︎Rigidity (cogwheeling = tremor super imposed on rigidity) ▪︎Tremor (‘pin-rolling’ of hands—worse at rest) ▪︎Postural instability Other clinical features: ▪︎Gait disorder (small steps) ▪︎Usually an asymmetrical disease ▪︎No pyramidal or cerebellar signs, but reflexes are sometimes brisk. ▪︎Non-motor symptoms are common and should be asked about. |
|
|
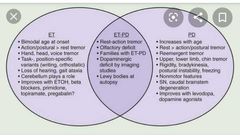
Non-motor symptoms of Parkinson’s disease ▪︎Depression (treat appropriately) ▪︎Anosmia (may be an early pre-motor feature) ▪︎Psychosis (may relate to medications; avoid typical antipsychotics, as they may worsen the motor features; atypicals such as quetiapine can be tried) ▪︎Dementia and hallucinations can occur in late stages, but drug side effects can cause similar problems. If features suggest Lewy body dementia, a trial of anticholinesterases may be warranted. ▪︎Sleep disturbance ~treat restless legs; ~review medications; ~advise about driving if sudden-onset sleep; ~daytime hypersomnolence may be treated with modafinil) ▪︎Falls (usually multifactorial; ▪︎Autonomic features are common in older patients. They should be sought and actively managed: ~Weight loss ~Dysphagia ~Constipation ~Erectile dysfunction ~Orthostatic hypotension ~Excessive sweating ~Drooling Investigations ▪︎Diagnosis is clinical and, once suspected, should be reviewed by a Parkinson’sdisease specialist. ▪︎Trials of treatment may be done, with review of the diagnosis if there is no improvement, but single-dose levodopa ‘challenge’ tests are no longer performed. ▪︎Brain imaging (e.g. CT) can be used to illustrate other conditions that may mimic Parkinson’s disease (e.g. vascular disease) ▪︎Specialist scans are becoming more widely used to assist diagnosis (e.g.consider123I-FP-CIT single-photon emission computed tomography (SPECT),commonly known as DatSCAN™ after the radiolabelled solution used). ▪︎Patients with essential tremor, Alzheimer’s disease, or drug-induced Parkinsonism have normal scans. |
•Parkinson’s disease: management Should be overseen by a Parkinson’s disease specialist clinic. Drugs ▪︎It is not possible to identify a universal first-choice drug therapy for either early Parkinson’s disease or adjuvant drug therapy for later stages. ▪︎Consider the short- and long-term benefits and risks of each treatment, along with lifestyle and clinical factors. Discussion with the patient is key. ▪︎Initiation treatment is started with one of the following: 1.Levodopa plus a decarboxylase inhibitor (prevents peripheral breakdown of drug) (co-beneldopa/co-careldopa). Start low and titrate to symptoms 2.Dopamine agonists (ropinirole, pramipexole, rotigotine). Psychiatric side effects, postural hypotension, and nausea may limit therapy 3.MAOI (selegiline, rasagiline). These drugs have many interactions with antidepressants and should be used with care by a specialist. ■Adjuvant treatment may be needed as the disease progresses. ▪︎Firstly, ↑ doses or add a second agent from the list already given; ▪︎then consider: □Catechol-O-methyltransferase (COMT) inhibitor (entacapone). Will smoothe fluctuations in plasma levodopa concentrations. Must be given with levodopa. Stains urine orange □Amantadine—weak dopamine agonist which can reduce dyskinetic problems. □Apomorphine—s/c injections. Specialist treatment— rarely useful in older patients □Anticholinergics (trihexyphenidyl, orphenadrine) are mild antiparkinsonian drugs, rarely useful in elderly patients due to severe psychiatric side effects. They do have a beneficial effect on tremor and are possibly the drug of choice where tremor is more of a problem than bradykinesia. Surgery □Ablation (e.g. pallidotomy) and stimulation (electrode implants) used in highly selected populations. Older patients often excluded due to high operative risk and rigorous exclusion criteria (e.g. cognitive impairment). Other therapeutic options ▪︎Patients and carers benefit from regular review by a specialist doctor or nurse.Many services now have specialist Parkinson’s disease nurses. ▪︎A course of PT can be helpful to boost mobility and reduce falls. ▪︎OT plays a vital role in aids and adaptations for disability. ▪︎SALTs, along with dieticians, can help when swallowing becomes a problem. ▪︎Inpatient assessment is rarely helpful, as hospital routines can rarely match home treatment and some patients deteriorate in hospital. Parkinson’s UK ( http://www.parkinsons.org.uk) has plenty of information and advice for patients and carers |
•HOW TO Manage a patient with Parkinson’s disease whocannot take oral medication ▪︎This situation arises quite commonly in advanced disease during a hospital admission.A patient with advanced disease admitted for another reason (e.g. sepsis)may miss an oral dose of medication (e.g. because they are unwell or because the drug is not immediately available). ▪︎In some, this will be well tolerated; in others, there will be a rapid decline in function and loss of swallow, with a downward spiral unless promptly managed. ▪︎Other situations in which oral medication may not be possible: Perioperatively when the patient is nil by mouth When an ileus or other cause makes poor drug absorption likely After a stroke ► Omission of medication will (for most patients with Parkinson’s disease)lead to a decline in function, so continuation of treatment is key. Reducing the risk ▪︎Plan ahead—patients should be educated about the importance of taking medication on time and to always bring their own medication with them if they come into hospital and be encouraged to self-medicate where possible If surgery is elective, then get specialist advice about medication as part ofthe pre-operative assessment. ▪︎Aim for local or regional anaesthesia, if possible. ▪︎Have protocols in place for the urgent care of Parkinson’s disease patients. ▪︎Ensure that wards have Parkinson’s disease drugs readily available; involve pharmacy colleagues. Early action ▪︎Use nasogastric tubes (NGTs) early if swallow is impaired. ▪︎Relax nil by mouth rules pre-operatively for Parkinson’s disease drugs ▪︎Use antiemetics (not metoclopramide) when vomiting ▪︎Medication Use a different preparation, e.g. ~levodopa dispersible down an NGT, ~buccal selegiline ~Use an enteral preparation, e.g. apomorphine (s/c delivery) or rotigotine(patch delivery). Advice will be needed from a specialist about doses that are equivalent to their usual medication, but a rough rule of thumb is that. 100mg levodopa daily is replaced by a 2mg/24h rotigotine patch |
|
|
Manging Challenging symptoms of PARKINSON'S disease |
1.Wearing off— improvement gained from a dose of medication does not last until the next dose begins to take effect. Due to disease progression. Patients require higher or more frequent dosing to produce the same effect; ▪︎use drugs from different classes; ▪︎try COMT inhibitors 2.Dyskinesias ▪︎Reduce dopaminergic drug dose if possible. ▪︎Add amantadine 3.Motor fluctuations with choreodystonic ‘on’ phases and freezing ‘off’ phases. These worsen with duration of treatment ▪︎Reduced levodopa dose more frequently(dose fractionation); ▪︎controlled-release preparations; ▪︎add entacapone; ▪︎add dopamine agonist 4.Other drug side effects (confusion and hallucinations, constipation, urinary retention, and nausea and vomiting) are a particular problem in elderly patients and often limit treatment to sub ideal levels ▪︎Treat hallucinations by reducing PD medications if possible, or adding rivastigmine or quetiapine ▪︎Domperidone is the best antiemetic, but use has been restricted due to concerns aboutarrhythmia 5.︎In general, patients prefer dyskinetic side effects than ‘off spells’— ▪︎relatives/carers may find the opposite easier to cope with, especially if patient confused or falling when ‘on’ Ensure you talk to the patient as well, even if it is easier to talk to the carer. Compromise may be necessary 6.Quantifying response to treatment is very difficult ▪︎Get patients/carers to fill in a 24h chart. ▪︎A formal quantified drug trial by therapists can be very helpful. 7.Morning stiffness ▪︎ Use a rapid-acting drug (e.g.Madopar® dispersible) in bed on waking, or try a long- acting drug last thing at night 8.End-stage disease ▪︎Ultimately drug responsiveness so poor and side effects so marked that ↓ and withdrawing therapy may be appropriate. ▪︎Palliative treatment and social support important. 9.Drooling ▪︎Use anticholinergic inhalers, ▪︎atropine eye drops, or botulinum toxin injections. ▪︎Hyoscine patches may cause hallucination |
Impulse control disorders Defined as failure to resist an impulse, drive, or temptation to perform an act that is harmful to the person or others Tend to occur after the initiation of dopaminergic therapy in those who are susceptible, and abate with dose reduction Examples include: ▪︎Hypersexuality ▪︎Pathological gambling ▪︎Compulsive buying ▪︎Aimless wandering ▪︎Repetitive activities (e.g. arranging objects) ▪︎Dopamine dysregulation syndrome (addictive use of dopaminergic drugs in a Parkinson’s disease patient with other impulse control behaviours) |
|
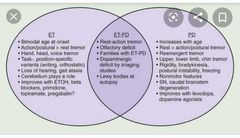
Diseases masquerading as Parkinson’s disease The majority of slow, stiff, or shaky older patients on geriatric wards do not have true Parkinson’s disease. As many as one in two diagnoses of Parkinson’s disease made by non-specialists are incorrect. It is important to get the diagnosis right or you will subject patients needlessly to the harmful side effects of medications. Coexistence of more than one syndrome can further complicate the diagnosis. 1.︎Atherosclerotic pseudo-parkinsonism/multi-infarct dementia: due to neurovascular damage—consider in those with stroke/TIA or with atherosclerotic risk factors, e.g. hypertension. Short-stepping, wide-based unstable gait with relative preservation of arm and facial movements (lower body parkinsonism). ▪︎Head scan may show lacunae or white matter change 2.Benign essential tremor: ▪︎often inherited (autosomal dominant), ▪︎worse on action (spills tea from tea cup), ▪︎improved by alcohol and β-blockers, may have head nodding or vocal tremor. 3.Lewy body dementia: ▪︎Lewy bodies are widely present throughout the cortex, not predominantly in the substantia nigra as with true Parkinson’s disease. ▪︎Psychiatric symptoms, e.g. visual hallucinations, tend to precede physical ones 4.Drug-induced parkinsonism: ▪︎neuroleptics are the most common cause, but remember that prochlorperazine for dizziness and metoclopramide for nausea are also causes. ▪︎Some irritable bowel treatments contain neuroleptics.
5.Other causes: Alzheimer’s disease, hydrocephalus, and even severe polyarthritis can sometimes cause diagnostic confusion. Rare differential diagnoses include ▪︎Wilson’s disease, ▪︎Pick’s disease, ▪︎carbon monoxide poisoning, ▪︎multiple head injuries (ex-boxers), ▪︎and post-encephalitis or anoxic brain injury 6.Parkinson’s plus syndromes This is a confusing array of rare disorders, including: 1》Multisystem atrophy (aka Shy–Drager syndrome, olivopontocerebellar atrophy) with early autonomic failure (incontinence and postural instability), ataxia, parkinsonism, and pyramidal signs. 2》Cognition intact Progressive supranuclear palsy (aka Steele–Richardson–Olszewski disease) with up- and downgaze palsy, axial rigidity and falls, dysarthria and dysphagia, and frontal lobe dementia. 3》Corticobasilar degeneration with asymmetrical ataxia, dementia, and speech/swallow disturbance. . |
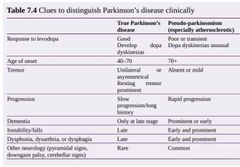
|
Q |
|
|
Epilepsy ▪︎1° epilepsy most commonly presents at around the time of puberty, but the incidence of new fits is actually higher in the over 70s (>100 per 100 000) because of the □↑ amount of 2° epilepsy (caused by, e.g. brain ischaemia, subdural haematomas, brain tumours).
□In addition, fits can be precipitated by: •Metabolic disturbance (e.g. hyponatraemia, hypoglycaemia) •Drugs (e.g. ciprofloxacin) •Infection (at any site, but particularly meningitis/encephalitis) •Withdrawal from alcohol or drugs such as benzodiazepines. •Wernicke’s encephalopathy (due to thiamine deficiency in malnourished, e.g.alcoholics) •Many of these conditions are more common in older patients who also have a lower fit threshold for any given level of stimulus.
Diagnosis An eyewitness account is the most useful diagnostic tool. Look particularly for post-event confusion/drowsiness which is rare in cardiac syncope. The classic features of prodrome, tongue-biting, and incontinence are not souseful in distinguishing cardiac from neurological syncope in older patients. Remember that cerebral hypoperfusion from any cause (e.g. bradycardia) can cause fits, so epilepsy can coexist with other causes of syncope. In these cases,treatment of the 1° syncope/hypoperfusion is more effective than antiepileptics Investigations Routine blood screening, CXR, and ECG to look for precipitants and differential diagnoses Lactate and creatine kinase (CK) are often elevated after a generalized seizure. CT scan is vital to exclude a structural lesion EEGs can be helpful when positive but very commonly have non-specific changes and low sensitivity, i.e. a normal EEG does not rule out epilepsy.
General management -Ensure the patient is not taking medication that lowers the fit threshold.common examples include -tricyclics, ciprofloxacin, tramadol, and phenothiazines. Think about over-the-counter drugs, e.g. Ginkgo biloba, St John’s Wort) -Correct any metabolic derangement (e.g. glucose, sodium, sepsis) -Advise about driving restrictions—do not assume they do not drive. ▪︎Detect and treat complications, e.g. aspiration, trauma, pressure injuries. ▪︎Driving regulations and epilepsy You have a duty to ensure that the patient informs the Driver and Vehicle Licensing Agency (DVLA). If you have reason to believe that the patient has not done so, then your duty to inform the DVLA yourself (after informing the patient of your intention) outweighs confidentiality concerns. Patients have at least a 6-month ban on driving for a first fit (unless a‘provoked fit’, e.g. following brain surgery or stroke, when it may be a shorter period—individual decision). They can then reapply for a licence as long as they remain fit-free. Patients must also refrain from driving for 6 months after withdrawing epilepsy medication. |
Epilepsy and stroke Onset seizures (within a week, most commonly within 24h) occur in 2–5% of strokes. More common with ▪︎haemorrhages, ▪︎large cortical strokes, and ▪︎venous infarction. ■Consider also alcohol/drug (especially benzodiazepine) withdrawal for early fits. Long-term anticonvulsants not usually prescribed unless fits recur. After the first week, stroke remains a risk factor for new epilepsy— first year5% fit, subsequently 1.5% annual incidence. ▪︎Many such patients develop transient neurological worsening (Todd’s paresis) or permanent worsening without CT evidence of new stroke. In these patients, it is usually worth considering long-term anticonvulsants. ▪︎Epilepsy may occur 2° to clinically ‘silent’ cerebral ischaemia, and 3% of patients with stroke have a past history of fits, most occurring in the preceding year. ▪︎Some epilepsy experts suggest that aspirin is prescribed for new-onset seizures in an elderly patient once structural lesions have been excluded. |
Epilepsy: drug treatment Acute treatment ▪︎Start with benzodiazepines (5–10mg rectal or 2–10mg iv diazepam, or 0.5–2mg lorazepam iv or im) ▪︎If fits continue, consider setting up a loading-dose infusion of phenytoin (use a cardiac monitor) or iv leve tiracetam until oral medication can be taken. ▪︎Rarely the patient may need intubating and paralysing to stabilize them or toallow an urgent CT scan Chronic treatment Because of side effects and long duration of treatment, most doctors will resiststarting anticonvulsants until after a second fit, especially if the diagnosis isunclear or if there is a reversible precipitant. Presence of an underlying structural abnormality or wishing to return to driving may tip the balance in favour of treatment. The choice of agent shows regional and personal variation Most commonly used agents are similarly effective Older agents include ▪︎phenytoin, ▪︎carbamazepine (both effective but may besedative), and ▪︎valproate (better tolerated, but plasma levels are unhelpful inmonitoring compliance or side effects) Newer agents such as lamotrigine and levetiracetam are ‘cleaner’ and increasingly used as first line. All anticonvulsants have significant side effects, e.g. sedation, confusion, rash,tremor, and ataxia. Serious liver, blood, and pulmonary side effects can also occur. ongoing monitoring to optimize dose and minimize side effects is necessary. Many anticonvulsants interact with each other, as well as other drugs, and can↑ toxicity or reduce effectivenessif in doubt, consult a pharmacist. Gabapentin and pregabalin are less likely to interact with other medications and can be useful alternatives, especially if their pain-relieving properties are also desirable. If a known epileptic presents with fits in the context of a new precipitant (e.g.sepsis), then short-term use of clobazam can aid control until the precipitant has been treated. Avoid abrupt withdrawal of antiepileptics fits may be provoked. Partial seizures (e.g. face/arm twitching) are rarely dangerous and often distress bystanders more than the patient, but they can progress to 2°generalized seizures. The same drugs can be employed. Partial seizures often indicate structural lesions and an early CT scan is advisable. Sometimes a trial of anticonvulsants in patients with recurrent unexplained collapse can be revealing. Refer to an epilepsy specialist if control is proving difficult and multiple drug sare required. |
|
|
Neuroleptic malignant syndrome A rare, but important, syndrome in patients taking neuroleptics (e.g. haloperidol,chlorpromazine, risperidone) with a triad of: Fever Rigidity and tremor Rhabdomyolysis with 2° renal failure ► Can be fatal (up to 30%), so early recognition is important. Diagnosis May arise at any time during treatment, i.e. the patient may have recently: ▪︎Started (most common) or ▪︎stopped neuroleptics ▪︎↑ the dose or ▪︎ been stable on them for a long time ▪︎Added a second drug, e.g. tricyclic antidepressant, lithium Reintroduction of the offending drug at a later date may not reproduce symptoms. Contributing factors such as intercurrent illness and metabolic derangement may be important in the aetiology. Clinical features The patient looks unwell with fever, severe lead-pipe rigidity, bradykinesia, occasionally tremor, and ↓ conscious level. Time course: onset usually over 1–3 days, starts with rigidity/altered mental state Seizures and abnormal neurological signs can occur Autonomic dysfunction causes sweating, tachycardia, and hypertension Multiorgan failure can occur; there is leucocytosis, and CK levels may be over 1000IU/L ■Lumbar puncture, CT scan, and EEG are often required to exclude other diagnoses such as CNS infection ► The most common cause of a similar presentation is sepsis in a patient with pre-existing cerebrovascular disease; CK measurement will aid the diagnosis. Management Stop all neuroleptics. Cooling using paracetamol, fans, and damp sponging. iv fluids with careful monitoring of electrolytes and renal function. Dantrolene(direct muscle relaxant) can speed recovery. Short-term dialysis is sometimes required. Bromocriptine is used in some cases, although there is limited evidence for efficacy. Early transfer to the intensive care unit may be wise—death most commonly occurs by hypoventilation/pneumonia or renal failure. There are sometimes persisting neurological sequelae. Serotonin syndrome A similar syndrome to neuroleptic malignant syndrome in patients taking serotonin reuptake inhibitors, especially if combined with tramadol, a tricyclic,or an MAOI. Patients tend to be agitated and delirious, rather than unconscious. Gastrointestinal symptoms (diarrhoea/vomiting) occur. Onset may be within 2h;resolution is usually quicker than neuroleptic malignant syndrome. |
Q |
|
|
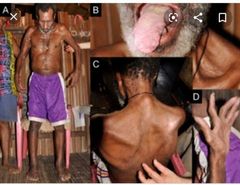
▪︎Motor neuron disease A progressive idiopathic disease with selective degeneration of motor neurons causing weakness and wasting.
▪︎There is a variety of manifestations, depending on the site of damage; ▪︎the most common site for lesions is in the anterior horn cells of the spinal cord (lower motor neuron (LMN)), ▪︎but the descending motor pathway (upper motor neuron (UMN)) may be affected in the corticospinal tracts, brainstem, and motor cranial nuclei.
► The combination of weakness and fasciculations should always prompt consideration of motor neuron disease (MND).
▪︎Onset rises steeply with age, with a peak incidence in late 50s/ early 60s. ▪︎Veryrare before age 40. Overall prevalence of 7 per 100 000, but incidence of 1 per10 000 at age 65–85. Underdiagnosed in older patients (confused with cerebrovascular disease,myasthenia, cervical myelopathy, motor neuropathy, syringomyelia, and paraneoplastic syndromes) ▪︎Slightly more common in ♂5% will have a family history (autosomal dominant is the most common butcan be recessive or X-linked)
History Weakness, cramps, and fatigue in limbs. Weakness usually begins in a focal area and spreads to contiguous muscles; onset in the upper limbs is the most common.
▪︎Palatal and vocal cord paralysis can cause stridor, dysarthria, dysphagia, and aspiration pneumonia.
▪︎Paresis of respiratory muscles can cause respiratory failure (may present to chest physicians/intensive therapy unit (ITU)). ▪︎Intellect, sensation, and continence are usually retained. ▪︎Some forms associated with frontotemporal dementia (<5%); ▪︎depression common.
Examination Look for wasting with fasciculations (LMN), especially in the tongue, shoulders , and legs. ► Fasciculations may be a normal finding in the hands and calves of older people.
▪︎Head drop/droop can occur. ▪︎Brisk reflexes, clonus, and up going plantars (UMN). ▪︎ This is one condition that can cause absent ankle jerks and upgoing plantars. ▪︎Atrophy and weakness are less specific signs `Donald Duck’ speech. □Sensory changes should make you question the diagnosis. . |
•Investigations ▪︎CK may be elevated ▪︎CT, MRI, and muscle biopsy are usually normal ▪︎Electromyography (EMG) shows denervation of muscles caused by anterior horn cell degeneration and is diagnostic. Clinical picturesDiverse presentations and rate of progression, including: ▪︎Amyotrophic lateral sclerosis (ALS) is the most common form—classicalpicture of mixed UMN and LMN. Term used commonly in the USAProgressive pseudobulbar or bulbar palsy—speech and swallowpredominantly affected ▪︎1° lateral sclerosis—UMNs predominantly affected ▪︎Progressive muscular atrophy—LMNs predominantly affected.
Treatment Riluzole (sodium channel blocker) 50mg twice daily (bd). Prolongs survival by a few months, but not function. Licensed and endorsed by NationalInstitute for Health and Care Excellence (NICE) for ALS only. Expensive and should be initiated by a specialist.
Monitor liver function, and check forneutropenia if febrile illness. Supportive Chest - antibiotics and PT, tracheostomy, and non-invasive nocturnal ventilation (for diaphragmatic palsy and sleep apnoea)
Speech—early referral to speech therapy for communication aids.
Nutrition—initially pureed food and thickened fluids. Malnutrition and aspiration are indications to consider artificial feeding .Muscle spasm—baclofen, PTMobility/independence—OT for wheelchairs and adaptationsPain/distress—opiates or benzodiazepines (but beware respiratory suppression) Other This is a devastating diagnosis to give to a patient—the mean life expectancy is 2–5 years.
Matters are often worse because there is often a considerable delay between symptoms and a concrete diagnosis being made (sometimes theinitial diagnosis may have been incorrect).
Emphasize the retention of cognition and aspects of supportive care available.
Offer regular follow-up appointmentsSpecialist neurology/MND nurses are available in some areas Refer to Motor Neurone Disease Association for supportConsider enduring power of attorney and advance directives (ADs). . |
|
|
|
Peripheral neuropathies Some minor degree of sensory loss in the feet and reduced or absent ankle jerks is so common in older patients (up to 50% of over 85 year olds) that some class this as a normal ageing change, but remember:Even mild, asymptomatic neuropathies can contribute to postural instability and falls. The diagnosis is often missed because of non-specific symptoms and insidious onset with slow progression.
Clinical features ▪︎There may be signs of LMN weakness with wasting and loss of reflexes. ▪︎Sensory loss often with joint position and vibration loss before touch and pain. This is classically in a ‘glove and stocking’ distribution, rather than dermatomal. Neuralgia-type pain may be present (especially diabetes and alcohol). Autonomic failure and cranial nerve involvement can also occur.
Severe cases may affect respiration
Classification Try to determine if the signs are focal or generalized and whether they are predominantly sensory or motor because this can help identify the likely underlying pathology.
Further classification by pathology (axonal or demyelinating) requires nerve conduction studies or biopsy.
The most common pattern produces wide spread symmetrical sensory loss(typically glove and stocking).
This may be combined with distal muscle weakness (mixed motor and sensory neuropathy) or sometimes there is a pure motor neuropathy.
Where signs are focal, consider mononeuritis multiplex.
Causes The causes are legion and often multiple in older patients. Idiopathic neuropathies are very common (25% defy diagnosis in most studies).
The following list is not exhaustive: Diabetes Paraneoplastic syndromes (e.g. small cell lung cancer) Alcoholism (often combined with vitamin deficiency) Renal failure B12 or folate deficiency Guillain–Barré syndrome (the most common acute onset) Chronic inflammatory demyelinating polyradiculoneuropathy (rare autoimmune motor neuropathy, considered the chronic counterpart of Guillain–Barré) Hypothyroidism Carpel tunnel syndrome Vasculitides (e.g. Wegener’s granulomatosis) Drugs (e.g. isoniazid, nitrofurantoin, amiodarone, colchicine) Paraproteinaemias and amyloid Investigations Always check B12 glucose, TFTs, serum and urine immunoglobulins, ESR,.and CRP before labelling a neuropathy idiopathic Look carefully for an occult tumour (e.g. breast examination, iron studies,CXR) Family history Nerve conduction studies will confirm nerve damage and distinguish demyelination from axonal damage (which sometimes helps with the differential diagnosis), but they are not always required in straight forward cases. Further specialist tests include immunology, tumour markers, lumbar puncture, molecular genetics tests, and nerve biopsy. Treatment The important thing is to identify reversible causes quickly, but even treatable causes rarely respond dramatically—the aim is usually prevention of further deterioration. Chronic inflammatory polyradiculoneuropathy is treated by steroids, plasma exchange, and iv immunoglobulins, but most other chronic neuropathies have no specific treatment. Supportive and symptomatic treatment(e.g. appropriate footwear, analgesia, environmental adaptation) is important. |
Guillain–Barré syndrome This is an acute inflammatory demyelinating polyneuropathy. Causes ascending paralysis, weakness beginning in the feet and hands and migrating towards the trunk. It can cause life-threatening complications, particularly if the respiratory muscles are affected or if there is dysfunction of the autonomic nervous system. ▪︎The disease is usually triggered by an acute infection. This is a medical emergency which responds to iv immunoglobulins or plasmapheresis. ▪︎These patients can deteriorate rapidly and should be managed in conjunction with specialist neurology units. ▪︎Even patients who look well should have their vital capacity measured daily to warn of impending respiratory failure. ► The main hurdle is recognizing the diagnosis. |
Q Q Q |
|
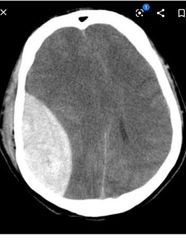
Subdural haematomaA condition which is much more common in old age because as the ▪︎brain shrinks, the veins which lie between it and the skull are much more likely to get torn following trauma (even minor injury).
▪︎Older people are also more likely tohave falls/head injuries and are more frequently on predisposing drugs (e.g.aspirin, warfarin, NOACs).
▪︎Other risk factors include alcoholism epilepsy, and haemodialysis. Features► Subdurals frequently present with very non-specific symptoms in frailconfused patients.
A high index of suspicion is required. Subdurals can occur acutely (and present within hours of an accident) or more slowly as the classical ‘chronic subdural haematoma’, although this distinction does not help guide management. A history of head injury occurs in only about half. Common features include drowsiness and confusion (rarely fluctuating),postural instability, progressive focal neurology (e.g. hemiparesis, unequalpupils), headache, and blurred vision. Rarely transient neurology (mimicking TIA) or parkinsonism can occur. Some patients are asymptomatic and large collections can be incidental findings. ▪︎Examine for -papilloedema, -focal neurology, and -long tract signs. DiagnosisCT head scan—look for crescent-shaped haematoma ▪︎compressing the sulci ▪︎(hypodense/black is old blood; ▪︎hyperdense/white indicates recent bleeding)and ▪︎midline shift■All patients who have new UMN signs with confusion and/or drowsiness should be scanned.It is harder to decide when to scan a confused patient without such signs—most agree it is reasonable to look for other causes of acute confusion before asking for a head scan, as long as the patient is being observed for any change in neurological signs or conscious level. Have a lower threshold for scanning patients on antiplatelets/anticoagulantsand for those who have evidence of falls, particularly facial bruising.
MRI canbe useful when CT changes are subtle (an isodense phase occurs on CT intransition between hyperdense and hypodense changes) or very small haematomas are suspected. Management ▪︎Decisions are usually made in conjunction with the local neurosurgical team(although in practice, only about one-third of patients will end up having surgery). ▪︎Stop antiplatelet agents/NOACs, and reverse warfarin therapy if possible. ▪︎Observation and supportive care are frequently used in: ~Asymptomatic patients. ~Those with small bleeds who are stable/improving. ~Those not fit for transfer/surgery. When conservative management is adopted, record the conscious level [Glasgow Coma Scale’, ] and any focal neurology at least daily or if there is any change. Any deterioration should prompt a repeat CT scanand reconsideration of surgery. Burr hole surgery is not complex and is done under local anaesthetic. Recovery after surgery can be dramatic. Complications include re-bleeding and seizures. Use symptoms (especially conscious level), not CT appearance, to decide on surgery. Mortality is around 10%—highest with a depressed conscious level and bilateral haematomas. Those left with residual neurology should receive rehabilitation as in stroke. |
Q |
|
|
Sleep and insomnia ▪︎With ↑ age, less sleep is needed (~1h less than young adults); the circadian rhythm is less marked, and sleep becomes more fragmented with greater difficulty getting to sleep.
▪︎ Deep (stages 3 and 4) sleep is reduced, but dreaming sleep/rapid eye movement (REM) is preserved. ▪︎Insomnia is a symptom which correlates poorly with observed actual sleep time (i.e. patients who complain of poor sleep may be observed by nurses/family to sleep well, while those who sleep very little do not necessarily complain). ▪︎It can be very distressing and is associated with ↑ morbidity and mortality. Around 25% of elderly people have chronic insomnia—even higher rates with psychiatric and medical conditions. ▪︎Insomnia is a particular problem in an unfamiliar noisy ward environment, and doctors are often under considerable pressure to prescribe hypnotics. Treatment of insomnia ▪︎First ensure that underlying causes are looked for and treated: □Pain at night—consider using analgesics with sedative side effects, e.g.opiatesNocturnal urinary frequency, e.g. due to polyuria, peripheral oedema,prostatism Comorbidities, e.g. orthopnoea, oesophageal reflux, ~Parkinson’s disease ~Depression/anxiety—very common and use of an antidepressant will improve sleep much better than a hypnotic. Alcohol dependence Drugs— corticosteroids, omeprazole, phenytoin, amiodarone, sulfasalazine, atorvastatin, ramipril, as well as psychiatric drugs,e.g.paroxetine ,haloperidol, and chlorpromazine, can cause insomnia. ▪︎β-blockers and levodopa cause nightmares. ▪︎The following non-pharmacological interventions (sleep hygiene) can be tried:Reduce or stop daytime ‘cat napping’ Avoid caffeine, heavy meals, and alcohol in the evening (alcohol helps to fall asleep but reduces sleep quality)
▪︎Use a bedtime routine Ensure the environment is dark, quiet, and comfortable. ▪︎Relaxation and cognitive behavioural techniques can be useful ▪︎Try warm milky drinks.
Manage expectations— older people will rarely sleep as much or well as younger people Drugs Benzodiazepines (e.g. temazepam 10mg) are licensed for short-term (<4weeks) management of insomnia and anxiety.They do work well when used correctly. The newer Z-drugs (e.g. zopiclone, zolpidem) are only for insomnia. They have shorter half-lives and fewer side effects (although zopiclone is still a cause of daytime drowsiness). Overall they are probably slightly superior to benzodiazepines, but the same cautions about dependence apply. Other hypnotics (e.g. chloral hydrate, clomethiazole, antihistamines) can be toxic, especially in overdose, and provide no major advantages. A new class of drugs that act on melatonin pathways may be beneficial. |
HOW TO . . . Use benzodiazepines for insomnia ▪︎Tolerance develops after only 4 weeks (i.e. benzodiazepines fail to produce a useful sedative effect). However, it takes only this long for dependence to occur. Dependence may be physical (with rebound insomnia, anxiety, or evendelirium) and/or psychological (the patient believes they will not be able to sleep without tablets). ▪︎The shorter the half-life, the greater the withdrawal effects. ▪︎Benzodiazepine use has been associated with ↑ falls, reducedfunctional status, road traffic collisions, depression, and memory impairment. ▪︎Although awareness of these problems has reduced the number of long-term benzodiazepine users, there is still over-prescribing. Prevention of dependence ▪︎Do not use benzodiazepines for mild or non-distressing insomnia—try non-pharmacological measures first ▪︎Never prescribe benzodiazepines for >4 weeks. ▪︎Never prescribe benzodiazepine medication at discharge from hospital ▪︎All patients/carers should receive warnings about benzodiazepine sideeffects (especially dependence) and the reason for limiting the course length at the outset. GPs should limit repeat prescriptions and audit their practice. Treatment of dependence ▪︎Explain and motivate the patient/carers. ▪︎Gradual reduction regimen ▪︎In difficult cases, switch to an equivalent dose of diazepam first—long half-life produces milder withdrawal symptoms ▪︎Continuing supportOccasionally acute withdrawal is undertaken by mistake (e.g. drugaccidentally not prescribed for a couple of weeks during acute admission.with a fractured neck of femur). In these cases, do not automatically restart.the benzodiazepines and do explain why to the patient or they will justrestart it when they return home. |
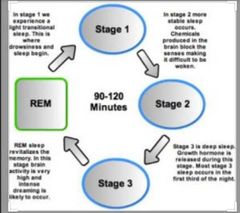
Sleep cycle |
|
Other sleep disorders ▪︎Hypersomnolence This is excessive daytime sleepiness despite a normal night of sleep. Causes include ▪︎brain disease (e.g. dementia, stroke), ▪︎cardiopulmonary disease (e.g.cardiac failure, COPD), ▪︎obstructive sleep apnoea, ▪︎hypothyroidism, ▪︎narcolepsy,and ▪︎sedative drugs. Restless legs syndrome A common (10% older people) unpleasant sensation in the limbs which ↑ with drowsiness and is eradicated by movement. Can be associated with limb jerking during sleep with sleep disturbance. Both symptoms respond to benzodiazepines. Check they are not iron-deficient (replacement can be curative). Treat with dopamine agonists. Circadian rhythm disorders Jet lag is the best known, but advanced sleep phase syndrome (sleepiness occurs too early in the evening, but there is early morning wakening) and delayed sleep phase (sleepiness comes too late at night) can occur without such a precipitant. Treat by gradually altering bedtime and bright light therapy when wakefulness is desired. Sleep apnoea in older patients Obstructive sleep apnoea and central sleep apnoea are very common in older patients and can contribute to daytime sleepiness, accidents, and heart failure. Unfortunately, periods of apnoea are less likely to be symptomatic than in the young and where symptoms do exist, they are often multifactorial, so diagnosis and compliance with therapy (non-invasive positive pressure ventilation) can be problematic. REM sleep behaviour disorder Dream-enacting behaviour during REM sleep, occurring because of a lack of muscle atonia that usually accompanies REM sleep. About half will go on to develop neurological pathology, e.g. Parkinson’s disease, Lewy body disease. Treatment with benzodiazepines may be successful.Further readingHarbison J. Sleep disorder in older people. Age Ageing 2002; 31: 6–9. |
Q |
|
|
|
Chapter 8 Stroke Definition and classification Predisposing factors Acute assessment HOW TO . . . Assess for inattention Investigations Acute management HOW TO . . . Manage swallow after stroke Stroke units HOW TO . . . Estimate prognosis after stroke Thrombolysis Intra-arterial therapies Ongoing management HOW TO . . . Protect your patient from another stroke Complications HOW TO . . . Manage urinary incontinence after stroke Longer-term issues Transient ischaemic attack clinics |
Q |
|
|
|
Definition and classificationDefinition▪︎Stroke is the sudden onset of a focal neurological deficit, lasting >24h or leadingto death, caused by a vascular pathology. ▪︎Infarction: emboli, in situ thrombosis, or low flow▪︎Haemorrhage: spontaneous (not associated with trauma). Excludes subdural and extradural haematomas but includes spontaneous subarachnoid haemorrhage. ▪︎Transient ischaemic attacks (TIAs) are focal neurological deficits (including monocular visual loss) due to inadequate blood supply that last <24h (in reality,most TIAs last just minutes). ▪︎Infarction and TIAs have the same pathogenesis, and the distinction is likely to become less helpful with time. Both stroke and TIA need urgent treatment, as it is impossible to distinguish TIA from stroke in the first few minutes and hours.Waiting to see if the focal neurology resolves causes neuronal loss at a rate of1.9 million per minute. It is useful to think of a spectrum of disease severity from TIA to fatal stroke where early intervention to save brain tissue has parallels with approaches to myocardial salvage in coronary syndromes. Stroke burdenIncidence of first stroke is about 200 per 100 000 per yearPrevalence is around 5–12 per 1000 population, depending on the age of the sample.It is a disease of older people (over two-thirds of cases occur in the over 65s; <15% occur in under 45s) Globally it is the second most common cause of death.
ClassificationVarious methods including:□Infarct or haemorrhage□Pathogenesis— large vessel, small vessel, cardioembolic (AF or LV muralthrombus), valve disease, infective endocarditis, non-atheromatous arterialdisease (vasculitis, dissection), blood disorders□Vessel affected—anterior circulation (mainly middle cerebral artery (MCA)),lacunar (deep small subcortical vessels), posterior circulation (vertebral andbasilar arteries) Classification systems include: Bamford’s classification—clinical features to define likely stroke territory.Used in major trials and gives prognostic information about each group TOAST classification—for subtypes of ischaemic stroke: large arteryatherosclerosis, cardioembolism, small vessel occlusion, stroke of otherdetermined aetiology, stroke of other undetermined aetiology
|

Predisposing factorsFixedAge: stroke risk ↑ with age (this is the strongest risk factor)Sex: ♂ > ♀Ethnicity: higher risk in people of African and South Asian origin than those of European origin. Probably due to ↑ obesity, hypertension, and diabetesFamily history: positive family history ↑ risk. Not simple inheritance complex genetic/environmental interaction Previous stroke/TIA: risk of recurrence is about 10–16% in the first year,highest in the acute phase. Other vascular disease: presence of any atheromatous disease (coronary,peripheral arterial, etc.) ↑ risk of stroke. Modifiable by lifestyle change▪︎Smoking: causal and dose related. Risk diminishes 5 years after quitting▪︎Alcohol: heavy drinking is a risk factor▪︎Obesity: ↑ risk of all vascular events in obesity—confounded by ↑ in otherrisk factors (hypertension, diabetes), but probably weak independent factor,especially central obesity▪︎Physical inactivity: ↑ stroke in less active—again confounded by presence of other risk factors in the inactive▪︎Diet: healthy eaters have lower risk but may have healthier lifestyles ingeneral. Low-salt, high-fruit and vegetable, high-fish, and antioxidant diets are likely to be protective, but trials have failed to show an effect from dietary interventions.▪︎Oestrogens: the oral contraceptive confers a slightly ↑ risk of stroke andshould be avoided in the presence of other risk factors. Post-menopausalhormone replacement therapy (HRT) has been shown to ↑ risk of ischaemic stroke, but not TIA or haemorrhagic stroke▪︎Medically modifiable▪︎Hypertension: clear association between ↑ BP and ↑ stroke risk across all population groups. Risk doubles with each 5–7mmHg ↑ in diastolic BP. Also ↑ with isolated systolic hypertension▪︎AF: risk of stroke significantly ↑ in AF. ▪︎Diabetes: risk factor independent of hypertension. ▪︎High cholesterol: weaker risk factor than in heart disease—likely due to diversity of stroke aetiologies▪︎Carotid stenosis: risk ↑ with ↑ stenosis and with the occurrence of symptoms attributable to the stenosis▪︎Other comorbidity: ↑ risk in some conditions such as ~obstructive sleep apnoea, ~sickle-cell anaemia, ~blood diseases causing hyperviscosity and vasculitides. |
Acute assessment A medical emergency increasingly recognized by the general public. Prompt assessment and treatment improves outcomes. History Is it a focal neurological deficit? Did it come on ‘at a stroke’ or is there a hint of progression (simple stroke may worsen over several days, but think of alternative diagnoses, e.g. tumour) Is there headache or drowsiness? (haemorrhage more likely) Was there a fall or other head trauma?► Think subdural and request an urgent scan. What are the vascular risk factors?What was the premorbid state? What are the comorbidities? (↑ chance of poor outcome) What are the medications? Where do they live, and with whom? Who are the significant family members?
Examination GCS -A standardized measure to assess neurological deterioration. Unconsciousness or deteriorating GCS suggests •haemorrhage, •a large infarct with oedema, or a •brainstem event.
National Institutes of Health Stroke Scale (NIHSS). Clinical evaluation instrument with documented reliability and validity. Used to assess severity of initial stroke (when making a thrombolysis decision), outcome, and degree of recovery in stroke. Grades the following areas: consciousness, orientation, obeying commands, gaze, visual fields, facial weakness, motor function in the arm and leg, limb ataxia, sensory, language, dysarthria, and inattention.
General examination General inspection (head trauma, signs of fitting—incontinence or tongue biting, frailty/general condition, skin, hydration) Temperature (especially after a long lie) Cardiovascular examination (pulse rate and rhythm, BP, cardiac examination for source of cardiac emboli, carotid bruits) Respiratory examination (aspiration pneumonia or pre-existing respiratory conditions) Abdominal examination (palpable bladder) Neurological examination (may need to be adapted if patient drowsiness ▪︎Cranial nerves: especially visual fields and visual inattention (if difficulty with compliance, test blink response to threat, and look for a gaze preference which may occur with hemianopia or neglect); assess swallow ▪︎Limbs: tone (may be diminished acutely), any weakness (grade power for later comparison). Is the distribution pyramidal—arm flexors stronger than extensors, leg extensors stronger than flexors? ♧♧♧♧If weakness subtle, assess for pyramidal/pronator drift and fine movements of both hands—(dominant should be better), coordination (limited if power is diminished), sensation. (gross testing by touching both sides with eyes closed), also sensory inattention, reflexes (initially may be absent, then become brisker with time). Plantars extensor on affected side ▪︎Gait: assess in less severe stroke—is it safe? If not safe, can the patient sit unaided?▪︎Speech: dysarthria (trouble enunciating because of, e.g. facial weakness or posterior circulation stroke) or dysphasia (cortical disruption of speech—may be receptive and/or expressive): ~Receptive dysphasia is an inability to understand language—test with one-stage commands—‘close your eyes’ and progress to more complex tasks ‘put your left hand on your right ear’. Do not do the action yourself or the patient will copy you—a test of mimicry, rather than dysphasia. If comprehension intact, reassure the patient that you know they can understand but are having difficulty finding the right words. ~Expressive dysphasia—problems producing speech. May be fluent (lots of words that make no sense) or non-fluent (unrecognizable words). Nominal dysphasia is part of an expressive dysphasia and is tested by asking the patient to name increasingly rare objects, e.g. watch, hand,second hand) |
|
|
HOW TO Assess for inattentionOccurs with parietal cortex damage where there are errors in awareness of self.—the patient’s ‘automatic pilot’ has gone wrong.In extreme cases (neglect), the patient will not recognize their own arm andonly wash half of their body. Lesser degrees (inattention) are more common and complicate the rehabilitation process, as the patient must constantly be reminded of the existence of the affected side. Can affect vision or the whole of one side of the body—most commonly non-dominant hemispheric (i.e.right hemisphere, left inattention).To test: •Establish that sensory input is present bilaterally, i.e. check that the patient can feel a touch to each hand individually and does not have a hemianopia.(may be hard to establish where extreme gaze preference exists)•Provide two stimuli at once (touch both hands together, or move fingers in both sides of the visual field) and see if the patient preferentially notices the sensory input on the good side. If so, there is inattention of the bad side. Even if formal testing does not reveal inattention, sometimes it will becomeapparent during rehabilitation, often noted by therapists. |
Investigations The rationale for investigations in acute strokeTest- Rationale 1.FBC -Anaemic or polycythaemic Elevated white count suggestive of infection. High or low platelet count 2.Urea and electrolytesLook for evidence of dehydration, and assess fluid replacement. 3.CK- Evidence of muscle breakdown (if prolonged lie on floor) 4.Glucose/HBA1c -Diabetic old or new diagnosis (elevated sugars initially may represent hyperglycaemic stress response)Hypoglycaemia ► may mimic stroke 5.Cholesterol -Vascular risk factor 6.ESR -Elevation in vasculitis or sepsis (including endocarditis) 7.CRP- Any evidence of sepsis (e.g. aspiration pneumonia) 8.Blood cultures -Consider if sepsis or new heart murmur heard (endocarditis) 9.Urinalysis- Diabetic, vasculitis, urinary infection 10.ECG- Assess rhythm (look for AF)Evidence of IHD/MI or previous hypertension. 11.CXR Often useful screening test—look for any sign of aspiration, what the heart size is,etc.12.CT brain Guidelines advise scan within 24h for all strokes, or sooner (<1h) if: ▪︎Thrombolysis candidate ▪︎GCS <13 or fluctuating neurology ▪︎Severe headache at onset ▪︎On warfarin/DOAC ▪︎Papilloedema, neck stiffness, or feverCT will distinguish stroke from non-stroke, e.g. tumour, identify whether bleed or an infarct, the likely cause of the event—carotid territory infarcts from stenosis,multiple infarcts from cardiac emboli.Blood appears white on early CT; infarcts may not show acutely (first few hours),develop into low-density areas after hours–days.Small infarcts may never be seen, and the diagnosis is made clinically or onmagnetic resonance (MR) scan► A normal CT does not exclude a stroke13.Carotid Doppler Request in carotid territory events where the patient has a good recovery and is a candidate for endarterectomy14.Echocardiogram Consider where multiple (? cardioembolic) infarcts, after a recent MI (looking forthrombus), or where there is a murmur. |
Acute managementGuidelines for acute care are published by the Royal College of Physicians (http://www.rcplondon.ac.uk). See also NICE Stroke guidelines (2014). Overall care should occur on an acute stroke unit.Diagnosis What is the lesion+where is the lesion ▪︎Diagnosis should be made clinically (including assessment of the likely cerebral area affected) and reviewed by a clinician with expertise in stroke. ▪︎CT scan should be performed unless there is good clinical reason for not doing so (e.g.dying patient for terminal care) ▪︎Medical interventions1]Thrombolysis with, e.g. tissue plasminogen activator (tPA) should be given promptly where indicated. 2]Intra-arterial clot retrieval has evidence of benefit (up to 6h of onset) where expertise exists either in combination with thrombolysis or where thrombolysis is contraindicated 3]Aspirin (300mg) should be given as soon as possible after the onset of stroke symptoms if haemorrhage is excluded (can be given nasogastrically (NG) or per rectum (PR)) and not a potential hemicraniectomy case. 4]BP- debate about the optimal BP in the acute phase high BP is harmful long term but may be required to provide perfusion pressure with altered cerebralautoregulation acutely ,trials ongoing.
Guidelines advise that BP should notbe lowered acutely in general, but existing antihypertensives continued. 5]Oxygen supplementation should be given to hypoxic patients only[Hypoxia is 6]Hydration should be maintained to ensure normovolaemia and biochemical normality, and monitred closely. 7]Glucose should be measured and, if >11mmol/L, controlled using iv variable dose insulin. 8]Pyrexia should be lowered with treatment of the underlying cause, fan,paracetamol, and sponging. High temperatures are associated with poorer outcomes, but the causal nature of this association is unknown. 9]DVT prevention is done with ▪︎early mobilization. ▪︎Compression stockings should not be used and the timing of low-dose low-molecular-weight heparindepends on individual risk/benefit analysis. ▪︎Intermittent pneumaticcompression devices are safe and effective in early disabling stroke.▪︎Neurosurgical opinion should be sought for ~hydrocephalus, ~posterior fossa haemorrhages/large infarcts, ~large haemorrhages with midline shift, and ~large MCA infarcts with malignant MCA syndrome. 10]Seizures should be treated. Multidisciplinary acute inputProtocols should be developed for early management (<24h), including▪︎monitoring consciousness level, ▪︎assessing swallow (not gag), risk ▪︎assessment for pressure sores, ▪︎nutritional status, ▪︎cognitive impairment, ▪︎bowel and bladder care(avoiding catheterization if possible), and ▪︎moving and handling requirements.Early SALT assessment should be done for all with swallow or languagedifficulties; early mobilization with a PT having expertise in strokerehabilitation. |
|
|
HOW TO . . . Manage swallow after stroke▪︎Aspiration of saliva or food is one of the most common complications ofstroke and a major cause of morbidity and mortality. ▪︎Patients who are drowsy and dysarthric and those with dysphasia are most at risk of aspiration.▪︎Assessment ideally all patients should have their swallow assessedpromptly by a professional with specific stroke dysphagia training. ▪︎ If the patient is low risk and you wish to do a bed side assessment:•Sit the patient upright, and listen to the chest to establish baseline.•Ask the patient to cough, and note the strength and effectiveness.•Give the patient a teaspoon of water, and ask them not to swallow.•Look for leakage of water from the closed mouth.•Ask the patient to swallow the water•Check for prompt, coordinated swallow with elevation of the tracheal cartilage.Watch for signs of aspiration—coughing and spluttering. These may not occur for several minutes, so do not leave immediately•If no problems, then try a half-glass of water (slowly) or a small amount ofyogurt.•Ask the patient to say name/address, and listen for a ‘wet’ voice. Management If the patient is high risk, there are concerns during the bedside test, or if there are problems encountered during feeding:Make the patient ‘nil by mouth’•Provide alternative means of hydration at once with an iv infusion or an•NGT•Refer for early SALT assessment, to stratify the impairment and make aplan for safe oral intake, reviewing at regular intervals. ■Early nutrition by NGT improves outcomes and allows medication to be given, in addition to feeding. ▪︎Looped/bridle NGTs are much less likely to be dislodged but are harder to insert. ▪︎Consider insertion on the inattentive side if recurrent NGT removal is a problem. Medium/longer-term management ▪︎if the patient continues to have swallowing problems, then a PEG or a radiologically inserted gastrostomy (RIG) tube can be inserted. ▪︎Patients on long-term feeding should be reviewed regularly, as swallow can return many months later and oral feeding can gradually be introduced and sometimes tubes can be removed. ► It is important to understand that artificial feeding by any method does not prevent aspiration and, in some cases, can aggravate it. |
• • • • • Stroke units Definition Geographically defined unit staffed by a coordinated MDT with expertise in stroke. The gold standard is to admit stroke patients directly and continue care through to discharge—known as a comprehensive stroke unit. Some units deal with the emergency management (including thrombolysis and hemicraniectomy) and are termed Hyperacute Stroke Units (HASU). Others provide ongoing acute care or deal with the post-acute rehabilitation phase only. Regions may organize a ‘hub and spoke’ model where a single HASU admits all stroke patients for the first 72h of care, transferring rapidly to other local units. There is evidence for stroke-specific early supported discharge schemes reducing death or dependency as part of the overall pathway. Benefits Stroke units, when compared with general ward care, result in lower rates of death, dependency, and institutional care, without lengthening hospital stay. The number needed to treat in a stroke unit to prevent one death or dependency is 18. Rationale The majority of improvement seems to occur in the first 4 weeks, and the mechanism is unclear. Key components of stroke units include: Meticulous attention to physiological homeostasis Attention to prevention of complications (such as thromboembolic disease and pressure sores) Early mobilization Coordinated MDT care Interest, expertise, and motivation of staff |
HOW TO . . . Estimate prognosis after stroke After first-ever stroke, death occurs in 12% by a week, in 31% at 1 year, and in 60% at 5 years. Indicators of a poor prognosis include: 1.Impaired consciousness 2.Gaze preference 3.Dense weakness 4.Cardiac comorbidity 5.Urinary incontinence 6.Pupillary abnormalities The risk of recurrent stroke among survivors is 10–16% at 1 year, thereafter falling to about 4–5% per annum. The risk is higher with ↑ number of risk factors. Risk can also be estimated using the Bamford classification. Recovery is usually slow, and a clear time frame established early on in the disease with the patient and relatives is helpful. Recovery is most rapid in the first 3 months, and this tends to be ‘front-loaded’, so the most dramatic improvements occur in the early weeks. Recovery then tends to slow but may continue for years. ► Each patient is different—recovery may be delayed by infections, depression, etc., and this time frame should be a guide only. ▪︎The risk of not returning to independence varies with stroke type. Overall,about 20–30% of survivors are dependent at a year, and 40–50% are independent. |
|
|
ThrombolysisRationaleIn acute ischaemic stroke, an artery becomes occluded by a thrombus in situ or an embolus, and blood supply is compromised. Death of surrounding brain tissueresults in deficits in function associated with that part of the brain. Early recanalization of the vessel by lysing the thrombus may limit the extent of brain injury.RisksTreatment with thrombolysis leads to an excess in death due to intracranialhaemorrhage (a fivefold ↑, compared with placebo).BenefitsDespite early excess of deaths due to haemorrhage, treatment with thrombolysis leads to 44 fewer dead or dependent patients per 1000 treated with recombinanttPA (r-tPA) within 6h, and 126 fewer dead or dependent patients per 1000 treated with r-tPA within 3h.ImagingThis must be done prior to giving thrombolysis to exclude haemorrhage.Perfusion- and diffusion-weighted MR scans may give more information thanCT. All need to be interpreted by someone with the appropriate experience priorto thrombolysis. Plain axial CT remains the mainstay of imaging prior to thrombolysis.Use Thrombolysis is recommended in centres with sufficient expertise in stroke and with facilities to deal with complications. In these centres, treatment isconsidered in all patients with definite ischaemic stroke who present within 4.5h of the onset of symptoms.
Where this expertise does not exist, the service may still be provided via tele medicine links with stroke ‘hubs’.Exclusion criteriaThere is a very long list of exclusions, but the following are the most common reasons to withhold treatment:▪︎Previous haemorrhage or active bleeding site▪︎Seizure at onset ▪︎Impaired coagulation▪︎Caution with very severe stroke▪︎Uncontrolled hypertension (>180/110mmHg) |
Intra-arterial therapiesRationaleIschaemic stroke patients may present where there is a bleeding risk to systemic thrombolysis, and more localized therapy is safer.
This can either be localized intra arterial tPA or, more commonly, clot retrieval.
This has been proven to besuperior to iv thrombolysis alone and is now being offered in more and more centres. In the UK, this service is provided by interventional neuroradiologists,but elsewhere trained cardiologists or stroke physicians are taking the lead. |
Ongoing management Should involve all of the MDT. Dieticians-Calculate food and fluid requirements for each individual patient; -adapt the diet for specific needs (e.g. diabetic, weight loss); -develop regimens for NG or PEG feeds; advise on provision of modified diets for stages of swallow recovery(thickened, pureed, etc.); -review nutrition as recovery alters needs. Doctors -Diagnosis; manage medical complications ; establish therapies. Nurses Monitor patient continuously; assist with basic care (physiological and physical); ongoing bowel and bladder management; ongoing skin care; facilitate practice of skills acquired in therapy; promote functional independence; first point of call for relatives. Occupational therapist Optimize functional ability (usually begin with upper limb work, coordinating with the PT); specific assessments of certain tasks (washing and dressing,kitchen safety, occupational tasks, etc.) as recovery continues; adaptation to home environment by a series of home visits, with and without the patient, and the supply of aids (rails, bed levers, toilet raises, bath boards, etc.); provision of wheelchairs where needed. Pharmacists Review charts; promote safe prescribing. Physiotherapists Assess muscle tone, movement, and mobility; maximize functional independence by education and exercise; monitor respiratory function; initial bed mobility, then work on sitting balance, then transfers, and finally standing and stepping; help prevent complications such as shoulder pain, contractures,and immobility-associated problems (pressure sores, DVT/PE). Psychologists Assess the psychological impact of stroke on the patient and family; allow the patient to talk about the impact of the illness; monitor for depression and other mood disorder, highlighting the need for medication; document cognitive impairment; assist in retraining where neglect is prominent. Social workers Psychosocial assessment of the patient and family; support with financial matters(accessing pension, arranging power of attorney (POA), financing placement,etc.); advice and support for the patient and family on accommodation needs especially finding a care home placement,link to community services (carepackage, community rehabilitation , day centres, etc.) Speech and language therapists Assess swallow and establish plan for safe oral intake; reassess, and plan nutritional route during recovery; language screening (dysarthria, dysphasia, and dyspraxia) with intervention to improve deficits. |
|
|
HOW TO . . . Protect your patient from another stroke Ensure that the following are addressed ▪︎Lifestyle issues—smoking, diet, and exercise ▪︎Antiplatelet therapy:Current advice is aspirin 300mg for 2 weeks, followed by clopidogrel 75mg there after. Adding clopidogrel to aspirin ↑ antiplatelet activity buthas been shown to ↑ the risk of cerebral haemorrhage, but may beconsidered for monotherapy failure ▪︎Lower BP—choice of agent debated. The important thing is just to lowerthe BP. If there are no contraindications, lowering BP per se is likely to be beneficial, but aim for <130/85 ▪︎Lower cholesterol—the Heart Protection Study clarified benefit post-stroke,including in older patients. The cut-off for treatment in t his trial was 3.5 ▪︎Anticoagulation for AF . In infarction,likely to be safe to start warfarin/DOAC after 2 weeks. With haemorrhage,judge each case individually. ▪︎Haemorrhage due to thrombolysis is not a reason to withhold anticoagulation. In 1° haemorrhage, anticoagulationmay never be justified. ▪︎Carotid endarterectomy—>70% symptomatic stenosis carries a stroke risk of about 15% per year and is an indication for endarterectomy where there is good recovery and the patient is fit for surgery (which can be done with local anaesthesia). Perform early for greater benefit. |
Complications ▪︎Contractures-Longer-term complication ▪︎Faecal incontinence. May be due to immobility, cognitive problems, or neurological impairment. ~Regulate bowel habit, where possible, with high-fibre diet and good fluid intake,and toilet regularly. If all else fails, then deliberately constipating the patient with codeine and using regular enemas can work. ▪︎Infection-Commonly chest or urine. Think of it early if a patient becomes drowsy or confused or appears to deteriorate neurologically. ~Prompt screening for sepsis and treatment with antibiotics, oxygen, and hydration are indicated in the majority of patients in the acute phase (stroke outcome very unclear initially) but may be withheld in a more established stroke where the prognosis can be assessed more confidently as dismal (decision made with the family).
▪︎Muscle spasm ︎Very common on the affected side. Arthritic joints are exacerbated by spasm,and antispasmodics may need to be used, alongside analgesia, for effective pain relief. Try baclofen or tizanidine. ↑ the dose slowly after a few days, if needed,but watch out for drowsiness and loss of tone in the affected side that can hinder therapy. Resistant spasm may benefit from a course of botulinum toxin injections.
▪︎Pain Commonly shoulder pain in a paralysed arm. Usually multifactorial, e.g. joint subluxation (treat with PT to strengthen muscles and arm support) interacting with muscle spasm and shoulder arthritis. Central post-stroke pain tends to affect all of the affected side and can be treated with neuromodulators (e.g. gabapentin)
Pressure sores Are avoidable with good care; however, pressure damage may occur beforeadmission but manifest only later (see ‘Pressure sores’, pp. 504–505). |
Psychological problems▪︎Low mood is extremely common post-stroke (at 4 months, 25% will bedepressed, and over half of these remain depressed at a year). ▪︎This is unrelated to the stroke type but is associated with a worse outcome (perhaps because of lowermotivation in therapy). ▪︎ It should be actively sought (the screening question ‘Do you think you are depressed?’ is quick and effective; it may also be noticed by nurses, therapists, or family; tools such as the GDS can also be used but may be confounded by dysphasia). Treatment is with psychosocial support and antidepressants (e.g. citalopram). Anxiety is also very common and often responds to explanation and empowerment.Post-stroke cognitive impairment is common (up to 80% prevalence).Delirium associated with acute stroke may improve, but cognitive deficits are often permanent (either fixed deficits or more global functioning). Non-dominant hemisphere syndromes Usually MCA strokes of the right hemisphere where language is crudely preserved, but there are issues with fluency, semantics, and interpretation of language (a kind of acquired neglect dyslexia). Deficits in assimilating information to make sense of reality can cause complex issues with insight and empathy and disease denial. Thromboembolism Very common post-stroke. Ensure well hydrated. Mobilize early. Intermittent pneumatic compression devices should be used in the acute phase. Low-dose low-molecular-weight heparin is used after the acute stage (when risk of haemorrhage into the brain diminishes). Have a low threshold for investigating a leg that becomes swollen or painful. Ensure careful VTE risk assessment is documented at each stage. |
|
|
HOW TO Manage urinary incontinence after stroke▪︎This is very common, more so after severe stroke. It does, however, improve over time, and a flexible approach is required to ensure that a patient does not get catheterized and remains so.▪︎Initially, try to manage with pads and regular toileting▪︎If the skin starts to break down, or if the burden on carers is heavy, then acatheter can be inserted for a limited time span▪︎Once mobility improves, try removing the catheter—ensure this is seen by all as a positive and exciting step back towards independence, as it can cause considerable anxiety▪︎If this fails, check for, and treat, UTI and then try again.▪︎If this fails, then replace the catheter and use bladder-stabilizing agents forabout 2 weeks (e.g. tolterodine) before removing it again.▪︎If all this fails, consider sheath catheter devices or bottles (with non-return valves for use in bed) in men; commodes next to the bed for women.► The need for a permanent catheter post-stroke should be reviewedregularly, as the condition is likely to improve. |
Longer-term issues ▪︎Return to the community Best coordinated by the stroke MDT. ▪︎Early supported discharge may be useful if the patient can transfer and there is a specialist community stroke team available.Later discharges are planned by the team, usually after careful assessment ofneeds (home alterations, care packages, etc.). The GP should be alerted tocontinue medical monitoring, in particular optimizing 2° prevention. Communityteams (district nurses, community rehabilitation teams, home carers, etc.) shouldbe aware of the patient’s needs (continence, diabetic monitoring, ongoingtherapy needs, etc.) and ideally be involved in the discharge planning. Thepatient and family should have adequate information and training, as well as acontact point in case of problems (stroke coordinators often take this role).Voluntary agencies (e.g. the Stroke Association) are helpful, and the patientshould be informed about them.Driving regulations with cerebrovascular diseaseTIA/stroke with full neurological recovery—1 month off drivingRecurrent TIAs—3 months off driving following last TIAStroke with residual neurological deficit after 1 month—the patient mustnotify the DVLA, and the decision is made on a case-by-case basis, withevidence from medical reportsHemianopia, inattention, and impaired cognition are definite markers of lackof fitness to drive (can be decided by a GP or hospital physician). Subtlevisual deficits need formal perimetry testingDysphasia is harder—cognitive state is difficult to assess, and associatedimpairments (such as problems reading street signs or misinterpreting theenvironment) are not readily identified out of contextPure limb weakness can often be safely managed with car adaptation. If thereare any doubts and the patient wishes to drive, then they should be seen in adriving assessment centre (the patient will have to pay)Stroke with seizure—this is treated as a provoked seizure. May be <1 yearban, depending on circumstances.
Some follow-up should be offered to all stroke survivors. The intensity andduration of inpatient care can contrast sharply with home. The realities of livingwith disability begin to sink in, and many questions and anxieties arise. Evenminor strokes or TIAs require a further point of contact, as they will have beencommitted to lifelong medication and will need monitoring of risk factors. Inaddition, stroke recovery continues (albeit at a slower pace) for years andmanagement plans made at discharge may need to be adapted. |
Checklist for follow-up(Usually 1–4 months after discharge)1.Secondary preventionCheck drugs, BP, diabetic control, and cardiac rhythm.2.ContinenceAre there continence problems?If a catheter is in situ, has mobility improved to a point at which trialremoval can be done?If the patient was discharged on bladder-stabilizing drugs and has remained continent, can these be tailed off?3.NutritionIs nutrition adequate? (If not, refer to a dietician.)If a PEG tube is in place, is it still required?Does the patient warrant another assessment of swallowing (by SALT) toallow oral nutrition to begin?4.Communication and speechAre there problems still?Is there a need for a SALT review?5.MoodIs the patient depressed?Do they need referral to a psychologist or (rarely) a psychiatrist?If discharged on an antidepressant, can it be discontinued?6.Physical progressIs there ongoing physical therapy?If not, is there continued improvement? If there has been deterioration, thenrefer back for assessment for further therapy .(Royal College of Physicians’guidelines)7.ContracturesAre there any contractures developing? (If so, refer to PT.)8.Muscle spasmsHave these developed or lessened since discharge?Review need for antispasmodic medication titrate down if no longer required9.PainCommonly in shoulder, or post-stroke pain.Has this developed or lessened?Review need for medications10.Daily livingAre there any issues in managing day to day?Is all the necessary equipment in place? (And is it still needed, e.g. acommode can be returned when the patient is able to mobilize to the toiletalone?)Is there anything that they would like to be able to do that they cannot? (e.g.read a book, take a bath)Would a further review by a therapist be helpful?Do they wish to drive? (see ‘Longer-term issues’, p. 196)11.SupportAre they in contact with a community stroke coordinator (if available)?Are they aware of voluntary organizations? |
|
|
•••••Transient ischaemic attack clinicsRapid outpatient assessment of TIA and minor stroke, to establish diagnosis,commence 2° prevention, and lower risk of subsequent event.How fast?This depends on how high a risk the patient is. Use the ABCD2 score (see Table8.3), and if the score is ≥4, the patient should be seen within 24h—in someservices, these patients are admitted to hospital, but larger centres may provide7-day/week outpatient services. Patients who have recurrent symptoms within aweek or those in AF on anticoagulation should also be seen urgently. Those atlower risk should be seen within 7 days.Currently, early antiplatelet agents, antihypertensives, and statin use have beenshown to substantially reduce the chances of stroke at 1 week. Anticoagulationfor patients with AF and early endarterectomy are also known to be beneficialfor a subset of patients.FunctionConfirm diagnosis—very variable, but up to half of referrals to a TIA clinicare non-stroke. Main alternatives are migraine, epilepsy, cardiacdysrhythmias, orthostatic hypotension, or rarely brain tumourArrange investigations—to aid diagnosis (e.g. CT brain) or investigate riskfactors (e.g. FBC, ESR, HbA1c, cholesterol, ECG, carotid Doppler, 24h tape,and echocardiogram)Modify risk factors—(see ‘HOW TO . . . Protect your patient from anotherstroke’, p. 193) set targets for BP and glucose control; advise aboutantiplatelet agents (there is significant regional variation in the therapychosen, including aspirin with modified-release dipyridamole, or aspirin withclopidogrel for 1 month and then clopidogrel alone) and anticoagulation inAF; advise about statin use; refer for carotid endarterectomy urgently; adviseabout smoking cessationEducation—of patients, relatives, and 1° care doctors. Discuss stroke diseaseand its modification, time frame for recovery, psychological aspects of stroke,and driving restrictionsStructureVaries enormously. Ideally would have:Rapid referral protocolStroke specialist nurse—can take history, including standardized risk factoranalysis, measure BP, provide education (leaflets, individual action plans), andcoordinate investigations and follow-up. Role can be extended into thecommunity—point of access for patientsTime for explanation—many patients will feel overwhelmed by the amount ofinformation they are being given. The specialist nurse can be very helpful inclarifying things, and information leaflets allow the information to be revisitedat home. There are often several new tablets, or even suggesting surgery for apatient who feels well. Comprehension is vital for concordanceRapid access to investigations—particularly carotid Doppler and CT scanning.Many clinics run a ‘one-stop’ service where all assessments, investigations,and conclusions are completed at a single visitPrompt communication to GP—advice about risk reduction must be relayedpromptly to the GP for maximum benefit. Ideally same day. The ABCD2 score for TIAScore A Age ≥60 -1 B BP ≥140/90mmHg -2 C Clinical features Unilateral weakness -2 Speech disturbance without weakness -1 D1 Duration ≥60min- 2 10–59min- 1 <10min- 0 D2 Diabetes Diabetes -1 ABCD2score—risk of stroke at 2 days: 0–3—1%; 4–5—4%; 6–7—8%. |
Q |
|
|
|
10 CardiovascularThe ageing cardiovascular system Chest pain Stable angina HOW TO Rationalize antianginals in older patients Acute coronary syndromes Myocardial infarction Hypertension Hypertension: treatment HOW TO Use antihypertensives in a patient with comorbid conditions Arrhythmia: presentation Arrhythmias: management Atrial fibrillation Atrial fibrillation: rate/rhythm control Atrial fibrillation: stroke prevention HOW TO Discuss warfarin for AF HOW TO Use digoxin Bradycardia and conduction disorders Common arrhythmias and conduction abnormalities Heart failure: assessmentHOW TO Investigate a patient with suspected heart failure Acute heart failure Chronic heart failure Dilemmas in heart failure HOW TO Manage the heart failure see-saw Heart failure with preserved left ventricular function Valvular heart disease Peripheral oedema Preventing venous thromboembolism in an older person Peripheral vascular disease HOW TO Measure ABPI Gangrene in peripheral vascular disease Vascular secondary prevention |
Q |
|
|
|
The ageing cardiovascular systemAdvanced age is the most potent risk factor for cardiovascular disease. Thisvulnerability stems from the following .1.︎Cumulative exposure to risk factors (extrinsic ageing)This was looked at in more detail in the WHO MONICA project , which looked at coronary risk factors in 38populations across 21 countries for a decade.This showed that not all risk factors ↑ with age—♂ smoking ↓ (although not♀), and cholesterol showed a small downwards trend.
Body mass index (BMI)↑ for both sexesThus, it is not inevitable and can be modified by behaviour, e.g. athletes who continue to exercise into older age may show fewer signs of cardiovascular ageing than an unfit younger person. 2.Disease acquisitionOften occultAffected by risk factor accumulation 3.Intrinsic ageing The relative contributions of these factors to the clinical picture are unclear.Table 10.1 addresses the three important questions:What are the common changes with age?How does that impact on function?What are the clinical implications? |
Table 10.1 An overview of age-related changes and their effectsAge-related change- Impact on function- Clinical implications1.Proximal arteries become thicker,dilated, elongated, and less elastic--》•Systolic pressure peak ↑, causinghypertension •↑ peripheral vascular resistance(variable) •Intimal thickening probablypredisposes to atheroma •Systolic hypertension common inolder patients □CXR may show enlarged aorticknuckle ‘unfolding’ of the aorta 2.Fibrosis and fat infiltration of thesinoatrial (SA) node and conductingsystem--》Slower conduction from the SA node and through the conducting system.•First-degree heart block and bundlebranch block common•Left axis deviation more frequentMore vulnerable to clinicallysignificant bradyarrhythmias. 3.Maximum heart ratefalls by 10% at rest and 25% during stress--》↓ capacity for cardiac output—largely compensated for at rest butlimits response to stress.▪︎Less able to mount a tachycardia, soless reliable sign of acute illness4.LV wall thickens as myocyte size ↑--》↑ cardiac filling pressures and allows compensation for drop in heart rateA degree of cardiac enlargementseen on CXR is normal. Worse with hypertension, so always check BP and treat as needed5.Left atrial size ↑ due to alterations in cardiac filling Predisposes to AF6.Myocardial contractility impairedat high demandContractility preserved at lowstimulation, but with stress cannot ↑.Means that (along with heart ratefactors) cardiac output cannot be ↑↓ cardiac reserve to stress—maybecome haemodynamicallycompromised in response to acuteillness earlier than younger patients 7.↑ circulating catecholamines withdownregulated receptors (especiallyβ-adrenergic)--》Impairs ability to mount a stressresponseAs above in this table—↓ cardiac reserve to stress. More prone to heart failure. 8.Impaired oxygen consumption on exercise Varies considerably between individual older patients—unchanged in those used to exercise, up to 60% reduction in unfit Contributes to reduced cardiovascular reserve to stress |
|
|
|
Chest pain A common complaint in all settings. May be 1° symptom (presenting to GPs and general medical take) or mentioned only in response to direct questioning. Also occurs during inpatient stays for other reasons. There are very many causes, the majority of which become more common with age. Many are benign, but some are serious and even life-threatening, so a thorough and sensible approach is needed. Common conditions not to be missed include: 1.cardiac pain; 2.pleuritic pain due to pulmonary infarction or infection; 3.peptic pain (including bleeding ulcers); and 4.pain from dissecting aortic aneurysm and 5.pneumothorax. (especially in COPD).
Other possibilities include: ▪︎muscular pain (e.g. after unaccustomed exertion); ▪︎costochondritis (local tenderness at sternal joint); ▪︎pain from injury (e.g. after a fall); ▪︎referred pain from the back and neck (e.g. osteoarthritis); and ▪︎referred pain from the abdomen.
Differentiating these depends on accurate history taking and careful examination, both of which can be more of a challenge in older patients Presentation may be atypical, and the patient may have many other problems, so teasing out which the important symptoms are can be difficult (experience improves the ability to ‘feel’ your way around the history). ► It may be the last symptom mentioned in a long list; however, mention of chest pain should always trigger a careful assessment.
History Is this a new symptom? (may suffer from chronic angina) If not, is it any different from the usual pain? (intensity, pattern) What is the nature of the pain? (pleuritic, heavy, tight. This is often hard to do,and hand gestures can help—a clenched fist for a heavy pain, a stabbing action for a sharp pleuritic pain) Where is it located? (including radiation) How acute is the onset and what is the duration? Are there any associated symptoms?Patients with cognitive impairment can be particularly difficult to assess, but allowing free conversation may reveal symptoms, followed by closed questions that may prompt appropriate answers. Family members may have noted signs or symptoms and are an invaluable aid to assessment, e.g. clutching the chest after walking. Remember that cardiac symptoms may differ for each patient and they will describe the pain in their own terms. Many older adults with ischaemia will deny ‘pain’ and instead describe ‘discomfort’. Some will just experience weakness,dyspnoea, or nausea.
Examination How does the patient look? A sweaty, clammy patient needs urgent and exhaustive assessment, whereas a patient drinking tea and chatting is less likely to have a devastating condition. What are the basic observations? Signs of shock alert to a serious condition—IHD, pulmonary infarction,dissection, sepsis, blood loss—but remember these may be late signs and are less useful in older patients. The patient may usually be hypertensive, so a BP of 120/80 may be very low for them; they may be on a β-blocker, so unable to mount a tachycardia, etc. Temperature may be raised in sepsis Low oxygen saturation always needs explaining (unless chronic) and may indicate an intra pulmonary problem Is the jugular venous pressure (JVP) elevated? (heart failure)
Look at the chest wall for shingles, bruising, and localized tenderness.
Different BPs in the arms may indicate dissection (but may also occur with atheroma)
Listen to the heart—are there any new murmurs (dissection or infarction) or arub (pericarditis)? Listen to the lungs-is there consolidation (sepsis) or a rub (consolidation or infarction)? Look at the legs—is there any clinical DVT?
Investigations-Some tests can be less useful in older patients and should be individually tailored to the patient. Sending off every single test on all patients with chest pain will only lead to confusion. ECG—should be done on the majority of patients with chest pain. Remember the baseline ECG may well be abnormal in an elderly person, and comparison with old traces is extremely useful. If your patient has a very abnormal ECG(e.g. left bundle branch block (LBBB)), it is useful to give them a copy to carry with them.
CXR—looking for lung abnormalities and widening of the mediastinum. Remember that the aorta often ‘unfolds’, so a careful look at the contours of the aortic arch and/or comparison with old films is needed to assess possible dissection. Remember that a patient can look fairly well in the early stages of aortic dissection. Blood tests—basic haematology, biochemistry, and inflammatory markers are often useful. Remember that in acute blood loss, the Hb level may not drop immediately and that an elderly septic patient may take a day or two to develop an elevated white cell count and CRP. Troponin—useful in a patient with suspected cardiac chest pain (for risk stratification). It is NOT a useful test if you do not think this is cardiac pain—there are many false positives that will only cause confusion. D-dimer—only useful if negative in cases of suspected thromboembolism.There are a huge number of causes of a positive D-dimer (including old age itself); a positive result does not imply the diagnosis of PE Further tests (e.g. CT thorax for suspected dissection, exercise testing for angina, lung perfusion scans for thromboembolism, etc.) depend on clinical factors.
► Always attempt to explain a chest pain—both for the patient and future clinicians. A ‘diagnosis’ of non-cardiac chest pain is rarely helpful. |
Treatment principles Life style modifications is the best basic. But hard to achieve target levels though good control is lot more beneficial than for the young. Avoid precipitants. Yet trial of medications should be done despite risks and stop if problems develop. Best drugs are there their that help avoid problems. Avoid precipitants. Revascularization as for the young. Palliation if cannot be treated. . Yet trial of medications should be done despite risks and stop if problems develop. Best drugs are there their that help avoid problems.
Treatment principles Life style modifications is the best basic.But hard to achieve target levels though good control is lot more beneficial than for the young.Avoid precipitants.Yet trial of medications should be done despite risks and stop if problems develop.Best drugs are there their that help avoid problems. Stable anginaCoronary artery disease is clinically evident in 20% of those >80 years.Management is often sub-optimal. It is unacceptable to leave a patient withsymptoms, however frail, until all available options have been looked at, and ithas been proven (by trying it) that a certain treatment cannot be tolerated. A stepwise, slow introduction of tablets allows insight into adverse effects and mayrequire multiple clinic visits. Symptom diaries can assist with this process.Stable anginaRisk factor reductionCholesterol and BP less likely to be lowered in older patients, but the riskreduction is equal to, if not greater than, for younger subjectsDiabetic control is less likely to be tight, in part due to justifiable concernsabout the dangers of hypoglycaemiaLifestyle advice (exercise, smoking, and diet) should be given Aggravating conditions More common in older people. Correcting such conditions, where possible, often more effective than increasing antianginal meds.Can be divided into:↓ myocardial supply, e.g. anaemia, blood loss, hypoxia, fever, hypotension↑ myocardial demand, e.g. tachycardia, fever, valvular heart disease, heart failure, hypertension, hyperthyroidismMedicationUnder-utilized, particularly aspirin (concerns about bleeding) and β-blockade,but there is evidence that they are both equally useful in reducing riskA trial and error approach to treatment is needed—add one or two treatmentsat a time to minimize the risk of side effects (most commonly orthostatichypotension), and stop if there are problems, trying something else instead. Start on low doses, and titrate upwards (e.g. bisoprolol 2.5mg) Long-acting agents (e.g. diltiazem modified-release) reduce compliance problems. Nicorandil (10–20mg bd) is often better tolerated than other antianginals in this age group.Choice of medication should be pragmatic-if a patient has a bradycardia, for example, a negatively chronotropic drug is usually inappropriate (consider using amlodipine 5–10mg).
If a patient has heart failure, a cardioselective β-blocker (e.g. carvedilol, metoprolol, bisoprolol) is a better choice than a fluid-retaining calcium channel blocker. GTN can cause considerable problems with hypotension, and instruction on correct use is essential. Tablets can be spat out once the pain starts to settle, so(in theory) the dose can be titrated to symptoms. In practice, the spray is often easier to use. It should be used sitting down, if possible, and prophylactically before significant exertion. Revascularization Should be considered (ideally after risk stratification by stress testing), as for younger patients, if symptoms persist despite maximal medical therapy . Palliation Consider if diffuse disease, not amenable to revascularization with ongoing symptoms (e.g. home oxygen therapy, opiates to allow sleep), particularly if angina occurs in the context of heart failure syndrome. |
Titrating down medicatons to optimum level. A level at which pulse,BP,symptoms and disease modification can be achieved. HOW TO . . . Rationalize antianginals in older patientsWhen?With advancing age and frailty, mobility may reduce and so angina symptoms may become less frequent, or even stopIt is not uncommon to find an octogenarian on four antianginals, who has not had angina for many yearsUse a low pulse or BP reading as a trigger to review the medication.Do not hesitate to rationalize this there are many pitfalls from polypharmacy, and requirements will change with timeHow?If cognitive impairment, confirm with carers that they are genuinely asymptomatic from angina. Review observations if bradycardic, stop β-blockade first; if hypotensive,start with symptomatic medications first (e.g. nitrates) before those with disease-modifying properties. Select one drug to modify, then reduce in a stepwise fashion (e.g.reducing dose and/or frequency). Patients and family may be resistant to medication changes after years of healthcare professionals emphasizing the importance of their tablets. Some are frightened of a recurrence of disabling symptoms, so explain to the patient, carers, and GP what you are doing. Clarify the contingency plan: ‘This is a trial, and if it does not suit you, you may get angina pains again. If that happens, please just start taking your old doses again’Ensure there is backup if there are concerns (e.g. telephone number) Review-Set a date for review of impact. Assess whether there have been any symptoms, and reassess the pulse and BP.If all is well, continue with careful reduction in medication. Goal-Aim to use as few medications as possible, while maintaining control of symptoms.If the BP allows, continue those with disease-modifying properties (β-blockade, ACE inhibitors), but in frail older people, it is more important to avoid orthostatic hypotension and falls. • Once the medication has been titrated down to an optimum level (balancing pulse, BP, symptoms, and disease modification), communicate the final list to others, especially the GP. |
|
|
Acute coronary syndromes Coronary heart disease (CHD) incidence rises with ↑ age. An acute coronarysyndrome describes a scenario in which the myocardial cells are not receivingenough blood and oxygen to meet their demandsztnn205s. This can be due to an acutecoronary event (type 1 ischaemia) or ↑ demand which outstrips supply (type 2ischaemia).‘Troponinitis’With ↑ sensitivity of tests for myocardial damage (e.g. troponin) and ↑ (ofteninappropriate) screening, older people are being increasingly diagnosed as ACScoinciding with other illness, rather than as 1° pathology. In these cases, ↑demand, rather than coronary artery disease, is driving the process and treatmentshould primarily aim at optimizing management of the other conditions (e.g.sepsis, dehydration, etc.) (see ‘Aggravating conditions’, p. 258).►► In these cases, the ACS treatment bundle (e.g. anticoagulant, antiplatelet,β-blockade, etc.) is often contraindicated and risks detracting clinicians from theunderlying pathology.ACS has a range of syndromes from unstable angina to non-ST elevation MI(NSTEMI) to ST elevation MI (STEMI). Management in general is as foryounger patients, but there are some points relating to older patients in particular.Atypical presentationMore likely to present with atypical or vague symptoms (e.g. intensedyspnoea, syncope, weakness, abdominal pain)Symptoms may be obscured by comorbidityECG changes may not be present in up to a quarter of acute MI, with the fulldiagnostic triad (chest pain, ECG changes, and biochemical changes) presentin under a third of those >85 yearsECG may be difficult to interpret because of pre-existing abnormalities(LBBB, pacing)Vital signs or symptoms may be obscured by medication (β-blockade, painmedication)Different pathologyMore pre-existing coronary artery disease with more multivessel diseaseNSTEMI more likely than an STEMIMore likely to develop heart failure, AV block, AF, and cardiogenic shock |
after a coronary event
Later presentation↑ prevalence of angina, so less alarmed by chest painsMay modify lifestyle to avoid symptoms (if climbing a hill gives them chestpain, they may just stop doing it)↑ occurrence of ‘silent ischaemia’ (especially in people with diabetes)↑ social/attitudinal factors (‘I didn’t want to bother the doctor’)A third of patients >65 with MI will present later than 6h after symptom onsetIncreased comorbidityMaking diagnosis difficult (e.g. a patient with COPD who has exertionalbreathlessness) and therapy less well tolerated (e.g. β-blockers with peripheralvascular disease)Also as comorbidities add up, so frailty ↑ and medications are generally lesswell toleratedOlder patients with ACS have a higher inpatient mortality so should beprioritized for specialist monitoring where there are limited resources; however,it is known that they are:Less likely to receive aggressive acute therapy (e.g. less angiography andangioplasty, and coronary artery bypass grafting (CABG), and maximalmedical treatment)Less likely to have full 2° prevention measures implementedManagement of the older cardiac patient is therefore more difficult and morelikely to result in death than in younger patients. Sometimes there are goodreasons for withholding therapy (e.g. patients presenting later are less ofteneligible for thrombolysis, side effects may restrict 2° prevention), but often thejustification is less robust. Lack of evidence in older people does not mean thatthere is no benefit—rather that it has not been proven.Common sense dictates when to use an aggressive approach, considering thepatient as a whole, including:Patient preference where possibleComorbidities (alter risk profile)Current medicationFrailty and likely life expectancyApparent biological age, rather than chronological ageThere are many well-defined treatment algorithms, and older patients should beincluded at every step unless there are good reasons not to. If you plan to exclude a patient from treatment, you should clearly document your rationale.There is some evidence that the excess IHD mortality for older people isdiminishing (see below), probably due to improved risk stratification and theapplication of more evidence-based interventions to this group. |
••••••Revascularization proceduresIncludes percutaneous coronary angiography and intervention (PCI) andCABG.When?Used when stable symptoms persist despite maximal medical therapy, whenunstable symptoms fail to settle, or for acute MI (1° PCI)Risk-stratify with exercise testing and troponin measurements. Olderpatients may be unable to exercise, but consider bicycle exercise, stressechocardiography, or an isotope myocardial perfusion scan to look forevidence of reversible ischaemiaWhat are the risks?PCI—higher risk of death, renal failure, and infarction in the elderly. Age isan independent predictor of ↑ complication, but so too are diabetes, heartfailure, and chronic renal impairment, all of which are more common inolder patientsCABG—↑ early mortality and stroke in older patientsWhat are the benefits?PCI—may be the only way to control intrusive symptoms in stable angina,with better quality of life but no change in mortality. 1° management ofSTEMI, rarely for NSTEMI that is not responding to other treatments.Variable evidence from studies—all agree ↑ early complications, butlonger-term benefits in older patients are reported as equivalent or evenbetterCABG—probably better with triple vessel disease, poor exercise tolerance,poor LV function, and diabetes. Generally well tolerated in the elderly, withsimilar long-term improvements in symptoms and quality of life to youngerpatients. New minimally invasive techniques that do not require bypass arelikely to reduce the early complications without impairing outcomeOverall recommendationsConsider all patients who fail medical treatment for revascularizationprocedures, regardless of age. The early complication rate is higher in olderpatients, but the eventual benefit is equal to, if not better than, for youngerpatients.Approach a cardiologist with a record of treating older patients. Crucial to include the patient in the decision, with a frank and individualized discussionabout risks and benefits. |
|
|
Q |
Q |
|
|
|
•Myocardial infarction This includes both NSTEMI and STEMI. Around two-thirds of all MIs occur in patients >65 and a third in patients >75. Overall, the incidence of MI has ↓, but this is not the case for older patients. Despite this, evidence regarding optimal management is lacking, as older patients tend to be under represented in clinical trials.Consult NICE guidelines (which do not suggest differences in approach for most areas for older people). While evidence is lacking in older patients, it ismore reasonable to extrapolate from a younger population than to deny treatment. This approach must, of course, be tempered with common sense and individually tailored decision-making. Prior to discharge Medication May include 1.︎aspirin ± clopidogrel, 2.statin, 3.β-blockade, 4.ACE inhibitor, and 5.GTN spray Use medications, unless they are contraindicated or the risk > benefit. Be alert for common side effects (more likely with advancing age), e.g.orthostatic hypotension, gastrointestinal bleeding
︎Education ▪︎Diet, nutrition, ▪︎cholesterol control ▪︎Smoking cessation ▪︎Activity restrictions and graded reintroduction ▪︎Recurrent symptoms and what to do ▪︎Routine follow-up after stents |
Cardiac rehabilitation Used after ACS and multiple presentations of congestive heart failure. Involves structured exercise programme. Proven to improve exercise tolerance and ↓ readmission. Underused for older cardiac patients—less referral and sometimes there are upper age limits in place. Benefit seen in older patients is equivalent to that in younger patients,although they start from a less fit baseline. Older people adhere well to programmes and seem to suffer no complications. Some adaptations are needed (more time to warm up and cool down, longer breaks, avoidance of high-impact activity, lower intensity for a longer time). Benefits include ▪︎improved fitness, ▪︎↑ bone mineral density, ▪︎improved mood ▪︎and fewer falls, as well as ▪︎ improved cardiovascular fitness.
MI treatment evidence for older people Therapy -Evidence 1.Aspirin - Equivalent risk reduction in elderly population. 2.1° PCI -This is usually the strategy of choice where presentation is early. It reduces death and recurrent MI, compared with a conservative strategy. Procedural risks are greater with frail patients, and the role for PCI in those >80 with multiple comorbidities has not been well studied. Resource limitations may impact.
3.Thrombolysis -Not indicated for unstable angina or NSTEMI. Has a role in STEMI, regardless of age. Must be administered <12h after onset (ideally <3h) and is used where PCI is not available or feasible. ↑ risk of complications, e.g. cerebral bleeding (but can predict those at higher risk if hypertensive, low body weight, previous stroke, or on warfarin)
Contradictory evidence regarding mortality early large RCTs suggest ↑ absolute risk reduction of mortality in elderly patients but had selected probably fitter patients and do not include the very old. Observational trials of actual practice suggest equivalent benefit in older patients, or possibly even a survival disadvantage. Probable equivalent proportional mortality reduction, so absolute reduction greatest in the elderly who have the highest mortality. Overall, the consensus is that thrombolysis can be used in older patients, with rare exceptions for the very frail or where individual risk seems to outweigh benefit.
4.Low-molecular-weight heparin Full dose is effective in NSTEMI, and possibly more effective in older patients.
5.GlycoproteinIIb/IIIa inhibitors Rarely used (e.g. for NSTEMI while awaiting reperfusion) Older people have ↑ risk of bleeding but have equal benefit overall. |
Q |
|
|
Hypertension Hypertension is an important risk factor for vascular disease. Historically under-diagnosed and under treated, especially in older patients, although improved in the UK when GPs had national guidelines/targets and financial incentives to treat. The incidence of hypertension overall rises with age, reaching a prevalence of 60–80% beyond 65. After this age, systolic BP (SBP) rises linearly, while diastolic BP (DBP) falls, leading to widening of the pulse pressure and relative frequency of isolated systolic hypertension. Isolated systolic hypertension reflects reduced arterial compliance, which is disease-related and not part of ‘normal’ ageing per se. It is treated in the same way as normal hypertension. Hypertension is an independent risk factor for stroke, IHD, peripheral vascular disease, congestive heart failure, renal failure, and dementia in all age groups,but in older patients, it is SBP and widened pulse pressure that are the strongest predictors of adverse cardiovascular outcome.
Assessment Ask about symptoms (including hypotensive), comorbidity, and smoking. Measure with a well-maintained, calibrated device, with an appropriate-sized cuff: Check supine and standing BP (orthostatic hypotension can cause symptoms when treatment initiated) Take at least two measurements in a single consultation and use the lowest reading Ambulatory or home BP measurement is now considered essential for confirming a new diagnosis in almost all cases. Ambulatory or home BP thresholds and goals are 5–10mmHg below clinic readings. Most cognitively intact older patients tolerate this well, and having a BP cuff at home can be useful to monitor for adverse effects during treatment Examine for evidence of target organ damage (stroke, dementia, carotid bruits,cardiac enlargement, IHD, peripheral vascular disease, renal disease, retinal changes) Investigations to look at target organs (urinalysis, blood urea, and electrolytes,ECG) and for risk factor analysis (glucose, lipids) Consider 2°hypertension-rare in older patients, but consider if drug-resistant,severe hypertension, or with suggestive examination or laboratory findings. Consider medications (NSAIDs, steroids, SSRIs), Cushing’s syndrome, sleep apnoea, 1° aldosteronism, phaeochromocytoma, or renal artery stenosis Treatment thresholds NICE suggests treating ▪︎<80 years who have stage 1 hypertension (BP >140/90in clinic (or 135/85 at home) if they also have other risk factors, e.g. diabetes mellitus, proteinuria, CKD, stroke, etc. ▪︎For patients aged >80, only treat if stage 2 hypertension (BP >160/100 in clinic or 150/95 at home) ▪︎Lower thresholds (>140mmHg) for diagnosis and treatment exist for very high-risk patients, e.g. CKD and diabetes. Treatment goals Target ranges are similar to treatment thresholds, i.e. 140/90 under age 80 and 160/100 for over age 80 In older patients, the most common limit to treatment is symptomatic postural hypotension; consider using alternative agents which may cause less orthostatic drop (e.g. ARBs, calcium channel blockers) There are even fewer data for very elderly (>90) people, and a pragmatic approach based on apparent biological age is appropriate. |
.Hypertension: treatment Similar approach to that used in younger patients, but it is important to bear the following in mind: Side effects are more common and more debilitating in older patients (due to more sluggish baroreceptors and reduced cerebral autoregulation) There is a greater risk of drug interactions, as older patients are more often victims of polypharmacy. Comorbidity is common and should direct the choice of antihypertensive agents. Hypertension should be seen as a risk factor, and the decision to treat should be weighed along with other risk factors. In a very frail older person with a limited life expectancy, the side effects might far outweigh any future benefits from risk factor modification. This, however, should be an active decision reached, if possible, with the patient, and not a simple omission. Begin with lower doses and titrate up slowly (‘start low and go slow’) to minimize adverse reactions. It is better to be on something at a low dose than nothing at all. Non-pharmacological measures Life style modifications are as important and effective in reducing BP in older patients as in the young. Salt restriction, weight reduction, and regular exercise are particularly effective. Moderate or absent alcohol intake is advised. Smoking cessation and ↓ saturated fat intake help with overall risk reduction. Choice of medication Many large trials have compared the different classes of antihypertensive, but with little consistency in results. Overall, it seems that it is lowering the BP perse that is the important factor, and this benefit continues up until at least 84 years(possibly beyond—evidence pending). In older patients, with much comorbidity,there may be compelling reasons for using, or not using, certain agents The 2011 UK guidelines suggest, in the absence of reasons to modify therapeutics, drugs should be introduced for all adults over age 55 in the following order: 1.Calcium channel blocker 2.Add ACE inhibitors or ARBs 3.Add thiazide diuretic In resistant hypertension, consider adding an 4.α-blocker, 5.spironolactone, or a 6.β-blocker |
••••••••••••••••HOW TO . . . Use antihypertensives in a patient with comorbidconditionsCalcium channel blockersUse rate-limiting options (e.g. diltiazem) to slow the heart rate in AF orreduce angina with normal LV functionMay make heart failure worse or cause constipationDihydropyridine calcium channel blockers (e.g. amlodipine, felodipine) areexcellent in isolated systolic hypertensionACE inhibitorsFor example: ramipril 2.5–10mg.Use for 2° prevention after vascular event (stroke, TIA, heart attack), and indiabetes, heart failure, and chronic renal impairmentAvoid in renal artery stenosisMonitor potassium and renal functionARBsFor example: losartan 50mg.Use when ACE-intolerant (usually cough) where an ACE is indicatedMay cause fewer orthostatic symptoms than ACE inhibitorsMonitor potassium and renal functionThiazide diureticsFor example: bendroflumethiazide 2.5mg.Useful first-line therapy in most older patients—may help with ankleswelling and heart failure symptomsAvoid if severe gout, urinary incontinence, or profound dyslipidaemiaMay worsen urinary incontinenceNeed to monitor for hyponatraemiaBeta-blockersFor example: atenolol 25mg.Useful with angina, AF, and stable heart failure (cardioselective better)Avoid with peripheral vascular disease, asthma, and heart blockAlpha-blockersFor example: doxazosin 1mg.Excellent for resistant hypertension in older patients Use if prostatic hypertrophyCommonly cause orthostatic symptomsMay exacerbate stress incontinence |
|
|
HOW TO Use antihypertensives in a patient with comorbid conditions 1.Calcium channel blockers Use rate-limiting options (e.g. diltiazem) to slow the heart rate in AF or reduce angina with normal LV function. May make heart failure worse or cause constipation. Dihydropyridine calcium channel blockers (e.g. amlodipine, felodipine) are excellent in isolated systolic hypertension. 2.ACE inhibitors For example: ramipril 2.5–10mg. Use for 2° prevention after vascular event (stroke, TIA, heart attack), and in diabetes, heart failure, and chronic renal impairment. Avoid in renal artery stenosis. Monitor potassium and renal function 3.ARBs. For example: losartan 50mg. Use when ACE-intolerant (usually cough) where an ACE is indicated. May cause fewer orthostatic symptoms than ACE inhibitors. Monitor potassium and renal function. 4.Thiazide diuretics For example: bendroflumethiazide 2.5mg. Useful first-line therapy in most older patients—may help with ankle swelling and heart failure symptoms. Avoid if severe gout, urinary incontinence, or profound dyslipidaemia May worsen urinary incontinence Need to monitor for hyponatraemia. 5.Beta-blockers For example: atenolol 25mg. Useful with ▪︎angina, ▪︎AF, and ▪︎stable heart failure (cardioselective better) ■Avoid with peripheral vascular disease, asthma, and heart block 6.Alpha-blockers For example: doxazosin 1mg. Excellent for resistant hypertension in older patients. Use if prostatic hypertrophy Commonly cause orthostatic symptoms. May exacerbate stress incontinence. |
Q |
Q |
|
|
•Arrhythmia: presentation Arrhythmias are very common in older people but are not so common as a presenting complaint. A patient with recurrent presyncope preceded by palpitations presents very little diagnostic challenge. What is much more common is for an arrhythmia to be the explanation for a rather more vague presentation such as: ▪︎Recurrent falls ▪︎Patient covered in bruises who has been explaining them away as clumsiness ▪︎General fatigue ▪︎Dizzy spells ▪︎Light-headedness‘ ▪︎Collapse query cause’ ▪︎Blackouts ▪︎Worsening/new angina or heart failure History It is important to ask about palpitations with any of these problems, (indeed it should form part of the systems review in all older people), but be aware of the following points: Clarify carefully what you mean. many people do not understand what we mean by ‘palpitations’ and may be describing an ectopic heart beat followed by a compensatory pause, or even just an awareness of the normal heartbeat, e.g.when lying in bed at night. Getting the patient to tap out what they feel can be very revealing. Do not exclude the possibility of an arrhythmia just because the patient does not complain of palpitations especially with confused patients. Where there are palpitations/light-headedness, establish an order wherever possible.
Postural hypotension is very common in older patients and can produce a similar set of symptoms (falling BP causing light-headedness, then a compensatory tachycardia)—in theory, the palpitations should come first in an arrhythmia. Are there any constant features? For example, dizziness occurring:On standing is more likely to be postural hypotension. On exertion may have an ischaemic component. On turning the head may be due to vestibular problems or carotid sinushypersensitivity . In any situation or at any time is much more likely to be due to an arrhythmia.
A history of SIGNIFICANT injury (especially facial bruising) with a blackout↑ the chances of finding an arrhythmia, particularly a bradycardia requiring pacing. Always take a full drug history antiarrhythmics can be pro-arrhythmogenic,drugs that cause bradyarrhythmias (commonly β-blockers, digoxin, or rate-limiting calcium channel blockers such as diltiazem), and antidepressants (especially the tricyclics) that can predispose to arrhythmias.
Medications containing ephedrine, thyroxine, caffeine, and β-agonists can cause tachyarrhythmias. |
Examination Should always include lying and standing BP; assessment of the baseline pulse character, rate, and rhythm; full cardiovascular examination to look for evidence of structural cardiac disease (e.g. cardiomyopathy, heart failure,valvular lesions), all of which may predispose to arrhythmias.
General problems require a full general examination-it is rarely appropriate to examine a single system only in an elderly patient. A rectal examination,for example, may reveal a rectal tumour causing anaemia, and hence palpitations. It may also be appropriate to examine the vestibular system and CNS.
Investigations •Blood tests-including FBC (anaemia), U, C+E (low potassium predisposes to arrhythmias), •thyroid function, and digoxin levels where relevant. •ECG—look for baseline rhythm and any evidence of conducting system disease (e.g. a bundle branch block or any heart block). Measure the P-R and the Q-T interval. Also look for LV hypertrophy (arrhythmias more likely) or ischaemia. A totally normal ECG diminishes the possibility of a clinically significant arrhythmia. •CXR—look at the cardiac size. •Holter monitoring a prolonged ECG recording. Usually a 24h periodinitially. Remember this is a very small snapshot and of limited value, especially if symptoms are infrequent. ■Can be useful if the symptoms are experienced while the monitor is on and the ECG trace shows normal sinus rhythm. If the suspicion of arrhythmias is high, then repeat the test or arrange for trans-telephonic event recording or even an implantable loop recorder where the symptoms are severe enough (e.g. sudden syncope). |
Arrhythmias:management Management of arrhythmias in older patients does not differ significantly from management in other age groups, but consider the following. ▪︎Precipitants Always check for common precipitants in older patients: □Electrolyte abnormalities (especially hypo- or hyperkalaemia and hypocalcaemia) □Anaemia □MI □Antiarrhythmic toxicity (especially digoxin) □Sepsis □Hypothermia □Any other acute illness. If the precipitant cannot be reversed quickly (e.g. sepsis), then the arrhythmiais likely to be recurrent.
Consider cardiac monitoring if cardiovascular compromise or if arrhythmia recurrence is likely.
Effect of arrhythmia Tachycardia may be less well tolerated than in younger patients, causing significant hypotension, angina, or heart failure. Hypotension itself may be less well tolerated than in younger patients (risk of cerebral injury), and so prompt action is required. Where there is heart failure because of an arrhythmia, fluids cannot be used for resuscitation, and so definitive action is required sooner rather than later. Begin by using standard treatment for acute heart failure (oxygen, iv diuretics,and opiates, etc.), while organizing cardioversion (usually electrical for speed)or rate limitation (appropriate for AF)
Diagnosis •There is more likely to be an underlying cardiac pathology.Always check for ischaemia and structural heart disease, even for apparently benign arrhythmias.(e.g. SVT) •Bundle branch block is common, and so there may be confusion between supraventricular and ventricular arrhythmias. There are numerous subtle ways of distinguishing between these, but in an emergency: ■If the patient is compromised, electrical cardioversion will treat both effectively. ■If the patient is unwell, but stable, an amiodarone infusion will treat both effectively and has the advantage of causing little myocardial depression.
Treatment Elderly patients are much more likely to be on an antiarrhythmic drug already. ► Check the medication carefully before administering any therapy. Electrical cardioversion is well tolerated by most older patients, usually effective and less likely to cause side effects than many medications. It should be considered early where there is significant compromise. Anaesthetic support is required, which can take some time to arrange, so prompt referral is recommended. It is less useful in acute sepsis where d arrhythmia is likely to recur, and it is hard to establish whether compromise is caused by the sepsis or the arrhythmia.
▪︎Implantable cardiovertor defibrillators are life saving for malignant ventricular arrhythmias (VT and ventricular fibrillation (VF)).
Regardless of patient age, they should be considered for survivors of sudden cardiac arrest,ventricular arrhythmias associated with EFs of <35%, and recurrent or refractory ventricular tachycardias with syncope. However, they are very expensive, can cause debility and pain, and do not allow a natural death (they must be turned off during palliative care). There is there fore reluctance to use them in older, frail patients in whom non-arrhythmic deaths are common. |
|
|
Common Pitfalls with antiarrhythmic medications |
1.Adenosine Its action is prolonged by dipyridamole (commonly prescribed with aspirin in stroke), so avoid using together. Exacerbates asthma and is antagonized by theophylline, so avoid in asthmatic people
2.Amiodarone Risk of ventricular arrhythmias when used with disopyramide,procainamide, and quinidine, so avoid concomitant use↑ plasma half-life of flecainide, so reduce dose.
3.Atropine Can precipitate glaucoma, so avoid in patients with this condition.
4.Flecainide Contraindicated when there is IHD, heart failure, and haemodynamic compromise. Probably best avoided in most older patients who may well have occult cardiac disease.
5.Verapamil Do not use iv in a patient already on a β-blocker (high risk of asystole and hypotension)
General guidance Most antiarrhythmic medication used concomitantly ↑ the risk of myocardial depression and arrhythmias. This effect is more pronounced in older patients. Caution when using >1, and consider using sequentially, rather than additively, if one alone is ineffective. |
antiarrhythmics side effects |
|
|
Atrial fibrillation Common arrhythmia, becoming more common with age (risk doubles with each additional decade of age, rising to 7% in >85 year olds).
Often associated with other disease (e.g. hypertension, coronary artery disease, mitral valve disease, thyrotoxicosis) but also occurs in 1–2% of otherwise healthy elderly people.
Unlike other arrhythmias, it is often chronic. Disorganized atrial activity with variable conduction to the ventricles leads to an irregularly irregular pulse rate and volume. Up to a third of older patients with AF will have AV nodal disease that limits the rate to <100bpm, often making it asymptomatic. It is therefore often noted incidentally during routine examination,but should never be ignored.
Assessment Should include examination for -hypertension and -valve disease, -blood tests for thyroid disease, and an -ECG to confirm the diagnosis. Paroxysmal AF may cause intermittent symptoms and should be looked for with Holter monitoring.
Complications AF causes an ↑ in morbidity and mortality, even if there is no underlying cardiac disease. Pulse >120 often causes ▪︎palpitations, ▪︎light-headedness, or ▪︎syncope ▪︎Rapid rate may also cause dyspnoea, angina, or heart failure
General malaise may also result from a chronically sub-optimal cardiac output.
AF is often associated with periods of AV conduction delay (‘pauses’) and if>3s, these may cause syncope.
The main complication is stroke from cardiac emboli.
Atrial flutter Rapid, regular atrial activity (usually 300/min) Characteristic sawtooth appearance on ECG (revealed by carotid sinus massage if rate high) Rate depends on degree of AV block (150bpm if 2:1 block, 75bpm if 4:1block, etc.) ► Always think of atrial flutter when the pulse rate is 150bpm. Commonly associated with COPD or IHD Similar embolic risk to AF, and as the patient will often flip in and out of flutter and fibrillation, they should be managed in the same way • Treat with rate control and stroke prophylaxis. This rhythm is usually amenable to cardioversion, but if there are significant comorbidities, structural heart disease, or ablation therapy, then sinus rhythm is unlikely to be sustained. |
Atrial fibrillation: rate/rhythm control
1.︎Acute atrial fibrillation Aim to return to sinus RHYTHM, i.e. cardioversion. Treat underlying condition, e.g. sepsis, ischaemia, heart failure. If compromised, consider electrical cardioversion. Otherwise, control rate (usually with cardioselective β-blocker, e.g. bisoprolol,or rate-limiting calcium channel blocker, e.g. diltiazem; add digoxin if resistant) and wait to see if resolves once precipitant has been dealt with. ▪︎Chemical cardioversion is an option give amiodarone (initially 200mg po tds for a week, then 200mg bd for a week, then 200mg od there after). ▪︎May be able to drop dose further to 100mg a day, or even every other day). ▪︎Long-term amiodarone for permanent AF rate control is no longer recommended. ▪︎Remember anticoagulation (stroke prophylaxis) during the episode.
2.Persistent/permanent atrial fibrillation ▪︎Persistent is lasting >7 days; it is classed as permanent when attempts at cardioversion have failed. ▪︎Guidelines suggest RATE control with cardioselective β-blockade (e.g.bisoprolol) or calcium channel blockers (e.g. diltiazem). ▪︎In practice, many frail, largely sedentary old patients will tolerate digoxin better, and this remains the first choice of many geriatricians . ► Remember that amiodarone interacts with warfarin, and regular monitoring of INR will be needed if the two are used together as is often the case. ▪︎Also can cause thyroid abnormalities, and this should be monitored with TFTs. ▪︎Dronedarone is a newer agent that is simpler to use than amiodarone but cannot be used in severe heart failure, and safety in permanent AF is unclear. ▪︎Electrical cardioversion for chronic AF should only be attempted after a period of anticoagulation (minimum 3 weeks). ▪︎It is more likely to succeed(and less likely to recur) where there are fewer of the following present: □Structural heart disease (hypertrophy, atrial enlargement, valvular heart disease, etc.) □Comorbidity (especially hypertension, heart failure) □↑ age □Recent evidence suggests that an older patient with several of the factors listed is better off being treated with rate control and anticoagulation only, without attempting cardioversion. ▪︎Atrial catheter ablation is used in selected, usually younger, patients, but consider referral if a patient remains symptomatic and has failed trials of medical treatment or to destroy/isolate abnormal electrical impulses.
▪︎Paroxysmal atrial fibrillation-Equal embolic risk, so consider anticoagulation as for chronic AF.
▪︎Remember that digoxin does not prevent paroxysms of AF. ▪︎Amiodarone, dronedarone, or cardio selective β-blockers (e.g. bisoprolol) are useful to prevent paroxysms of AF.
▪︎Ablation therapy may be useful in those with normal-sized atria and few comorbidities, although the recurrence rate is probably higher than in younger patients. |
Atrial fibrillation: stroke prevention
This is a complex and often emotive issue. Many older people will have very strong views about stroke (‘I’d rather die than ever have a stroke’) or warfarin (many know it as ‘rat poison’ or have known someone who had a bleed while on warfarin). As we are dealing with population risks and benefits, it is impossible to accurately predict for a single individual what will happen to them if they do,or do not, take preventative therapy. Conveying this concept is difficult, but because the decision is complex, involvement of the patient becomes key. This takes time and patience. A decision aid with visual aids, such as that available from NICE clinical guideline CG180, can help.
It is important to have a simple way of explaining the facts as they are known,perhaps writing them down for clarity, then allowing time for them to sink in before coming to a final decision. There is no enormous urgency the stroke risks quoted are per annum, and it is worth giving the patient time to think things over, if required.
Address each of the following questions. ▪︎What is the risk of stroke in this patient with atrial fibrillation? Overall, the risk is about five times greater than a person with similar health and age who does not have AF. Paroxysmal AF carries the same embolic risk. Risk may be more accurately quantified by using CHA2DS2VASc scoringsystem . CHA2DS2VASc scoring system Cardiac failure -1 Hypertension 1 Age over 75 -2 Age 65–74-1 Stroke, TIA, or thromboembolism- 2 Vascular disease- 1 Sex ♀ -1 Total (maximum 8) Without anticoagulation, annual stroke rate is ~1% for a score of 1, 2% for ascore of 2, 3% for a score of 3, 5% for a score of 4, 7% for a score of 5, and 11%
• NICE recommends anticoagulant treatment for anyone with a CHA2DS2VAScof ≥2 (and as age >74 scores 2, that means almost everyone, all older patients), unless there is a very high bleeding risk. ► In clinical practice, most older patients with AF will have a risk of stroke that warrants consideration of anticoagulation. What is the risk of anticoagulant therapy? ▪︎Warfarin, when taken correctly and monitored carefully, has around 1% peryear risk of major bleeding in 1° prevention of stroke, and 2.5% risk in 2°prevention. ▪︎Direct, non-vitamin K thrombin inhibitors (NOACs/DOACs), e.g. dabigatran,require no INR monitoring and no dietary changes, and have lower bleeding risks (probably about half the rate of warfarin). ▪︎Falls alone are not a contraindication to anticoagulation. Risk can be more accurately assessed using HAS-BLED criteria ▪︎Hypertension—systolic >160mmHg-1 ▪︎ Abnormal liver function- 1 ▪︎Abnormal renal function -1 ▪︎Stroke -1 ▪︎Bleeding tendency- 1 ▪︎Labile INR- 1 ▪︎Elderly (age >65) -1 ▪︎Drugs, e.g. aspirin, NSAIDs -1 ▪︎Alcohol abuse- 1 Total (maximum 9)Annual major bleed risk on anticoagulant is 1% for score of 1, ~2% for score of2, ~4% for score of 3, and ~9% for score of 4. How effective is therapy at reducing risk?Anticoagulation reduces stroke risk in AF by around 60–70% Aspirin at any dose is no longer used in AF as monotherapy but is sometimes co-prescribed with anticoagulants if there is a separate reason (e.g. coronary artery disease). What are the recommendations? Other options There is limited trial evidence that occlusion of the left atrial appendage using a percutaneous device is equivalent to warfarin in the prevention of stroke. Paceand ablate therapies can also be used to control AF. This may be an option for high-risk patients in whom anticoagulation is not feasible. |
|
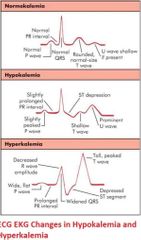
HOW TO Use digoxin Indications ▪︎Rate control of AF in sedentary patients intolerant to β-blockers. ▪︎Mild positive inotrope sometimes used in heart failure ▪︎Not useful for Paroxysmal AF prevention. Exercise-induced fast AF ▪︎Loading with digoxin 1mg in divided doses over 24h. This dose is always required, regardless of renal function modify maintenance doses only in renal impairment. Example: Day 1—8 a.m. digoxin 500 micrograms po, 8 p.m. digoxin 500 micrograms po Day 2—8 a.m. digoxin at maintenance dose Deciding a maintenance dose Main determinant is renal function-digoxin is excreted this way, so use a low dose with renal impairment. Consider also body mass, e.g. start with 62.5 micrograms for a small older woman—the dose can always be ↑ if there is inadequate rate control. Dosage is determined clinically, but serum levels can be used to assess toxicity or adherence. Digoxin toxicity Hypokalaemia predisposes to this, so always monitor potassium and supplement if needed. Target [K+] >4.0mmol/L Symptoms include confusion, nausea, vomiting, arrhythmias (especially nodal bradycardia and ventricular ectopics), and yellow or green visual haloes ▪︎ECG may show ST depression and inverted T wave in V5 and V6 (reversedtick pattern) or any arrhythmia Treat by stopping medication, rehydrating, and correcting hypokalaemia Life-threatening poisoning can be treated with digoxin-specific antibody fragments. |
•Bradycardia and conduction disorders As the heart ages, the function of the cardiac pacemaker (the SA node) and the conducting system (bundle of His and Purkinje fibres) tends to decline, due to: Declining numbers of cells in the SA node ↑ prevalence of disease (atheroma, amyloid, and hypertension) Degeneration with fibrosis and fat infiltration This is not inevitable, but around 50% of older patients will have some ECG evidence of conduction delay (prolonged PR and QT interval, left axis deviation)& be prone to symptomatic bradycardia and conduction disorders. Causes ▪︎Medication- digoxin, amiodarone, β-blockers (including some eye drops),calcium channel blockers, donepezil, tricyclic anti depressants ▪︎Sick sinus syndrome—isolated sinus node dysfunction, very common in older patients, with uncertain cause (theories include vascular insufficiency or amyloid infiltration, but often no cause is found) ▪︎IHD ▪︎Structural heart disease, e.g. hypertrophic cardiomyopathy ▪︎Systemic disease—hypothyroidism, liver failure, hypothermia, hypoxia, hypercapnia, cerebral disease (e.g. stroke, raised intracranial pressure,haemorrhage)
Presentation Often picked up incidentally on an ECG When symptomatic, bradycardia causes low output syndromes ranging from fatigue, dizziness, dyspnoea, and presyncope, syncope, and falls, angina to heart failure and shock. May be intermittent (with paroxysmal bradyarrhythmias), chronic (with stable arrhythmias), or occur acutely (usually post-MI) |
ManagementThis depends on the clinical presentation, not on the rhythm; a patient with complete heart block can be moribund or asymptomatic, and treatment will obviously be dramatically different in these scenarios. Seek advice from the local cardiology service if in doubt.Is the patient acutely compromised? If so, then urgent treatment is required in order to minimize cerebral injury. For shock, lie the patient down, and elevate the legs. Use iv fluids and/or try to ↑ the heart rate using atropine (0.6mg iv, repeated up to a total dose of 3mg), an isoprenaline infusion, or temporary pacing (external pads are quickand often well tolerated; if the situation persists, insert a temporary pacing wire) Always look for underlying causes, e.g. sepsis, stroke,hypothermia, hypothyroidism; treating an underlying cause may be equal or greater priority than the bradycardia. Reversible bradyarrhythmia commonly occurs after acute inferior MI, in which case support the patient during the acute episode as needed (non-invasively if feasible). Acutely, hypothermia can also cause bradycardia which reverses when patient is normothermic again.Tailor your treatment to each individual if the cause of the bradycardia is a catastrophic intracerebral event, then putting the patient through a temporary wire is not appropriate. A stable patient with intermittent symptoms Symptoms can be insidious, and sometimes patients do not recognize their symptoms until after the bradycardia is corrected. Ask specifically about tiredness, exercise tolerance, worsening symptoms of heart failure or angina,as well as syncope and presyncope symptoms.Check medication. □Digoxin (especially with toxicity), amiodarone, β-blockers,and calcium channel blockers can all cause or exacerbate bradycardia. □Referral for a pacemaker should not occur unless patients remain bradycardic off any culprit drugs for at least a week. □Check and correct thyroid function. Very few patients need admission and urgent permanent or temporary pacemaker insertion, but consider admission for frequent syncope or severe persistent symptoms. Most cardiology services have a ‘fast track’ outpatient service to avoid admission for symptomatic patients who cannot wait for a routine list. Patients or their relatives can sometimes reject the idea of a pacemaker because they believe it is a ‘big’ operation or it will stop them dying. A pacemaker is a relatively low-risk intervention which can dramatically improve quality of life, even in frail patients or those with cognitive impairment, so ensure they fully understand the proposed procedure before rejecting it. Asymptomatic patients • Older patients can be truly asymptomatic and can tolerate pulses of 50bpm for many years. Action is not required unless there is a high risk of future asystole. Asystole is a major risk with complete heart block and also with second-degree heart block type II where there is also bundle branch block even if asymptomatic, a pacemaker should be offered for these two conditions. |
|
|
•Permanent pacemakers IndicationsOver 85% are used for patients >64 years old50% are for sick sinus syndrome and AV blockOccasionally used for vasodepressor carotid sinus hypersensitivityOccasionally used for recurrent syncope where no cause is foundUsed with AV node ablation with refractory AFResynchronization therapy for heart failurePacemakersDual-chamber pacemakers are preferred, as they produce a better cardiacoutput and less AF than single-chamber onesPermanent pacing should be programmed to minimize paced beats,allowing the intrinsic rhythm to get through as much as possibleIn LV dysfunction, pacing may need to include multisite pacing, as rightventricular pacing can worsen dyssynchrony and exacerbate LV failureCardiac resynchronization therapy (biventricular pacing) may reduce heartfailure symptoms in patients with reduced LV function, if they have wideQRS complexesInsertionRelatively simple procedure, done while awake using local anaesthetic,which is usually well tolerated by even very frail patients; however,technical problems can occur with insertion if the patient cannot lie still andflatRare complications during insertion include arrhythmias (commonly AF)and rarely perforation of the right ventricleLater problems include sepsis and failure of pacemaker outputRegular follow-up is required to check the pacemaker function and batteryreserve. . |
Common arrhythmias and conduction abnormalities Condition- Clinical features Treatment 1.Sinus bradycardia Intrinsic SA nodal disease Pulse rate <60 Common incidental finding Drugs are common cause Acute onset associated with inferior MI and raised intracranial pressure Consider hypothyroidism ~Treat only if symptomatic (rarely causes problem) Check thyroid function. 2.Supraventricular ectopics Narrow complex QRS without a P wave, followed by a compensatory pause. Patient may be aware of this and describe an ‘early beat’with a gap afterwards. Benign. Reassure the patient. ~No action required.
3.SA block Intermittent inability of SA node to depolarize the atrium. ECG shows pauses that are multiples of the PR interval. Usually asymptomatic. ~Treat only if symptoms.
4.Slow AF Combination of AF and SA nodal disease very common Symptomatic pauses frequent ~Anticoagulation for stroke prevention ~High index ofsuspicion for pauses if suggestive symptoms-check with Holter monitor and treat with pacemaker.
5.Sick sinus syndrome SA node dysfunction (degenerative or due to IHD)causing a bradycardia—includes sinus bradycardia and slow AF. Often associated with other conduction problems. Treat symptoms 6.Tachy-bradysyndrome Combination of slow underlying rate (sinus bradycardia or slow AF) with tendency for runs of SVT that often terminate with a long pause. ~Symptoms due to both slow and fast pulse ~Usually require spacemaker for bradycardia and rate-limiting drugs to control tachyarrhythmias.
7.First-degreeheart block PR >0.2s Benign if isolated,but always check for coexisting second- or third-degree block. ~No action required
8.Second-degree heart block,Mobitz type I(Wenckebach) PR interval ↑ progressively until a QRS is dropped. Often occurs transiently post-MI Usually appropriate to monitor until resolves.
9.Second-degreeheart block,Mobitz type II Fixed PR interval, but conduction to the ventricles does not occur on every beat. Usually in a fixed pattern(conducting every second, third or fourth beat) Often associated with bundle branch block. Often symptomatic. High risk of progression to complete heart block. ~Usually require selective pacing.
10.Complete heartblock (third-degree) Complete dissociation of atrial and ventricular activity. P waves visible, but not conducted. QRS originates at ventricular pacemaker (escape rhythm). If this is in the AV node, then the rate is around 60 and the QRS morphology narrow. If it is more distal, then the rate tends to be around 40 with wide QRS complexes. ~Usually symptomatic,although if rate >50,may only be on exertion. ~If chronic, limit activity until permanent pacing arranged. ~If acute (e.g. post-MI), likely to resolve. ~When associated with hypotension angina, or heart failure at rest, may need urgent pacing.
11.Bundle branch block Widened QRS due to delayed conduction. Not related to rate, usually asymptomatic. RBBB is a common finding in healthy elderly and is usually benign, but if acute, consider acute PE. LBBB is associated with hypertension and IHD. Acutely, LBBB may indicate acute infarct. If found incidentally,tell the patient (aids future emergency treatment) and consider giving a copy of the ECG to the patient. 12.Trifascicular block Prolonged PR, RBBB, and left anterior or posteriorfascicular block (causing left axis deviation) High risk of intermittent complete heart block. Pacemaker is considered. |
|
|
|
Heart failure: assessment Heart failure is very common, occurring in 1 in 10 of the >65s and accounting for 5% of admissions to medical wards and 1–2% of all health care costs. Overall prevalence is 3–20 cases per 1000 population, but this doubles with each decade after 45 years. Becoming more common as the population ages and survival from coronary events improves. Pathology ▪︎Poor LV function (systolic or diastolic) -↓ cardiac output, resulting in ↑pulmonary pressures and oedema ▪︎Sympathetic nervous system activated (↑pulse, myocardial contractility peripheral vasoconstriction , and catecholamines) ▪︎Renin–angiotensin system activated (↑ salt and water retention) ▪︎Vasopressin and natriuretic peptides (↑) Causes-Usually due to CHD (especially in Caucasians—CHD risk factors are markers for heart failure) and hypertension (especially African Caribbeans). Other causes include valve disease, arrhythmias, pericardial disease, pulmonary hypertension(e.g. with COPD or multiple PEs), high output states (look especially for anaemia, thyroid disease, and Paget’s disease), and cardiomyopathy (checkalcohol history). Diagnosis Heart failure is a complex clinical diagnosis, with no universally agreed diagnostic criteria. Often difficult to diagnose accurately, particularly in older patients with ↑ comorbidity and symptoms. Many older people are put on a diuretic for presumed heart failure, but diuretic use as a marker of heart failure is 73% sensitive but only 41% specific. This predisposes to postural symptoms and adds to polypharmacy. Symptoms Ask ‘are these symptoms cardiac’ and ‘what is the underlying disease causing them?’ ▪︎Exertional dyspnoea is 100% sensitive for heart failure (i.e. every case has it), but only 17% specific (i.e. many other causes of exertional dyspnoea exist the main one being respiratory). ▪︎Fatigue and ankle swelling are also very common in heart failure but occur in many other diseases too. ▪︎Orthopnoea and paroxysmal nocturnal dyspnoea are much more specific but, occurring late in the disease, are not sensitive. Signs Again, early signs are sensitive, but not specific (e.g. tachycardia, pulmonary crepitations, peripheral oedema). ▪︎Later signs are more specific, but not sensitive (e.g. elevated JVP—98% specific, 17% sensitive; gallop rhythm—99% specific and 24% sensitive). ► Overall, clinical features tend to be sensitive OR specific, but not both. The multiple pathology of older patients poses a particular challenge in diagnosis. Clinical suspicion should then be supported by investigation before embarking on a trial of treatment. |
HOW TO Investigate a patient with suspected heart failure Use investigations to support a clinical diagnosis and establish the cause. 1.ECG—abnormal in over 90% of cases (Q waves, T wave/ST segment changes, LV hypertrophy, bundle branch block, AF). 2.Consider Holtermonitor if paroxysmal symptoms 3.CXR—look for cardiac enlargement (although this is absent with acute onset, e.g. post-MI or PE), upper lobe blood diversion, fluid in the horizontal fissure, Kerley B lines, bat wing pulmonary oedema, pleural effusions (usually bilateral; right > left if unilateral), and any other cause for breathlessness ► Combination of a normal CXR and ECG makes heart failure very unlikely indeed. 4.Blood tests— B-type natriuretic peptide (BNP) , FBC (?anaemic), biochemistry (? renal function, sodium low in severe heart failure), glucose and lipids (CHD risk factors), liver function (?congestion), thyroid function 5.Echocardiography—echo should be done for all with suspected heartfailure (NICE guidelines) to help -confirm diagnosis, -establish cause, and -grade disease severity. Looks for LV function,estimates EF, and looks for evidence of valve disease, cardiomyopathy,regional wall abnormalities from IHD, pericardial disease, intracardiac shunts, LV aneurysms, or cardiac thrombus. Open access echo (i.e. direct GP referral) has improved the number of patients who have an echo, but the results are only as good as the echo technician and there can be problems with interpreting results (e.g. diastolic problems). Patients may need a repeat echo after several years, especially if symptoms progress or change. 6.Pulmonary function tests—may help distinguish cardiac from respiratory breathlessness (peak expiratory flow rate (PEFR) and forced expiratory volume in 1 s (FEV1) reduced in heart failure, but less than in COPD). Remember many patients have both conditions. |
B-type natriuretic peptide Three types of natriuretic peptide known, with effects on the heart, kidneys,and nervous system. B-type is found mainly in the heart and ↑ with volume and pressure overload of the heart, acting as a biochemical marker for heart failure. BNP rises slightly with age but remains below 100pg/mL (which is the most common threshold used for diagnosis). ► Overall, a ‘negative’ (i.e. very low) result in a breathless patient makes heart failure very unlikely, and other causes should be sought. BNP is a highly sensitive test (negative predictive value of 96%). Causes of false positives include -pulmonary hypertension, e.g. PE, sepsis, acute kidneyinjury. BNP concentration correlates with the severity of heart disease. very highlevels carry a poor prognosis. It is equally useful in systolic and diastolic heart failure. Where and how is it used? ▪︎1° care screening. BNP should be used in all patients before referring for heart failure services. Patients with a normal BNP should not be referred to cardiology, as they are extremely unlikely to have heart failure causing their symptoms, and other diagnoses should be considered. ▪︎Acute first presentation of heart failure. A single BNP measurement can be helpful, alongside clinical features and other tests (e.g. echo). Remember that acutely unwell patients (e.g. sepsis and kidney injury) can have false-positive elevations, and in these patients, an interval measurement when stable is indicated, especially if only a minor elevation is detected acutely. ▪︎Chronic heart failure. A single measurement is all that is required to aid diagnosis (usually by the GP as above) and does not usually need repeating. ▪︎Future uses. Research shows BNP is a powerful predictive tool for future morbidity and mortality in lots of different populations (including asymptomatic patients). BNP may therefore have a role in population screening and targeting interventions for the highest-risk groups. It can be used by cardiologists to monitor response to treatment. |
|
|
Acute heart failure-Treatment ■ 1.Immediate treatment with oxygen, 2.iv loop diuretic, and 3.nitrates given iv or sublingually (if adequate BP), 4.opiate, and 5.antiemetic
■Address the cause (e.g. acute MI, arrhythmia) ■Stop any exacerbating medications (NSAIDs, β-blockers) ■Consider thromboprophylaxis (e.g. low-molecular-weight heparin) ■Consider ventilatory support (non-invasive positive pressure ventilation,which can be done in a non-ITU setting, can produce dramatic resolution of symptoms) ■After improvement is seen, begin to plan ongoing treatment—write up ~regular loop diuretic and usually an ACE on the drug chart, and make plans for further assessment. ~Monitor electrolytes, BP, and weight.
Prognosis Patients with acute heart failure look extremely unwell yet can often make apparently ‘miraculous’ recoveries as the precipitant is dealt with. Remember that it is the premorbid state and the nature of the acute injury, never age alone,that should determine how aggressively to manage the acute condition. Rapid atrial fibrillation and heart failure A common combination in older patients. It is rarely clear which came first.Treat both simultaneously. Digoxin may slow AF in this situation without depressing the myocardium. (may occur with β-blocker or amiodarone). Load 1mg of digoxin over 24h in divided doses, e.g. 500 micrograms iv or po, repeated at 12h, followed by the maintenance dose. Always look for a precipitant sepsis, MI, PE, etc. |
Chronic heart failure A specialist multidisciplinary approach is preferable, as management is bestdone by those with an interest and with facilities for ongoing follow-up. Settings can vary, e.g. DHs (particularly suited to the frailer patient), heart failure clinics,and by heart failure specialist nurses or GPSIs. Education and involvement of the patient are key to successful monitoring(e.g. daily weighing) and management (improves adherence and satisfaction). Subtypes LV failure Right ventricular failure/cor pulmonale LV failure with preserved LV function, previously known as diastolic heart failure There is a strong evidence base for management of LV failure subtype with life prolonging therapies. Diagnosis Use a combination of clinical features, ECG, CXR, BNP, and echo. Always review a historical diagnosis of heart failure, particularly if the appropriate investigations were not completed. Lifestyle interventions Address cardiovascular risk factors, stop smoking, stop/reduce alcohol intake,and ↑ aerobic exercise (ideally as part of a rehabilitation programme). Offer annual influenza vaccination annually and pneumococcal vaccine once.
Treating underlying cause comorbidities ▪︎AF should be slowed and ▪︎Anticoagulation started (cardioversion unlikely to succeed once LV dysfunction) ▪︎Hypertension should be treated until disease progression drops cardiac output and hence BP. ▪︎Valvular disease should be assessed for surgical correction where appropriate (especially aortic valve disease-discuss with the patient early after onset of heart failure) ▪︎Optimize management of anaemia (iv iron or erythropoietin), thyroid disease,and diabetes.
Medication There is a large evidence base for many drugs. It may be tempting to limit the number of drugs in older patients, justified by concerns about side effects, but if the diagnosis is secure, then all classes should be attempted. It is probably better to prescribe a low dose of an ACE with a β-blocker, rather than a maximum dose of an ACE when the BP may be insufficient to introduce the β-blocker.
Monitoring Should include: ▪︎Clinical assessment of symptoms and functional capacity (how far they can walk without stopping, etc.) ▪︎BP, including postural measurements. ▪︎Fluid status (weigh regularly. ▪︎Estimate the dry weight, record it, and use this to titrate future management. ▪︎Examine for JVP, oedema, and lung crepitations) ▪︎Cardiac rhythm (clinically and by ECG) ▪︎Cognitive state (commonly impaired in heart failure due to vascular disease and low BP) ▪︎Nutritional state (malnutrition common in heart failure. Ask about appetite,assess muscle bulk, check albumin—consider build-up drinks or dietician input) ▪︎Medication review (are they on all appropriate drugs at maximum tolerated doses?)
Side effects (especially ask about postural symptoms, check U, C+E) Psychological and social review, done by an MDT (how are they and carers coping with problems of chronic disease? Do they need any social support?) Routine monitoring with tests such as BNP, CXR, and echo are expensive and largely unhelpful. |
Medication for chronic heart failure 1.Loop diuretics—(e.g. furosemide) used to control symptoms. Begin with 40mg (20mg in the very elderly) and titrate upwards (guided by symptoms and examination findings). Monitor renal function and electrolytes 2.ACE inhibitors—should be started early in all with a diagnosis of systolic heart failure, unless valvular cause or renal artery stenosis suspected. Again begin low (e.g. ramipril 1.25mg) and titrate upwards, monitoring renal function and postural symptoms. 3.β-blockers-should be started in all stable patients with LV systolic dysfunction after diuretics, regardless of whether there are continuing symptoms (improves prognosis). Use a β-blocker licensed for heart failure,e.g. carvedilol 3.125mg, bisoprolol 2.5mg, and titrate upwards.
4.Spironolactone—use for continuing symptoms despite loop and ACE. Watch potassium levels, especially in combination with ACE.
5.Digoxin—use in AF and where there are continuing symptoms despite maximal other therapy in sinus rhythm. 6.Thiazide diuretics—can be added to loop diuretics in end-stage heart failure, e.g. bendroflumethiazide 2.5mg or metolazone 5mg (monitor electrolytes closely; may be used on alternate days if causes excess diuresis)
7.Warfarin—use in AF or where echo has shown intra cardiac thrombus
8.ARBs—(e.g. candesartan) useful where ACE-intolerant; this class has benefits in heart failure, but its equivalence to ACE inhibition is debated.
9.Aspirin/statins—used for risk factor modification where the cause is IHD.
10.Ivabradine—useful in some patients in sinus rhythm who have a tachycardia but cannot tolerate a β-blocker. |
|
|
Dilemmas in heart failureTerminal careChronic heart failure is a grim diagnosis with a poor outlook (only 25% will survive 3 years—worse than many cancers)Consider broaching this with all patients in clinic (ideally in a stable phase of the illness) to allow future plans to be made and resuscitation issues discussed.Monitoring of any cognitive impairment allows the timing of this conversation to be carefully judged.As the disease progresses, ensure appropriate palliative care measures are taken with careful review of symptoms and patient/family anxieties.Consider hospice care if available•Opiates (e.g. morphine sulfate solution 2.5–10mg) can be given to help relievethe distress of dyspnoea and allow sleep.•Continuous oxygen therapy may ease discomfort.•Intermittent iv boluses of furosemide in DH or sc at home can keep people out of hospital. Mixed cardiac and pulmonary diseaseIt is very common to see older patients presenting with mixed pathology, e.g. a 92-year-old ex-smoking ♀ may well have breathlessness due to a combination of: •Significant emphysema •LV impairment •Kyphoscoliosis with restrictive ventilatory problems •Chronic anaemia •Rhythm disturbance with poorly controlled AF •Physical deconditioning In this sort of case, the signs can be confusing or contradictory, and trying to balance management is very challenging. Recognition of all the contributingfactors is important ,small changes across all factors may be a much moresuccessful strategy than intense focus on a single element. A geriatrician may be better placed to do this than involvement of a multiple organ specialist. |
HOW TO . . . Manage the heart failure see-saw One of the most common dilemmas is balancing drugs in a patient with both heart failure and chronic renal failure. When the see-saw is unchecked, thiscan lead to multiple unnecessary admissions to hospital and, moreimportantly, reduced quality of life for the patient. Aggressive diuretic use will improve the heart failure but lead to thirst,malaise, hyponatraemia, uraemia, postural hypotension, and ultimately anuria. Hydrating to improve renal function will lead to worsening pulmonary and peripheral oedema.Each patient will need a carefully planned balance, accepting moderate elevations in urea and/or a bit of oedema, which enables the patient to exist in the greatest comfort. This balance will take time and skill to achieve. The following will help: Make all changes slowly and wait for impact large-dose changes ↑oscillation between wet and dry states. If a patient is still losing/gaining weight on a treatment regimen, they are likely to continue to do so. Keep the patient under continuing close review until stability is reached. Get to know the ideal weight, and use this to guide therapy. Involve patients and carers wherever possible. Get to know the patient continuity of care is very helpful, and community heart failure nurses may play a key role in this. The patient must know how to access a review and advice quickly when they detect a swing in their symptoms. Communicate between different health care settings. Many mistakes are made when a carefully constructed medication balance is not continued when a patient moves from hospital to home, or vice versa. |
•Heart failure with preserved left ventricular function Previously known as ‘diastolic heart failure’. Clinical syndrome of heart failure with normal LVEF seen on echocardiography (>50%) where there is no major valvular disease. The use of BNP in combination with echo has helped identify these patients (BNP equally sensitive in heart failure with preserved left ventricular function(HFPLVF)) NICE guidelines suggest diagnosis should be done by a specialist. Accounts for around a third of clinically diagnosed heart failure and is more common in older patients, especially women, hypertensive patients, and thosewith LVH Not a benign condition.Ambulatory patients do better than those with systolic heart failure, but the mortality is equivalent in older patients or hospitalized. Fourfold ↑ in mortality when compared with controls without heart failure. ► Important to recognize and treat, and not to discontinue heart failure treatment on basis of normal LV function alone. Pathology Thick-walled LV with a small cavity. Slow to relax and slow filling in diastole,causing ↑ diastolic pressures (hence pulmonary pressures and so dyspnoea) and a low cardiac output (hence fatigue). Diagnosis Clinical suspicion (history as for systolic heart failure) and examination findings (elevated JVP, pulmonary oedema, hypertension, murmur in the aortic area, fourth heart sound) are key. CXR may show pulmonary congestion, and the ECG may have evidence of hypertension. Echocardiography shows preserved LV function and, in skilled hands, may show evidence of abnormal diastolic relaxation (Doppler studies show are duced ratio of early (E) to late or atrial (A) ventricular filling velocities—E:A <0.5 is suggestive of diastolic dysfunction). However, these changes are very common in older patients (E:A often <1). Thus, treatment is based on symptoms, not echo. BNP useful particularly in cases where there are multiple possible aetiologies of dyspnea, as a negative result makes heart failure unlikely, helping to limit polypharmacy. Management Prevention by BP control at population level. Relieve precipitants treat arrhythmias, anaemia, thyroid disease, ischaemia,and malnutrition. Acute symptoms control BP with oral agents as priority. Use loop diuretics,as for LV failure. Chronic disease management—much less evidence than for systolic heart failure. Control of BP is key. Diastolic relaxation agents (e.g. β-blockade) not proven, but rate control with these, especially where there is dual indication,e.g. AF or IHD. Diuretics improve symptoms, but ensure you do not dehydrate patients as these patients are vulnerable to reductions in preload. Improve exercise tolerance with physical activity. |
|
|
Valvular heart disease The majority of valvular heart lesions in the UK are degenerative (e.g. senile calcification is the main cause of aortic stenosis), so valve defects are very common in older patients. The following must be remembered:Listen to the heart of all elderly people—many valve lesions are detected incidentally. Think of valvular disease when a patient presents with dyspnoea, heart failure,angina, palpitations, syncope, or dizziness. Examine them carefully.When a murmur is heard, an echo should be requested in order to document the valve lesion and formulate a management plan. Following a review of evidence by NICE in 2008, antibiotic prophylaxis is nolonger recommended for invasive dental procedures. ► Remember endocarditis if a murmur is heard in the context of an unexplained fever. Surgical treatment Once the valve lesion is known, decide whether valve replacement is indicated yet. In many lesions (e.g. mitral valve disease and aortic regurgitation), the progression of symptoms alerts to the need for pre-emptive surgery. In aortic stenosis, however, a prompt response to the development of early symptoms is required, and so the approach is different. Consider whether or not the patient is a surgical ‘candidate’. Remember that many lesions are amenable to percutaneous interventions which are feasible in much frailer patients. Bioprosthetic (pig) valves can be used in older patients, which have a shorter lifespan than metal valves but obviate the need for anticoagulation. If surgery is not yet indicated, then ensure there is some sort of call-back/surveillance system in place whether repeat echocardiography or clinic review, or simply ensuring that the patient knows what symptoms should trigger medical review. Talking to patients about surgical intervention ▪︎Always ask the patient what they think often there will be strong views that come as a surprise to the physician. Many older people are terrified at the prospect, others are keen to proceed, and others will not want to decide ‘whatever you think, doctor’. Make it clear that obtaining a surgical opinion is not committing the patient to surgery, indeed the surgeon may feel that the risks outweigh the benefits, butt hat it is an important first step. Having a frank and useful discussion about risks and benefits is often difficult but should always be attempted before referral is made. Ensure you have enough time and take it slowly, giving plenty of opportunity for questions. They may wish to go away and think about it, perhaps returning with a family member—encourage this and do not force a decision |
Common valve lesions- Valve lesion Symptoms -Complications Treatment -Who to consider for surgery? 1.Mitral stenosis Dyspnoea Fatigue Palpitations Chest pain Haemoptysis ~AF- ~Systemic emboli ~Pulmonary hypertension ~Pulmonary oedema. ~Pressure effects from large LA ~Endocarditis ▪︎Rate control of AF (digoxin and/or β-blocker) ▪︎Anticoagulation for AF ▪︎Diuretics if heart failure ~Symptoms despite medical management ~If pliable valve, may be candidate for balloon valvuloplasty. 2.Mitral regurgitation Dyspnoea-Fatigue Palpitations ▪︎Pulmonary oedema ▪︎AF ▪︎Endocarditis/ ~Rate control of AF ~Anticoagulation if AF ~Diuretics if HF ~Deteriorating symptoms aim to replace valve before extensive LV damage. ~Condition progresses slowly,so not if asymptomatic. 3.Aorticsclerosis None None None Not applicable 4.Aortic regurgitation, Dyspnoea Palpitations, Heart failure Pulmonary oedema Endocarditis ~Diuretics if •heart failure •Worsening symptoms, •worsening cardiomegaly, •ECG deterioration •Aim to replace valve before extensive LV damage. |
Q |
|
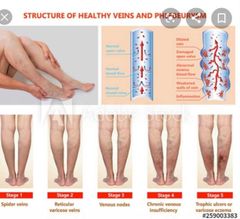
Peripheral oedema Swollen ankles are extremely common in older patients. As with all geriatric medicine, a careful assessment, diagnosis, and appropriate treatment should becarried out. ► Swollen ankles do not always indicate heart failure starting a diuretic must not be an immediate reaction, as this treats only one of several causes and may cause harm. Causes ▪︎Often, mild ankle swelling occurs in an otherwise fit person .this tends to be worse on prolonged standing and in the heat (sometimes referred to as‘dependent oedema’). It is likely that there is some minor venous disease causing the oedema, but it is essentially benign. ▪︎Peripheral venous disease chronic oedema due to damage to deep veins causing venous hypertension, ↑ capillary pressure, and fibrinogen leakage. Usually bilateral, but one side is often worse than the other. ▪︎Heart failure-usually bilateral. Look for associated signs, e.g. raised JVP,cardiac enlargement, pulmonary crepitations, etc. ▪︎Superficial thrombophlebitis-acute oedema with a red, hot, very tender venous cord with surrounding oedema. ▪︎DVT-acute-onset, painful swollen calf with pitting oedema of the ankle. Review thrombotic risk factors. ► Always consider DVT with new-onset unilateral swelling. ▪︎Drug side effect-commonly calcium channel blockers (especially amlodipine) and NSAIDs. ▪︎Low serum albumin- nephrotic syndrome, gastrointestinal loss, malnutrition,chronic disease, acute sepsis, etc. ▪︎Lymphatic obstruction -consider obstructing pelvic tumours. If oedema is severe, perform rectal and groin examination. ▪︎Traumatic-after forcefully dorsiflexing the foot (usually when walking),leading to rupture of the plantar tendon or injury to the gastrocnemius. This oedema tends to be unilateral, tender, above the ankle, and with associated bruising to the calf. Treat with rest and NSAIDs ▪︎Other, e.g. hypothyroidism, osteoarthritis of the knee, ruptured Baker’s cyst,post-stroke paralysis. |
Assessment- History- how acute was the onset? Is it unilateral or bilateral? Is it painful,red, and hot? What are the associated physical symptoms importantly dyspnoea (may indicate PE or heart failure)?
Examination-look for physical signs. ▪︎Always listen to the heart and lungs,and look for sacral oedema when ankle swelling is found. ▪︎Consider rectal/groin examination. Investigations-be guided by your clinical suspicion. -Consider ECG (unlikely to be normal in heart failure), -urea and electrolytes, -albumin, -FBC, - TFTs ► D-dimer in elderly patients with swollen ankles is rarely helpful. While a negative result effectively rules out DVT, many elderly patients will have an elevated D-dimer. only use the test if you would proceed to ultrasound scanning in the event of a positive result.
Treatment All patients with ankle swelling should have a careful assessment for disease,with treatment dependent on cause: ▪︎Stop drugs if they are responsible (consider alternatives) ▪︎If heart failure, then full assessment and treatment are required. ▪︎For chronic venous disease, use leg elevation and compression bandaging or stockings. ▪︎With severe lymphoedema, massage and pneumatic boots can be useful. Consider referral to specialist lymphoedema clinics. ▪︎If low albumin, treat the cause and ↑ dietary intake. ▪︎If no disease is found, then management is pragmatic. ▪︎Patients may find the ankle swelling unsightly, have difficulty fitting on their shoes, or even complain of an aching pain. Support hosiery may help. Many patients may be happy to tolerate the minor inconvenience once they have been assured that there is no serious pathology. It may then be appropriate to start a low dose of thiazide or loop diuretic, but this will necessitate occasional monitoring of electrolytes and clinical review to ensure the benefits of treatment still outweigh the risks. Ankle swelling and nocturnal polyuria During the day, a large amount of fluid can collect in the interstitial space in the ankles.At night, when the legs are elevated, this fluid is partly returned to the circulating volume and can cause a diuresis-hence nocturnal polyuria.
Paradoxically, treating such a patient with diuretics to limit the swelling may ultimately help with the polyuria. |
|
|
|
Preventing venous thromboembolism in an older person VTE, which incorporates DVT and PE, is a major cause of morbidity and mortality in hospitalized patients on both medical and surgical wards. Recognition Older patients present atypically, so diagnosis is often delayed. They less frequently complain of pain and ↓ ability to walk, and their legs more commonly have asymmetrical swelling due to other conditions, e.g.chronic venous insufficiency, arterial or joint disease, etc.
Prophylaxis Most hospitals now use a standardized risk assessment score/tool for all patients. Age >60 is known to be a risk factor for VTE, as is reduced mobility, compared to normal, so almost all older patients will qualify for prophylactic treatment unless risks of treatment (bleeding risk) outweigh benefits. ▪︎Contraindications are sometimes absolute (e.g. immediately after stroke,haematemesis) but more commonly are relative (e.g. extensive bruising after a fall), and the geriatrician must weigh risks and benefits on a case-by-case basis. ▪︎Aspirin is no longer considered effective VTE prophylaxis, but patients on aspirin for other reasons are at higher risk of some bleeding complications (especially gastrointestinal) when low-molecular-weight heparin is given.
Review-Reassessment is required every few days or when there is a material change in the patient condition.Patients who move into rehabilitation settings often regain mobility, and it is unclear from the evidence when prophylactic low-molecular-weight heparin is no longer required. Options include stopping at: •Premorbid mobility level •Steady state •Discharge •Heparin injections should usually be stopped for patients receiving palliative care.
Thromboprophylaxis options for older patients Prophylactic treatment Risks/disadvantages in older patients Notes 1.General measures, e.g. promote mobilization, avoid dehydration ~Earlier mobilization may ↑ falls risk and↑ nursing time.
2.Graduated anti-embolism stockings ~Contraindicated in arterial disease,neuropathies, skin fragility/breaks,and heart failure ~Not always well tolerated Older patients need help to put on and maintain correct position, so ↑nursing time Incorrectly applied stockings (wrinkles)can worsen venous return Proven to be of no help in patients with stroke.
3.Low-molecular-weight heparin Bleeding risks more common in older patients, include risk of falls,low platelets, and anticoagulant treatment A reduced dose should be used in patients with low eGFR or weight
4.DOACs Low dose used for prevention after elective hip or knee surgery
5.Inferior vena cava(IVC) filter Only in very high-risk (e.g. PE despite warfarin) patients who need additional or alternative prophylaxis to full anticoagulation. |
Q |
|
|
|
•Peripheral vascular disease Peripheral vascular disease is common in older patients causing symptoms in 10% of those over 70 years. Symptoms Only a third of older patients will have the classic symptoms of ▪︎intermittent claudication, and often ↓ activity levels will mask developing disease. It maybe difficult to distinguish the pain from that of osteoarthritis. Claudication pain may progress to ischaemic rest pain (night-time pain, often relieved by hanging the foot over the bed), then to ulceration (due to trauma with poor healing—small, punctate, painful ulcers at pressure points, e.g. toes, lateral malleolus, metatarsal heads), and possibly gangrene. Around 80% of patients with claudication remain stable or improve; only 20% deteriorate, and 6% require amputation (ongoing smokers and diabetics are most at risk) Examination Loss of pulses (best discriminator is an abnormality of the posterior tibial pulse) Possibly bruits Coolness to touch Slow capillary refill (over 2s) Shiny, hairless skin with atrophic nails and poor wound healing. Peripheral vascular disease as a marker of other vascular disease. 5-year mortality in peripheral vascular disease is 30%, mostly due to cardiovascular disease. It is easy to detect peripheral vascular disease non-invasively by measuring the ankle–brachial pressure index (ABPI).The impact of broad vascular risk management on subsequent vascular disease burden has yet to be quantified but is likely to be substantial ► If a patient complains of leg pains, screen for peripheral vascular disease and if detected, initiate full vascular 2° prevention. Management Although very common, many elderly people will modify their lifestyle to reduce symptoms. It is important to actively seek out symptoms. Adopt the following treatment approach: ▪︎Modify risk factors (especially smoking) ▪︎Advise ↑ exercise (NOT ↓). This is best done in a supervised exercise programme. ▪︎Commence antiplatelet agent—commonly aspirin; clopidogrel may have slightly more efficacy. ▪︎ Consider other drugs-naftidrofuryl oxalate is the only vasodilator drug supported by NICE. The herbal remedy Ginkgo biloba probably has no impact but does no harm. Do not necessarily stop β-blockers (traditionally thought to worsen claudication). The evidence for this is weak and β-blockers have a major role in modifying cardiac risk. ▪︎Refer for revascularization when appropriate. Percutaneous revascularization is relatively low-risk and should be considered for lifestyle-limiting claudication that does not respond to medical therapy, where there is a focal stenosis, a non-healing arterial ulcer or when there is limb-threatening ischaemia. ▪︎ Elective surgery is usually reserved for low-risk patients (under 70 years with no diabetes and no distal disease) who are fit enough to tolerate the procedure. Age has a significant impact on surgical risk -relative risk of mortality ↑ by 1.62 with each decade. |
HOW TO . . . Measure ABPIWhen?To confirm peripheral vascular disease as a cause of claudicationTo diagnose vascular disease before implementing 2° preventionTo diagnose the aetiology of (venous) ulcersTo ensure compression bandaging is safeEquipmentBP cuff with sphygmomanometerHand-held Doppler probe MethodInflate the cuff around the upper arm as usual, and use the Doppler probeover the brachial artery to measure the SBPRepeat in the other arm, and use the highest valueNext inflate the cuff around the ankle, and use the probe to measure thesystolic pressure in the dorsalis pedis and posterior tibial arteriesTake the highest of the four ankle readings, and divide by the higher of the two arm readings to give the ABPI Interpretation>1.3—non-compressible, calcified vessels; reading has limited value1.0–1.3—normal range<0.9–angiographic peripheral vascular disease very likely0.4–0.9—likely to be associated with claudication<0.4—advanced ischaemia |
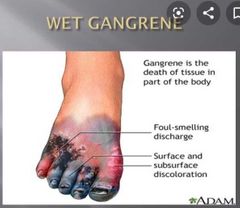
Gangrene in peripheral vascular disease The onset of gangrene is relatively common in people with severe peripheral vascular disease and causes considerable distress. It often poses management difficulties, as many frail older patients are judged inappropriate for open surgery.
Slowly progressive disease with dry gangrene Cyanotic anaesthetic tissue with necrosis Distal, with clear demarcation Often a low-grade inflammatory response (elevated white cell count and CRP) The patient may feel unwell and anorexic Non-urgent surgical review is appropriate, but the approach is often to allow auto-amputation—a lengthy, and sometimes distressing, process, for the patient and family. This may be managed as an outpatient Surgical amputation may be considered, but as there is often inadequate local circulation to allow healing, this may need to be extensive or combined with bypass (the latter carrying a significant operative risk). Sometimes amputation vastly improves the quality of life for a bed-bound patient with gangrene. Discussion about possible amputation should be approached with tact and sensitivity patients are often very against loss of a limb, even when they have not walked for years. Ensure that the patient and family are aware of the rationale for treatment and that there is regular review of analgesia requirements. Wet gangrene Moist, swollen, and blistered skin—usually in people with diabetes ► This is a life-threatening condition, and urgent surgical review is required in all but the most terminal of patients. The usual approach to management is to start iv antibiotics prior to debridement and amputation. Acute ischaemia ► This is a limb-threatening condition and demands urgent action. Usually due to embolization. Distal emboli cause so-called ‘blue toe syndrome’. The main object of treatment is to prevent recurrence, as little can be done to salvage occluded small vessels. This may include angiography to establish the source, and/or anticoagulation depending on the fitness of the patient. Proximal emboli cause diffuse acute ischaemia. Revascularization of the limb is nearly always attempted (unless there is already irreversible ischaemic changes), and the approach can be tailored to the frailty of the patient ranging from thrombolysis and percutaneous thromboembolectomy (possible under local anaesthesia) to emergency bypass procedures. |
|
|
Q |
Q |
|
|
|
Vascular secondary prevention Atheromatous vascular disease (cardiac, peripheral vascular, and cerebral)accounts for a huge amount of morbidity, mortality, and expenditure. 2° prevention measures evolve continually, as individual clinical trials reach completion. Although some interventions are primarily applied to a specific pathology, most impact on all vascular systems. The cumulative effect of therapies is not yet known, but the consensus is that they will substantially reduce the burden of future vascular events.Traditionally, older patients have been under-provided with 2° prevention measures, for a number of reasons: There is often polypharmacy, and reluctance among patients and health-care professionals to add to this. It was thought that 2° prevention benefits were seen only with long-term(perhaps 5–10 years) treatment. Unless life expectancy was greater than this,therapy was not begun.It may be ‘shutting the door after the horse has bolted’ the damage has already been done.But consider several contrary points:Recent evidence suggests that some therapies (statins and ACE inhibitors) act quickly, possibly due to endovascular stabilization.
Although older patients are under represented in clinical trials, where older patients have been included (e.g. the Heart Protection Study), the benefits have been equal, if not greater than in younger patients.Do not assume that a disabled person will not benefit from prevention of further events. In a bed-bound stroke patient who has to be spoon-fed, a further stroke that removes swallowing ability altogether may deprive that person of their only pleasure in life.As with much of geriatric medicine, frank discussion with the patient is advised. Many patients will have strong views and fall into one of two groups the fatalists, who prefer not to take medication ‘just in case’, and the ‘belt-and-braces’ patients who welcome all possible measures. Drug doses are determined by clinical trial evidence often a high dose in generally robust patients. Adopt a pragmatic approach in frail patients with lower doses, as these may be effective with fewer side effects. Reduce doses of renally cleared medication with low glomerular filtration rate (GFR). We do not advocate the blind prescription of all 2° prevention to all of older patients such an approach would be clinically inappropriate for some and unlikely to be cost-effective. What we do suggest is that each patient is considered on a case-by-case basis and, where possible, included in the discussion to reach an individually tailored action plan. |
The main secondary prevention agents Agent- Dose -Action -Outcome -Special points 1》Aspirin-75–300mg od Antiplatelet activity Prevention of all vascular events ~Use lower doses ~Beware gastric irritation Consider co-prescription of proton pump inhibitor/H2 antagonist Enteric-coated formulations unlikely to be very useful.
2》Clopidogrel 75mg od -Antiplatelet activity Mainly as an alternative to aspirin where not tolerated, or as an addition in cardiac and some stroke disease. Reportedly, lower gastric side effects. Slightly higher efficacy than aspirin as monotherapy.
3》Dipyridamole 8MR 200mg bd Antiplatelet activity Addition to aspirin in cerebrovascular disease. NOT for cardiac disease(can exacerbate angina). Start slowly as commonly causes headache. Low bleeding risk. 4》Antihypertensives- Various agents Blood pressure reduction Prevention of all vascular events The lower the BP, the greater the benefit, but take care to avoid hypotensive side effects ACE inhibitors e.g.ramipril10mg od Blood pressure reduction and possible endovascular effect Prevention of all vascular events Dose stated is often not tolerated in older patients aim as highas tolerated, but start with 1.25mg. 5》Statins e.g.atorvastatin 40mg od Cholesterol lowering and possible endovascular effect Prevention of all vascular events Should be prescribed for vast majority Lower doses may be better tolerated. watch for myalgia and raised LFTs Note: consider also smoking cessation, increasing physical activity, and for stroke–endarterectomy and AF prophylaxis. |
|
|
|
Q |
Q |
|
|
|
Chest medicine The ageing lung Respiratory infections Influenza HOW TO . . . Treat influenza-like illness in older people Pneumonia Pneumonia: treatment HOW TO . . . Manage the patient with pneumonia who fails to respond to treatment Vaccinating against pneumonia and influenza Pulmonary fibrosis Rib fractures Pleural effusions Pulmonary embolism Aspiration pneumonia/pneumonitis Chronic cough Lung cancers Tuberculosis: presentation Tuberculosis: investigation Tuberculosis: treatment Asthma and COPD: assessment Asthma and COPD: drug treatment HOW TO . . . Improve drug delivery in asthma or COPD Asthma and COPD: non-drug treatment Oxygen therapy Asbestos-related disease |
Q |
|
|
|
•The ageing lung Most of the functional impairment of the lungs that is seen in older people is due to disease, often smoking-related. Intrinsic ageing leads only to mild functional deterioration. The respiratory system has a capacity well in excess of that required for normal activity, so intrinsic ageing:Does not lead to symptoms in the non-smoker without respiratory disease,although reduced physical activity can lead to a reduction in physical fitness. In those with respiratory disease (e.g. emphysema) will cause progressively worsening symptoms with age, even if the disease itself remains stable. In acute disease, e.g. pneumonia, may cause earlier decompensation or a more severe presentation. Specific respiratory changes Seen in healthy older people are similar to those seen in mild chronic obstructive pulmonary disease and include: ▪︎↓ elastic recoil causing small airways to collapse at low lung volumes and ↑residual volume ▪︎↑ chest wall stiffness, due to:Degenerative change in intercostal, intervertebral, and costovertebral joints. ▪︎Osteoporosis and kyphoscoliosis. ▪︎Weaker respiratory muscles that may have lower endurance. ▪︎Reduced gas exchange and ↑ ventilation–perfusion (V/Q) mismatch, due to collapse of peripheral airways while perfusion remains intact. ▪︎Impaired chemoreceptor function, leading to lessened ventilatory response to↓ PaO2 or ↑ PaCO2. ▪︎Impaired microbial defence mechanisms. ▪︎Less effective mucociliary clearance and less sensitive cough reflex.
Observed consequences of these changes These include: ▪︎↑ susceptibility to infection (underventilation of, and inability to clear sputum from, dependent lung zones) ▪︎Lower maximum minute ventilation (weaker musculature acting against a stiffer chest) ▪︎An approximately linear fall in PaO2 with age (~0.3%/year). Since alveolar oxygen tension remains stable, the alveolar–arterial (A–a) oxygen gradient rises. ▪︎Reduced exercise capacity. However, oxygen consumption and cardiac output decline in proportion to lung function, so the lungs are rarely the limiting factor in exercise performance. ▪︎Breathlessness in older people is often multifactorial. Chronic breathlessness in an individual may be the result of, e.g. ↓ fitness,obesity, an inefficient gait (osteoarthritis or stroke), kyphosis, previous lung damage (e.g. apical fibrosis due to tuberculosis (TB)), and intrinsic ageing. In this example, note that only one of the factors is specific to the lung.
In the acutely breathless patient, pathologies commonly coexist, e.g. infection,fast AF, and heart failure.
The classic treatment triad of digoxin, furosemide,and amoxicillin is not a sign of diagnostic indecision but is often entirely appropriate treatment. |
Respiratory infections Cough with or without sputum, shortness of breath, fever, or chest pain are a very common presentation in older patients. It is very important to try to distinguish which part of the airway is primarily affected because this implies completely different pathogens, prognoses, and treatment strategies.
Try to avoid aggregating all such patients together using the imprecise term ‘chest infection’. Upper respiratory tract infections These are caused by viruses, e.g. rhinovirus, respiratory syncytial virus,influenza, and parainfluenza.
Symptoms include nasal discharge and congestion,fever, and sore throat.
These may extend to the lower tract and then include cough, wheeze, sputum production, or worsening of existing cardiopulmonarydisease. With ↑ age: Upper respiratory tract infection becomes less frequent, but more severe. The risk of complications ↑. These include: ▪︎Lower tract infection such as bronchitis or pneumonia, which may be bacterial or viral. ▪︎Bronchospasm ▪︎Extrapulmonary manifestations such as falls, immobility, and delirium. ▪︎Post-infection weakness, fatigue, and anorexia are more severe and prolonged,maybe lasting several weeks. ▪︎Frequency of hospital admission and death ↑ substantially.
Acute bronchitis Occurs with inflammation of the bronchial tree, with little or no involvement of lung parenchyma (which is pneumonia). Is more common in those with chronic airways disease
Compared with pneumonia, bronchitis: Has fewer systemic features and a better prognosis Has no chest symptoms and signs, e.g. pleuritic pain or crepitations, but may have prominent cough and wheeze.
CXR not routinely indicated Can be managed less aggressively, with more reliance on supportive treatment and bronchodilators than antibiotics. Often viral in origin; if an antibacterial is thought appropriate, give amoxicillin to cover Streptococcus pneumoniae (erythromycin or clarithromycin if penicillin-sensitive). |
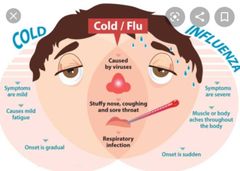
••Influenza This is the most serious viral respiratory tract infection in older patients and is often a severe, systemic illness with pulmonary bacterial super infection (Staphylococcus aureus, Haemophilus influenzae, S. pneumoniae). It occurs most commonly in December–February. Antigenic shifts result in periodic pandemics. Presentation is similar in young and old, i.e. rapid onset of fever (rigors,chills), myalgia, headache, and fatigue, with variable degrees of prostration.
Compared with less threatening viruses such as rhinovirus: Nausea, vomiting, diarrhoea, high fever, rigors, and ocular symptoms (e.g.photophobia) are more common.
Rhinorrhoea is less common. Less common serious complications include ▪︎myocarditis and ▪︎meningoencephalitis. Mild meningism is common and, if combined with other sinister features (e.g. altered conscious level), is an indication for CSF sampling.
Diagnosis is usually based on combining clinical assessment with epidemiological data, particularly current influenza incidence. Some other viruses can cause an identical clinical syndrome, and serological test results are not immediately available. Thus, an initial assessment cannot produce an absolutely confident microbiological diagnosis. The syndrome may therefore most precisely be labelled ‘influenza-like illness’. Positive virological diagnosis in the context of ↑ community incidence or a care home outbreak is helpful by prompting vigorous attempts to reduce transmission of infection.
•Reducing viral transmission All >65yr olds in the UK are offered yearly influenza immunization. Massoutbreaks of respiratory viral infection are common in care homes andhospitals. They can occur at any time of the year but are most common fromautumn to spring. Viruses are spread by aerosol or hand-to-hand contact(sometimes indirect, via fomites such as cutlery or drinking vessels).During an outbreakReduce transfers of healthy patients into, or symptomatic patients out of,the affected areaReduce staff movement across work areas (especially applicable to short-term staff who may work in many clinical areas in a short time)Care for symptomatic patients in single rooms, or in ward bays withsimilarly infected patientsExclude visitors with respiratory or viral symptoms from the wardEnsure that care staff have been immunized against influenzaEnsure that scrupulous hand-washing procedures are followedConsider using face masks for staff caring for symptomatic patients |
|
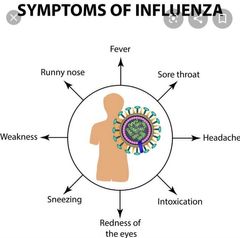
•HOW TO . . . Treat influenza-like illness in older people The following guidance is generic and should be tailored to the patient, their illness, and their care environment. If the highest-quality care cannot be provided, then a prompt step-up of care should be arranged. This may include hospital admission. Do not underestimate the disease. Mortality and morbidity ↑ exponentially with age and frailty. Give excellent supportive and symptomatic care. Its effectiveness should not be underestimated.
Fluids. Reduced intake and ↑ losses (fever) lead to volume depletion and end-organ dysfunction. Encourage frequent oral fluid, and suspend any diuretic treatment. Consider early initiation of s/c or iv fluids if a vicious spiral of dehydration and poor intake seems likely to ensue.
Nutrition. Encourage high-calorie, high-protein drinks or solids. If theillness is especially severe, prolonged, or complicated, or if the patient is especially frail or malnourished, consider a period of NG feeding. Involve a dietician early.
Paracetamol. If fever, discomfort, or pain occur.
Maintain mobility. Bed rest may sentence the patient to death or dependency. Carers may need clear, firm advice about this.
Identify and treat complications promptly Carers may need information about important warning signs and the need to seek prompt medical advice. Perform regular observations of BP, pulse, and temperature where possible. Common serious complications include ▪︎delirium, ▪︎2° bacterial infection, ▪︎bronchospasm, ▪︎pressure sores, and ▪︎circulatory collapse
Antiviral agents (the neuraminidase inhibitors zanamivir and oseltamivir)may reduce both the severity and duration of influenza. They are indicated in patients with severe symptoms if they are pregnant, >65 years, or with certain comorbidities (especially if hospitalized)
Zanamivir is inhaled; oseltamivir is taken po. |
Pneumonia This is a syndrome of acute respiratory infection with shadowing on CXR. May be lobar, bronchial, or mixed pattern. Symptoms may be mild and are often non-organ-specific, e.g. fever, malaise. Common presenting scenarios include cough (often unproductive), delirium, reduced conscious level, lethargy, anorexia, falls, immobility, and dizziness. Rarely patients can present with shock, coma, and adult respiratory distresssyndrome (ARDS) Chest pain, dyspnoea, and high fever are less common than in younger people.
Signs may be minimal: ▪︎The patient may be well or unwell. ▪︎Assess severity using the CURB criteria Fever is often absent, but vasodilatation is common Tachypnoea is a sensitive sign, as is at least moderate hypoxaemia (≤95%on air) on oximetry.
Tests often guide management: Chest radiograph may reveal only minimal infective infiltrate. Also look for malignancy, effusion, or heart failure. Blood cultures should be sent, but sputum culture is rarely useful, unless TB is suspected. White cell count may be raised, normal, or even depressed. CRP is often normal early in the illness. U, C+E guide fluid management. Kidney impairment is a sign of poor prognosis. Arterial blood gas (ABG) sampling is not usually necessary, unless oxygen saturations are <90%; oximetry is much better tolerated and usually sufficient to guide oxygen therapy. Organisms vary depending on the clinical setting in which pneumonia occurs. Often no causative organism is identified. Pneumococcus is common in all settings, including hospital. Viral pneumonia, especially influenza, is under-recognized and is the second most common cause of community-acquired pneumonia. Legionella and Mycoplasma pneumonias are uncommon. Mycoplasma is much more frequent during epidemics, occurring every 3 years or so. Unusual organisms are more common in frail patients, in higher-dependency environments, and in those who have recently received courses of antibiotics. Organisms include Gram negatives (which colonize the oropharynx) and anaerobes (a result of aspiration of gut contents). MRSA pneumonia can occur in hospital or community. Health care-associated pneumonias can occur after discharge. |
Pneumonia pathogens in various care settings (in approximate order of frequency) 1》︎Community-acquired ▪︎S. pneumoniae (>30% ofcases) ▪︎Viral, e.g. influenza,parainfluenza, respiratory syncytial virus ▪︎H. influenzae ▪︎Gram-negative aerobicbacilli, e.g. Klebsiella, P.aeruginosa ▪︎Legionella pneumophila. ▪︎Mycoplasma pneumoniae if epidemic ▪︎Other, e.g. TB Following influenza,think of 2° bacterial infection, especially withS.pneumoniae (most common), H. influenzae, or S. aureus 2》︎Care home-acquired ▪︎S. pneumoniae (>30% of cases) ▪︎Viral, e.g. influenza, parainfluenza, respiratory syncytial virus ▪︎Gram-negative aerobic bacilli,e.g. Klebsiella, Pseudomonas aeruginosa ▪︎H. influenzae Viral, e.g. influenza,parainfluenza, respiratory syncytial virus ▪︎Anaerobes, e.g. Bacteroides, especially in those at risk ofaspiration, e.g. immobility,swallowing difficulty, prolonged recumbency, or impaired conscious level. ▪︎Others TB ▪︎Following influenza, think of 2° bacterial infection, especially with S. pneumoniae (most common), H.influenzae, or S. aureus 2》︎Hospital-acquired ▪︎Gram-negative aerobic bacilli, e.g. Klebsiella, Pseudomonas aeruginosa ▪︎aerobes, e.g. Bacteroides, especially in those at risk of aspiration, e.g. immobility, swallowing difficulty, prolonged recumbency, or impaired conscious level ▪︎S. aureus (including MRSA) ▪︎S. pneumoniae and H. influenzae. NB. These may be the most common pathogens—in non- acute settings, e.g. rehabilitation wards—in the well, less frail patients. ▪︎Viral, e.g. influenza, parainfluenza, respiratory syncytial virus. |
|
|
Pneumonia: treatment Multifactorial treatment should be started promptly. Ideally, antimicrobials should be administered within 1h, alongside the following: ▪︎Assess and optimize fluid volume status; give po, sc, or iv fluid as appropriate. Concurrent heart failure is common, but volume depletion more so. ▪︎If there is subjective dyspnoea or moderate/severe hypoxaemia, then supplement oxygen, titrating the inspired oxygen concentration upwards to achieve arterial oxygen saturations of 94–98%. ▪︎For lesser degrees of hypoxaemia, it is not necessary to subject patients to claustrophobic, uncomfortable oxygen masks,simply monitoring saturations may be sufficient. ▪︎Exercise caution in COPD and other conditions that predispose to carbondioxide (CO2) retention. Target saturations of 88–92%. ▪︎Observe the patient closely, both clinically and with serial ABG sampling. ▪︎Avoid the use of nasal specs acutely; if ventilatory drive is poor, inspired oxygen concentrations are very uncontrolled. ▪︎Encourage mobility. ▪︎If immobile, sit upright in bed, and sit out in a chair. ▪︎Request physiotherapy if there is a poor cough or lobar/lung collapse. ▪︎Use saline nebulizers to loosen secretions which are difficult to expectorate,and bronchodilator nebulizers when wheeze suggests associated bronchoconstriction. ▪︎Minimize the risk of thromboembolism, unless contraindicated, through prophylactic heparin and early mobilization. ▪︎Assess pressure sore risk and act accordingly. ▪︎If dyspnoea, anxiety, or pain is very distressing, consider opiates. ~Side effects include respiratory depression, sedation, and delirium, so begin with small doses and assess effect. ▪︎Anticipate possible deterioration, and judge in advance the appropriate levels of intervention. ▪︎Would renal dialysis, ventilation, and/or cardiopulmonary resuscitation be effective and appropriate? Keep the family informed. Where possible, enlist their help, e.g. in encouraging eating and drinking |
•Characteristics of severe pneumonia: the CURB-65 score Five key criteria (acronym ‘CURB-65’) determine prognosis and can helpguide location of treatment: 1.Confusion (AMTS ≤8) 2.Urea (serum urea >7mmol/L) 3.Respiratory rate (≥30/min) 4.Blood pressure (<90 systolic and/or ≤60mmHg diastolic) 5.65 years of age or more. The score has a six-point scale (0–5 adverse prognostic features): 0 or 1: low risk of death (0–3%) 2: intermediate risk of death (13%) 3, 4, or 5: severe pneumonia, with high risk of death (score 3: mortality17%; score 4: mortality 41%; score 5: mortality 57%) A five-point scale using only four criteria (CRB-65; urea excluded) can be applied outside hospital and also discriminates effectively between good and poor prognoses (e.g. mortality score 1: 5%; score 3: 33%). Antimicrobials Refer to local guidelines, reflecting pathogen sensitivities and drug costs. □Community or care home settings- Amoxicillin po is usually effective (vs S. pneumoniae and H. influenzae).
□Erythromycin or clarithromycin if penicillin-allergic Add clarithromycin (or erythromycin which has more gastric side effects) if there are features of ~atypical pneumonia, there is a ~Mycoplasma epidemic, or the patient may have had ~influenza. □Co-amoxiclav po has added activity against some Gram-negatives and S.aureus, and may be more effective in the frail patient or where aspiration is likely. □Ciprofloxacin alone should be used rarely- it has Gram-negative activity but is less effective against S. pneumoniae, an important pathogen in most settings. If an antimicrobial is sought that will cover both chest and urinary sepsis, a better choice may be co-amoxiclav or trimethoprim. □iv antibiotics are only necessary if the patient is very unwell (CURB-65 scoreof 3 or above). Examples include co-administration of co-amoxiclav and clarithromycin. □If you suspect MRSA pneumonia, add vancomycin. □Convert to oral therapy and change broad- to narrower-spectrum drugs when the patient’s condition improves and/or culture results are known, to minimize complications, e.g. CDAD (see ‘Clostridium difficile-associated diarrhoea’). ■Often, only 48h or less of broad-spectrum iv therapy is needed. Hospital-acquired infection ▪︎This presents a difficult dilemma. Hospitalized patients, especially those who are more frail and have spent longer in hospital, are prone to Gram-negative and anaerobic pulmonary infections. ▪︎However, they are also susceptible to the adverse effects (especially diarrhoea) of broad-spectrum antimicrobials. ▪︎A hierarchical approach is sensible, considering likely pathogens and illness severity, guided by local protocols: In the less frail patient who remains well, begin amoxicillin alone, co-amoxiclav, or a combination of amoxicillin and a quinolone (all po). ▪︎Broaden the spectrum only if the patient deteriorates or culture results suggest that thelikely pathogen is insensitive. If a patient is at high risk of Gram -negative infection (frail,dependency, prolonged stay, invasive procedures, aspiration risk), begin with iv piperacillin/tazobactam (or equivalent). ▪︎Narrow the antimicrobial spectrum when the patient’s condition improves and/or a pathogen is identified. If the patient has received multiple courses of treatment, seek microbiology advice. ▪︎In all cases, take blood cultures, and monitor the patient carefully. |
How to Manage the patient with pneumonia who fails to respond to treatmentIs the diagnosis correct?▪︎Consider other chest pathology such as heart failure, PE, pleural effusion, empyema, cancer, or cryptogenic organizing pneumonia. Extrathoracic pathology mimicking pneumonia includes ▪︎acidosis (tachypnoea) and ▪︎biliaryor ▪︎pancreatic pathology Review the history, examination, and investigations. Consider admission to hospital and further tests. Is there a complication? For example, effusion, empyema, heart failure, silent MI, or PE. Is the antibiotic being taken regularly and in adequate dose? Is adherence a problem? Could a friend or relative help prompt tablet-taking, or would a Multiple Dosing System help? Syrups may be swallowed more easily than tablets. If swallowing remains ineffective, or drug absorption in doubt (e.g.vomiting), then consider iv/im therapy. Is the organism resistant? Take more blood cultures. Consider a change in antimicrobial, taking into account likely pathogens and their known sensitivities. Consider atypical infection; send urine for Legionella antigen test,especially if the patient is immunocompromised or if a patient appears disproportionately unwell. Remember MRSA pneumonia, especially in those known to be previously colonized. Could other elements of care be more effective? For example, fluid balance, oxygenation, nutrition, posture, and chest PT. Would this be more effectively delivered on ITU/high dependency unit(HDU)? Is this an end-of-life situation? Is treatment to extend life now inappropriate, the failure to respond a sign that the diagnosis is of ‘dying’. If the patient cannot tell you their wishes, determine their likely views by discussing with family and friends,a decision informed by your judgement of where in their life trajectory your patient sits. In determining prognosis, consider comorbidity—is this an abrupt, potentially reversible illness in an otherwise fit person, or a further lurch downhill for a patient with multiorgan failure? Not for nothing is pneumonia referred to as ‘the old man’s friend’, sometimes bringing to a brisk and welcome end a period of irrevocable decline and suffering. |
|
|
Vaccinating against pneumonia and influenza Vaccine delivery ▪︎Both vaccines should be offered simultaneously in October or early November to all aged >65 years, especially: ▪︎Care home residents. ▪︎The immunosuppressed ▪︎Those with comorbidity, e.g. heart failure, COPD, diabetes. ■Pneumococcal vaccination is given once, whereas influenza vaccination should occur annually. Reliable delivery of these vaccinations depends upon effective information management systems in GP, and substantial efforts by patients, carers, district nurses, and 1° care nurses. A common reason to have missed immunization is to have been a long-term inpatient (e.g. undergoing rehabilitation) during the autumn immunization period. Hospitals should ensure that these in patients are immunized. Vaccinating health-care workers, especially those working in long-term care settings, reduces the spread of infection, and there fore death due to influenza,among patients. Pneumococcal vaccine Pneumococcal polysaccharide vaccine (PPV) is effective against 65% of serotypes. Immunity remains for at least 5 years, perhaps for life. Bacteraemia is reduced by at least 50%. The effect on incidence of pneumonia itself is less clear. Influenza vaccine The trivalent vaccine is prepared from currently prevalent serotypes. Immunity develops in <2 weeks, and it is therefore useful during epidemics Immunity remains for up to 8 months. The risk of pneumonia, hospitalization, or death due to influenza is reduced byover half. Post-exposure antiviral prophylaxis Pharmacological prophylaxis of influenza is currently recommended when an unimmunized, high-risk group adult (e.g. care home resident) has had close contact with a person with influenza-like illness during a period when flu is prevalent. 1Treatment with neuraminidase inhibitors must be initiated promptly (within24h) Consider why immunization was not performed. Is it too late to administer this year (this contact may not have ‘flu, but the next one might)? If not, then optimize the chances of immunization next year. |
Q |
Q |
|
|
•Pulmonary fibrosis This common problem is much under diagnosed in older people due to a combination of under-investigation and overlap of clinical signs with common pathologies such as heart failure. Consider when breathlessness coexists with profuse fine chest crepitations,with or without clubbing, or is slow to recover from apparently minor respiratory infection. On CXR, there may be bilateral pulmonary shadowing indistinguishable from pulmonary oedema, but with little supporting evidence(e.g. normal heart size, absent Kerley B lines).
Causes Idiopathic. The most common type in older people, known as usual interstitial pneumonia Connective tissue disease, e.g. rheumatoid arthritis (most common), systemic lupus erythematosus (SLE), sarcoidosis. Lung involvement is sometimes the first manifestation of the multi system disease. Drugs, e.g. amiodarone, nitrofurantoin rarely Occupational exposure, e.g. asbestos, silica If localized, consider TB,bronchiectasis, and radiotherapy
Tests The diagnosis is usually confirmed by high-resolution CT scanning, which can also help distinguish subgroups likely to respond to immunosuppressive treatment. Respiratory function tests may be useful (a restrictive picture with ↓ transfer factor is usual) but typically adds little in the frail older person. Consider referral to a respiratory physician to confirm diagnosis and guidance on management
Prognosis This is very variable-about a third are clinically stable, a third improve, and a third deteriorate at rates that vary greatly between individuals. Some can live with pulmonary fibrosis for years without significant functional impairment. Treatment Treat or remove any underlying cause, e.g. drugs A minority respond slowly (over weeks) to immunosuppression (e.g.prednisolone and azathioprine or pirfenidone). Ensure bone protection with calcium, vitamin D, and bisphosphonates. Home oxygen therapy is often useful Give opiates for distressing dyspnoea. In those in whom dyspnoea progresses, consider end-of-life issues, including treatment limitation, and a change of focus from life-extending measures to a purely palliative approach. |
Rib fractures Common in older people.Often a result of falls or even minimal bony stress such as coughing in aperson with osteoporosis. Consider the possible contribution of alcohol, which causes both falls and osteoporosis. Diagnosis Rib fractures should be diagnosed clinically.Point tenderness and crepitus are often found. Pressure over the sternum may provoke the pain in a lateral rib. CXR, even with multiple projections, may miss the fracture but is useful in excluding early complications such as pneumo- or haemothorax. Radioisotope bone scans are very sensitive but not specific (hot spots are often found without clinical fracture), so they are rarely indicated.
Management Rib fractures heal without specific treatment. The major problem is pain, which commonly leads to voluntary splinting of the injured area. There is hypoventilation and a failure to clear secretions, and 2° pulmonary infection can occur.The patient should be encouraged to breathe deeply and to cough. Supporting the injured area when coughing, using a small pillow, minimizes pain. Strapping of the affected area is no longer done, as it ↑ complication rates. Regular analgesia should include paracetamol, plus a weak opiate in most cases. A short course of NSAIDs with gastric protection may be helpful. Anticipate and treat complications (usually infection) promptly. In cases of severe pain (e.g. multiple fractures), consider strong opiates or intercostal/paravertebral blocks. Involve the local pain team.
Surgical repair is an option, e.g. for flail chest, multiple fractures.The patient is often reassured with a diagnosis and explanation that the injury itself is not severe and will heal without immobilization, and that coughing will prevent complications, not cause further damage. |
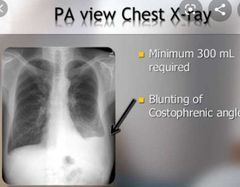
•Pleural effusions A frequent (clinical or radiological) finding, sometimes incidental. Common causes are heart failure, post-pneumonia, PE, and malignancy (especially lung1°, mesothelioma, leukaemia, lymphoma, and metastatic adenocarcinoma (ovary,stomach)). The differential diagnosis is wide, but narrowed when the results of CXR and pleural fluid aspiration are known. Pleural aspiration (diagnostic and therapeutic) is now mainly done under ultrasound guidance by a clinician with appropriate competencies. Light’s criteria is used to differentiate fluid type. Exudate is likely if one of the following is present: ▪︎Effusion protein:serum protein ratio >0.5 ▪︎Effusion lactate dehydrogenase (LDH): serum LDH ratio >0.6 ▪︎Effusion LDH level > two-thirds the upper limit of the laboratory’s reference range of serum LDH
Differentiating cause by fluid type Transudate Heart failure Hepatic cirrhosis Hypoproteinaemia, e.g. malabsorption, sepsis Nephrotic syndrome (Exudative causes if low serum protein)
Exudate Malignancy Infection, including TB Gastrointestinal causes,e.g pancreatitis Multisystem disorders,e.g. rheumatoid (Heart failure after diuresis)
Empyema, malignancy, and TB produce exudates with low pH (<7.2), low glucose (<3.3mmol/L), but high LDH. Transudates are usually not due to focal lung pathology and so usually affect both lungs. Unilateral effusions due to transudates occasionally do occur,more commonly on the right side. Effusions due to heart failure are typically bilateral, with cardiomegaly; they can be unilateral, but usually a tiny contralateral effusion is seen, manifesting as blunting of the costophrenic angle; if the angle remains sharp, other causes are more likely. A massive unilateral effusion is usually due to malignancy. A uniformly bloodstained effusion is usually due to infection, embolism, malignancy, or trauma.
Chronic effusions. If bilateral transudates, should be treated as for heart failure. If the diagnosis is not clear after CXR and aspiration, consider CT chest and/or chest physician referral. For large, recurrent effusions, consider chest physician referral for continuous out patient external fluid drainage via a semi-permanent intra pleural catheter or pleurodesis. Frail patients may not tolerate, or desire, the more invasive tests. In this case,consider: ▪︎Repeated aspiration, combining diagnostic with therapeutic taps and sending larger volumes of fluid for cytology and acid-fast bacilli culture‘ ▪︎Watching and waiting’, with regular clinical review. ▪︎A trial of diuretics, especially if the effusion is a transudate. |
|
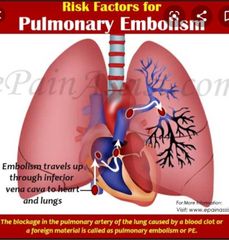
Pulmonary embolism PE is common yet, as ‘the great pretender’ (of other pathology), is under-diagnosed and underreported on death certificates. It commonly coexists with,and is confused with, other lung disease, e.g. pneumonia, heart failure, and COPD—and is a common cause of deterioration in such patients. Presentation The classic symptom triad of pain, dyspnoea, and haemoptysis is seen less commonly in older people.
Common presentations include: Brief paroxysm(s) of breathlessness, or tachypnoea. Collapse, cardiac arrest, syncope, presyncope, or hypotension. Pulmonary hypertension and right heart failure, presenting as chronic unexplained breathlessness. Puzzling signs, e.g. fever, wheeze, resistant heart failure, arrhythmia,confusion, or functional decline. Investigations Determining the likelihood of PE rests on combining clinical judgement (the product of history, examination, and immediately available tests such as CXR)with appropriate imaging such as V/Q scan or CT pulmonary angiography(CTPA). The common clinical features of PE—tachypnoea, tachycardia, and modest degrees of hypoxaemia—are common in ill older people, so clinical judgement alone is rarely enough. Moreover, a confident diagnosis is essential because in older people: The risk of anticoagulation is higher The risk of a missed diagnosis is higher (less physiological reserve) Possible PE in older people should be investigated in the usual way, with the choice of tests guided by local facilities and expertise. The following issues are especially relevant: In a patient without known lung disease, the combination of breathlessness and a CXR showing clear lung fields strongly suggests PE.
Further test(s)(V/Q or CTPA) are indicated. Ensure adequate hydration prior, to minimize the risk of contrast nephropathy.
CXR abnormalities may be minor (atelectasis, raised hemidiaphragm, small effusions) or major (usually reflecting comorbid conditions, rather than PE itself). Classical wedge shadows or unilateral oligaemia are rare. PE in the absence of lower limb DVT is common (10–20% of cases), so do not be put off by an absence of clinical signs of the leg or a negative Doppler ultrasound. D-dimer can be a useful screening test to rule out PE, but because many older people have coexisting conditions, e.g. infection,false positives are very common (i.e. sensitivity high, specificity low) ABGs have some value in diagnosis, but the common abnormalities (low PaO2, low PaCO2, and ↑ A–a oxygen gradient) are neither sensitive nor specific. In healthy older people, an ↑ A–a gradient is common. In older people following PE, a normal A–a gradient is seen in >10%.
Echocardiogram may be normal following PE. However, in a patient with a high clinical probability, typical features of PE on echocardiogram may provide sufficient diagnostic confidence to permit anticoagulation without further imaging. In the patient with unexplained right heart failure, consider PE-obtain an ECG and echocardiogram (ask for PA pressures) and request imaging that details the lung parenchyma (high-resolution CT: pulmonary fibrosis?) and the vasculature (CTPA: PE?) In the patient who does not respond to treatment for chest infection, heart failure, or acute exacerbation of COPD, consider whether PE may be responsible. |
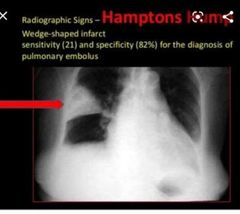
Treatment AnticoagulationStandard treatment is low-molecular-weight heparin (e.g. dalteparin,enoxaparin), followed by warfarin, with a goal for INR of 2–3. DOACs (e.g.apixaban, dabigatran) are also recommended and achieve full anticoagulationafter the first dose. Once the possibility of PE is raised, it is essential to treatwith treatment-dose low-molecular-weight heparin pending investigation results,unless there are particular treatment risks.To minimize bleeding risk:Anticoagulate with caution. Check baseline clottingBeware the older patient with mild anaemia/low MCV—do they have occultblood loss?In the very frail, sick, unstable patient in whom oral anticoagulation wouldpresent significant risk, consider a period of anticoagulation with low-molecular-weight heparin. Start an oral agent when clinical stability returns ThrombolysisConsider thrombolysing, balancing risks and benefits, where there is life-threatening PE manifesting as acute right heart strain and systemic hypotension.Both risk and benefit ↑ with age, so age itself is not a contraindication.Inferior vena cava filterAn IVC filter can be inserted under local anaesthesia by an interventionalradiologist. Follow-up should be arranged to consider timing for removal.Indications include:Strong contraindication to anticoagulation, e.g.:Active bleedingA high risk of bleeding, e.g. newly diagnosed peptic ulcer or very recenthaemorrhagic strokeMassive thromboembolism with contraindication to thrombolyisOngoing thromboembolism despite anticoagulationEmbolism from a septic focus |
|
|
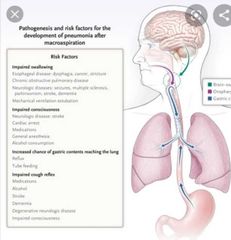
Aspiration pneumonia/pneumonitisThe involuntary entry of extrinsic material into the pulmonary airways. This is a common problem, ranging from subclinical micro-aspiration of oropharyngeal mucus to major inhalation of gastric contents. Risk factors ▪︎Swallowing problems ▪︎Gastro-oesophageal disorders leading to reflux. ▪︎Impaired conscious level, including seizures. ▪︎Sedative drugs ▪︎Previous aspiration or non -aspiration pneumonia ▪︎Clinically assisted nutrition—either NG or gastrostomy DiagnosisCommonly, the occurrence of pneumonia in a patient with risk factor(s) suggests the diagnosis. CXR may show consolidation in dependent lung zones, e.g. right lower lobe,although any zone may be affected. TreatmentThe role of antibiotics is debated. Much of the radiographic response may be chemical pneumonitis, i.e. inflammatory reaction to caustic gastric contents,rather than infective pneumonia.The choice of antibiotics is also contentious. Many cases respond well toamoxicillin or co-amoxiclav, but consider broad-spectrum iv antibiotics tocover Gram-negatives and anaerobes in: ▪︎The unwell and frail ▪︎High-dependency settings ▪︎Where aspiration has been majorIf possible, treat the underlying cause. If risk factors persist (e.g. impairedswallow or continual seizures), consider a ‘nil by mouth’ order until they are addressed. Where the swallow may be impaired, perform a formal swallowingassessment and manage according to the results.In palliative care;Consider anticholinergics to dry secretions. In advanced dementia, it is often appropriate to accept the risk ofaspiration. Insertion of a gastrostomy (commonly a PEG) risks medicalizing the final months while achieving nothing—aspiration is common in patients with a PEG.It is often cruel and futile to deny a dying patient food that he or she mayenjoy, even if the risk of aspiration and a life-shortening pneumonia exists.‘Nil by mouth’ orders are usually inappropriate in end-of-life situations.Instead consider using thickened fluids, pureed diet, supervised feeding, and avoiding eating in a recumbent position, with straws or feeder cups |
Q |
|
|
|
Chronic cough A common problem, with causes ranging from the trivial to the sinister. Even where the underlying cause is benign, chronic cough can be both distressing and disabling. Causes 1.Asthma. (Cough is a common presenting symptom in older people.) 2.Silent pulmonary aspiration 3.GORD 4.Post-nasal drip can be due to sinusitis or chronic rhinitis. Frequently allergic in origin, but in older people, symptoms are often not seasonal. 5.Drugs, e.g. ACE inhibitors (may take weeks or months to develop), 6.β-blockers(leading to bronchospasm) 7.Persistent benign cough following upper respiratory tract infection. May persist for 2–3 months 8.Chronic pulmonary pathology, e.g. COPD, TB, bronchiectasis 9.Heart failure, with high pulmonary pressures 10.Thoracic malignancy, either 1° or 2°
Investigation Consider both tests and trials of treatment. Their pace and extent depend on the differential diagnosis following careful history and examination. Consider the following tests: CXR (mandatory) Sinus imaging Spirometry, with assessment of response to bronchodilators Monitoring of PEFR, looking for morning drops suggesting asthma Sputum microscopy and culture are unlikely to be helpful.
Next, consider a trial of treatment for the most likely cause, e.g.:Bronchodilators (and inhaled steroids) for possible asthma. A PPI for possible GORD. Assess the effect of treatment of possible chronic rhinitis with: Nasal corticosteroids. Probably the treatment of choice, e.g.beclometasone, budesonide Decongestants. Should be used in short courses only (since rebound phenomenon) Antihistamines. Most useful for obvious allergic rhinitis. Can be topical spray or tablet. Should be used with caution; select those with fewer anticholinergics properties, e.g. cetirizine or loratadine In all cases, trials of treatment need to be prolonged (≥8 weeks). Treatment This is of the underlying cause. Where this cannot be treated effectively (e.g.advanced malignancy), specific treatments aimed at reducing cough may be of benefit. These include opiates such as codeine or morphine. Simple coughlinctus may be useful for irritating dry cough following an upper respiratory tract infection. |
Q |
|
|
|
A disease of the old. Early suspicion especially with pneumonia in elderly Look for sinister features Early investigations Now treatment methods have improved both for extending life and Palliation
Lung cancers The most common cause of cancer deaths, and largely a disease of older people. Symptoms may be non-specific (e.g. fatigue, weight loss), or else pulmonary in origin but attributed to existing non-malignant pathology (e.g. dyspnoea in a patient with COPD)
Have a high index of suspicion and a low threshold for further investigation.
Have an even higher degree of suspicion in older smokers presenting with pneumonia. Sinister features in those presenting with pneumonia include: ▪︎Haemoptysis, especially if significant, e.g. with persistent blood clots ▪︎Regional or generalized symptoms of cancer (e.g. hoarse voice, weight loss) ▪︎Cough and consolidation without obvious infective symptoms (e.g. fever) ▪︎Symptoms that continue to be troublesome despite antibiotics. If sinister features are present, it is unacceptable to wait (up to 6 weeks) before repeating a CXR to confirm resolution. Refer promptly for urgent specialist assessment, and consider CT scanning, bronchoscopy, or lung biopsy.
Treatment Treatment has improved and is now more effective, both in extending life and in palliating symptoms. Therefore, ‘benign neglect’, i.e. simply observing an older person with probable lung cancer, is now only rarely acceptable. It maybe appropriate, for example, in cases of extreme frailty or severe cognitive impairment. Older people with probable lung cancer remain under-investigated and under treated:Tests such as bronchoscopy and a histopathological diagnosis are less commonly obtained. This makes palliative treatment and prognostication difficult. Treatment such as surgery or chemotherapy are less commonly considered or administered. To an extent, this reflects appropriate decision-making based on functional status. Treatment decisions should be made by expert MDTs that consider the patient’s functional status, comorbidities, and cancer characteristics ► Refer all patients with suspected or confirmed lung cancer for a specialist opinion. |
Non-small cell carcinoma (squamous cell, adeno-, and large cellcarcinoma) Surgery may lead to cure if: There is adequate pulmonary function (arbitrarily, FEV1 ≥1.5L) There is no distant spread (but >50% of cancers have spread at presentation) The patient is relatively well with good functional status and no serious comorbidity. Surgical procedures are high-risk, but the condition is always fatal without treatment, so the patient’s view is critical.
Radiotherapy. When surgery is not feasible, either because of the nature of disease or the fitness of the patient, then radiotherapy may be used either palliatively (to control symptoms), neoadjuvantly (to reduce tumour volume and sometimes to convert a non-operable tumour into an operable one), or occasionally curatively
Molecular characterization of tumour tissue enables tailored treatment. Chemotherapy, molecularly targeted therapy, and/or immunotherapy can be useful.
Small cell carcinoma Relatively more common in older people: >20% of cases. Most cases are advanced at presentation. Most patients are treated with combination chemo- and radiotherapy. Frail patients are unlikely to tolerate aggressive treatment and it risks reducing the quality of the brief life that remains. Therefore, regimens are tailored to the patient, determined by structured assessment of performance status. Ingeneral, frail patients undergo fewer, but similar, therapy cycles, compared to the more robust. Prophylactic cranial irradiation ↓ the incidence of brain metastases and prolongs survival in patients with limited disease. Very advanced disease is managed supportively or with chemotherapy alone. Surgery is seldom useful because tumours are rarely localized at presentation. Palliative interventions ▪︎Radiotherapy for superior mediastinal obstruction, bronchial obstruction, chest pain, haemoptysis, or painful bony metastases. This is generally well tolerated, although ~10% develop radiation pneumonitis weeks after treatment, and it is on average more severe in older people ▪︎Opiates for cough ▪︎Aspiration of pleural effusion for breathlessness ▪︎Endobronchial therapy (e.g. stenting, diathermy) |
How do opiates work for cough Does it have an effect on breathlessness |
|
|
•Vague presentation •Mostly due to reactivation so multi drug resistant TB is rare in elderly •Milliary more common in elderly •Taking a sputum sample may be difficult in elderly •Other modalities of investigations are available - active pulmonary-sputum culture - active gu-urine -latent- mantoux/ interferon gamma in blood -type of organism- pcr
•Tuberculosis: presentation In older people: Incidence of TB is much higher, especially in the very old and certain immigrant populations Outcomes, including mortality, are much less good. TB is most commonly due to reactivation of previous disease, the 1° infection having been asymptomatic or unrecognized. In the early twentieth century, 1°infection of young adults was common. By the mid-late twentieth century, 1°infection in younger people had diminished. When this cohort reaches old age,TB reactivation will be much less common. Reactivation (post-1° disease) occurs due to ↓ immunity, it self due to intrinsic ageing, disease (e.g. diabetes mellitus, renal failure), malnutrition (e.g. chronic alcohol excess), or drugs (e.g. steroids) A few patients develop new infection from open cases. Care home residents are most vulnerable, infection passing from fellow residents or from care home staff. Consider HIV infection when TB diagnosed. Presentation
Pulmonary disease ▪︎Sometimes similar to that in younger people, i.e. cough, sputum, fatigue,weight loss, and anorexia. ▪︎Night sweats, fevers, and pulmonary symptoms may be less commonly present as pneumonia that fails to resolve or as an incidental finding suggested on CXR.
Extrapulmonary disease Most (>75%) presentations are pulmonary, but extra pulmonary cases are relatively more common in older people, e.g.:Miliary. Diffuse, overwhelming infection with fever, weight loss, and hepatosplenomegaly. Pancytopenia can occur.
Urogenital and renal. May affect any part of the renal tract. Sterile pyuria,haematuria, abdominal or back pain, genital sinuses, or pelvic masses may occur, or disease may be asymptomatic.
Meningeal. Consider this in the very frail, malnourished, or immunosuppressed patient with non-specific cerebral signs (e.g. confusion,dementia-like syndrome, headache, or reduced conscious level). Meningism may be absent, and the CSF virtually acellular.
Skeletal. Bone infection most commonly affects the spine (usually thoracic or lumbar), presenting as pain and tenderness. TB arthritis usually affects large weight-bearing joints.
Other, e.g. lymph nodes, intestine.
Sequelae of previous treatment Lung collapse therapy was used widely in the treatment of pulmonary TB in the1930–50s. Procedures included therapeutic pneumothorax, thoracoplasty, and plombage (expanding the extrapleural space with artificial materials). Sequelae include empyema, sinus formation, bronchopleural fistulae, and ventilatory failure. TB pyogenic or fungal organisms may be isolated. Early specialist input is essential. |
Tuberculosis: investigation 1》Chest X-ray Changes are more variable than in younger people and may mimic other benign or malignant disease (e.g. bacterial pneumonia, cancer). Usually upper zone infiltrates with cavities, but more common features in older people include mid-/lower zone infiltrates, and miliary (diffuse nodular) and bilateral changes.
Healed old disease is usually seen, i.e. calcified hilar nodes, a peripheral 1°complex, pleural thickening, and diffuse apical fibrosis and calcification. ▪︎Pleural effusions are common. ▪︎Rare changes include mass lesions or isolated lymphadenopathy.
The CXR may be normal, e.g. occasionally in miliary or endobronchial disease. If clinical suspicion persists, repeat at an interval. 2》Sputum for microscopy and culture The standard method of confirming TB:Conventionally, three early morning sputum specimens are obtained and stained by acid fast staining (e.g. Ziehl–Neelsen). The quality and persistence of the microscopist is important, as the scanty organisms can be easily missedon cursory examination. If a patient cannot expectorate, obtain ‘induced sputum’ through PT or nebulized normal saline (rarely nebulized hypertonic saline).
If this fails, or clinical suspicion is high despite negative smear and culture, consider bronchoscopy with washings.
3》Other tests Raised ESR and CRP are usual FBC-Mild (normocytic) anaemia and reduced white cell count are more common in older people. Lymphocytosis or pancytopenia can occur. Obtain three early morning urine specimens in case of possible genitourinary infection.
4》Tissue sampling. Where possible sample tissue including lymph node, pleura, bone marrow. Send samples to both microbiology (microscopy and culture) histology. Typical histological features of caseous necrosis with granuloma formation (with or without acid-fast bacilli) support strongly the diagnosis of TB
5》Polymerase chain reaction (PCR) testing on smear-positive sputum can identify the type of Mycobacterium and help guide appropriate antimicrobial therapy.
6》Tuberculin skin testing (Mantoux test) is used to diagnose latent TB and is done in high-risk individuals where treatment would be considered. Tuberculin purified protein derivative (PPD) is injected in a standardized manner, and the reaction assessed quantitatively. ~Previous bacille Calmette–Guérin (BCG) vaccination can cause a reaction, but this usually reduces over about a decade. ~Age can reduce the immunological response to tuberculin, and the test and its interpretation are best done by specialist clinicians. 7》Interferon γ release assay is a newer type of blood test for TB that is becoming more widely available and is used to help diagnose latent TB
|
•Tuberculosis: treatmentGiven the complexities of treatment, specialist referral is mandatory. This is a notifiable disease, and public health local authorities will investigate contacts,especially in the care home setting. Respiratory isolation precautions are necessary for patients with pulmonary TB treated in hospital. ▪︎Pulmonary disease is treated for a total of 6 months: ▪︎Usually 6 months of rifampicin and isoniazid, with pyrazinamide and ethambutol for the first 2 months only. ▪︎Longer-treatment periods may be needed for extrapulmonary disease. ▪︎In older people:Drug resistance is rare, as most infections are recurrences of 1° disease,contracted decades ago. ▪︎Failures of treatment are usually due to poor adherence. ▪︎Combination drug preparations may improve this. ▪︎Side effects are more common, including ~ocular toxicity from ethambutol (reduce dose in renal impairment) and ~hepatitis from isoniazid. ▪︎Close monitoring is important Atypical mycobacteria, e.g. Mycobacterium avium intracellulare or kansasii,can occur in those with structural lung disease such as bronchiectasis. ▪︎This requires even broader and more prolonged courses of antibiotics, but isolation is not required as it does not spread from person to person |
|
|
Q |
Q |
|
|
|
Asthma and COPD: assessment Presentation :Are both diseases characterized by airflow obstruction Commonly coexist, e.g. in the childhood asthmatic who has smoked May be both mimicked by other common diseases, e.g. cancer, PE, heart failure May present late-older people are less aware of hypoxaemia, breathlessness, or bronchoconstriction, or may interpret it as ‘normal’ ageing. Are under-diagnosed & undertreated , especially in older people. Asthma May present in old age as true ‘late-onset asthma’. There are also ↑ numbers of people who have grown old with their asthma. In older people, cough may dominate, symptoms fluctuate less, triggers (e.g.cold, smoke, allergens) are less frequent, and the association with hay fever or eczema is less strong.
Nocturnal cough or dyspnoea, including paroxysmal nocturnal dyspnoea, maybe caused by asthma.
NSAIDs and β-blockers (oral or ocular) may worsen bronchoconstriction. COPD Is much more common in older age, the consequence of intrinsic ageing and progressive disease. Is caused by environmental exposure, usually to tobacco smoke, in genetically susceptible people. Significant disease can develop in those who have not smoked for years, as acquired lung damage depends more on ‘total pack years’smoked, rather than duration alone
Symptoms are usually more chronic and slowly progressive, without significant variability. If bronchitis is significant, there is a productive cough. Fatigue and sleep disturbance are common.Daytime somnolence suggests ventilatory failure.
There may be associated anaemia of chronic disease, osteoporosis, malnutrition, and depression.
Investigations 1》Pulse oximetry will determine the presence and degree of hypoxaemia. In moderately or severely hypoxaemic patients (oxygen saturation <92%), consider ABGs to determine whether long-term oxygen therapy (LTOT) maybe of benefit and, in the acutely unwell, to guide oxygen administration.
2》CXR, ECG, and FBC will help to exclude other pathology, e.g.anaemia, dysrhythmia. 3》α-1 antitrypsin should be considered in atypical cases 4》PEFR, measured regularly (bd–qds) for up to 2 weeks, helps determine whether variable airways obstruction (asthma) exists. Variability of ≥20% is significant. Older people may find using PEFR meters and charting the results difficult. Ask them to demonstrate the technique, reading the device, and charting in clinic. 5》Spirometry. Obtain at least FEV1 and forced vital capacity (FVC). An FEV1:FVC ratio of <0.7 suggests obstruction. Older people often have difficulty performing pulmonary function tests; an experienced technician in a respiratory laboratory will help provide accurate results. ▪︎Assessments for bronchodilator responsiveness using inhaled bronchodilators are now considered less helpful, as they are poorly predictive of the response to treatment and do not distinguish reliably between asthma and COPD. However, airflow obstruction that completely and repeatedly resolves after bronchodilator administration does exclude. ▪︎COPD Assessments for steroid responsiveness can be helpful in distinguishing between asthma and COPD (response is greater in asthma than COPD,although there is overlap). Perform spirometry before and after steroids(either2 weeks of prednisolone 30mg od or 6 weeks of inhaled beclometasone 400 micrograms bd) Some patients show improvement in FVC or functional status (walking distance or speed) despite significant change in FEV1. |
Distinguishing asthma from COPD Asthma COPD ASTHMA-Modest degree of fixed airways obstruction (this is uncommon in younger people) COPD-Greater degree of airways obstruction ASTHMA-Significant or full reversibility COPD-No, or only minimal,reversibility
ASTHMA- ≥20%variability in PEFR COPD-<20% variability in PEFR Greater age Significant Smoking history |
•Asthma and COPD: drug treatment In general, treatment principles are similar to those in younger people and are described in detail in British Thoracic Society guidelines. However, some differences and some similarities benefit from emphasis.
Bronchodilators Older people perceive symptoms less reliably, so where there is evidence of variable airways obstruction, give bronchodilators regularly, rather than as required. ▪︎These include short-acting β2-agonists (SABA), short-acting muscarinic antagonists (SAMA), long-acting β2-agonists (LABA), and long-acting muscarinic antagonists (LAMA) ▪︎Protocols exist for up-titration of these, starting with short-acting agents, then adding in the long-acting agents in a stepwise fashion. ▪︎In older age, response to antimuscarinics, e.g. ipratropium, tiotropium, may be better than to β-agonists, e.g. salbutamol. ▪︎High-dose β-agonists, e.g. from nebulizers, may cause tremor, tachycardia, or rate-related angina. ▪︎Nebulizers may not be required-try higher inhaled doses via a spacer or long-acting agents. ▪︎Antimuscarinic bronchodilators uncommonly cause side effects such as drymouth or blurred vision, more often with higher (nebulized) doses and with long-acting preparations. ▪︎Acute glaucoma is a rare, but important,complication-reduce ocular exposure by nebulizing via a mouth piece, rather than a face mask. ▪︎Corticosteroids Inhaled corticosteroids have a role in the protocols. Long-term oral steroids are rarely beneficial. In those receiving regular courses of oral steroids for acute exacerbations, give osteoporosis prophylactic treatment. Inhaled steroids alone probably do not cause osteoporosis. ▪︎Theophylline Toxicity is common in older people. Plasma levels are ↑ by febrile illness,heart failure, and drugs, e.g. erythromycin/ciprofloxacin. ▪︎Serious side effects,e.g. convulsions, may be the first sign of toxicity. ▪︎Check levels when titrating the dose. ▪︎Most of the therapeutic effect is seen at the lower end of the therapeutic range, so target this first. Before introducing oral theophylline, optimize inhaled bronchodilator and steroid therapy, including the use of long-acting and higher-dose preparations,if necessary. ▪︎Other Influenza and pneumococcal vaccine should be given Exercise extreme caution in the use of respiratory depressants, e.g.benzodiazepines or opiates. ▪︎In general:In acutely unwell patients with CO2 retention, stop them, reintroducing only if withdrawal effects occur. ▪︎In stable patients with or without CO2 retention, withdraw or reduce them where possible. In severe end-stage COPD, if dyspnoea or cough are distressing and cannot be otherwise relieved, consider giving opiates. ▪︎Give small doses initially,but ↑ as needed to relieve distress, even if respiratory function deteriorates. ▪︎Explain the rationale to staff, relatives, and the patient, if appropriate. |
|
|
HOW TO . . . Improve drug delivery in asthma or COPD ▪︎The traditional metered-dose inhaler alone is rarely adequate, due to difficulties in coordinating device activation and the onset of inhalation.
▪︎Adding a large-volume spacer device reduces the need to coordinate activation with inhalation, improving drug delivery and reducing side effects (e.g. oral thrush). Spacers are generally better tolerated by older patients who do not tend to attach the same social stigma as youngsters.
▪︎Breath-activated devices provide an alternative to the metered-dose inhaler,although lung volumes may not be adequate to activate the device. They vary widely in design, and patients vary greatly in the ability to use them. Assess and advise on the technique regularly, involving both hospital and community teams (doctor, nurse, and pharmacist), as well as the family and other carers. Nebulizers are rarely required.A metered-dose inhaler via a large-volumes pacer device is usually just as effective. Patients in whom nebulized drugs are being considered should be referred for specialist assessment.
Where adherence is an issue, e.g. in a person with dementia living alone:Give long-acting preparations where possible. Give combined preparations. Once-daily inhaled steroid is better than none. Supervise the taking of medication as often as possible, but accept that for pulmonary drugs, taking medications irregularly is probably better than taking them not at all. Rarely, inhaled drugs are administered too frequently by cognitively impaired people. This very rarely causes side effects, but relatives may need reassurance that this is the case.
▪︎Occasionally, oral β-agonists are useful in patients in whom all inhaled preparations have been unsuccessful. |
•Asthma and COPD: non-drug treatment ▪︎Smoking cessation should be advised, except in the very advanced or terminal phase where it may lack benefit and be unkind. Consider referring for support and/or nicotine replacement therapy. ▪︎Exercise is beneficial, sometimes available as part of a pulmonary rehabilitation scheme. Elements should include aerobic and strength-based exercises, as well as specific breathing exercises ▪︎Pulmonary rehabilitation is as effective as inhaler therapy and should be a key part of treatment. It is a complex intervention tailored to the individual, with ~exercise, ~behavioural, and ~educational components. ◇Individual action plans can be followed by older people, facilitating self- management and early intervention. ▪︎Weight reduction is beneficial in the obese. However, weight commonly falls in advanced disease as the work of breathing exceeds calorific intake, and nutritional supplements may be needed. ▪︎Comorbidities, including depression, are common and should be treated aggressively. Social and practical interventions. ▪︎A comprehensive multidisciplinary assessment may be warranted. ▪︎Provide appropriate mobility aids, e.g. electric wheel chairs, stair lifts, and alarm systems, e.g. pendant alarms. ▪︎Treat social isolation ▪︎Assisted ventilation Consider this in cases of respiratory acidosis, delirium, exhaustion, or deteriorating respiratory function despite full treatment. Hypercapnia, rather than hypoxaemia, is usually the key contributor to delirium; sedation is likely to worsen ventilation and precipitate coma. For some patients with acute- on- chronic deterioration, ventilation will be futile and inappropriate. Make such a decision after considering: •The nature of the chronic illness and recent deterioration. •The presence of reversible factors. •The patient’s current physiological status. •The views of the patient or others who represent them. •If in doubt, request guidance from the ITU team. Non-invasive ventilation (NIV), e.g. nasal intermittent positive pressure ventilation, provides an acceptable alternative to invasive ventilation (usually endotracheal intubation). NIV is often well tolerated, can be delivered on specialist or high- dependency wards, and provides a modest level of ventilatory support that can be weaned promptly as the patient recovers.
Palliative interventions ▪︎Reassure-many patients are frequently terrified. Assure them that their symptoms of suffocation can, and will be, treated. •Consider advance care planning •Positioning—sit up, day and night. •Involve the palliative care team. Their advice and support are often valuable and can continue into the community if discharge occurs. |
|
|
|
Oxygen therapy ▪︎Oxygen is a drug ,it has clear indications and common and important side effects. Precision and care in prescribing maximizes benefit and reduces harm. ▪︎Controlled oxygen therapy targeted to pulse oximetry (94–98% for most, 88–92% for those at risk of type 2 respiratory failure). ▪︎In older people, dyspnoea may be more frequently accepted, leading to under prescribing. However, indiscriminate prescribing, particularly the use of high -concentration oxygen, risks respiratory depression and CO2 narcosis. This is common in older people with COPD. High-concentration oxygen therapy. Previously used in emergency situations (15L/min via a non-rebreathe bag); this is rarely required to achieve the target saturations of 94–98%.
Hyperoxaemia can be harmful (it can cause vasoconstriction and depressed respiratory drive).
Oxygen delivery systems Supply patients with an administration device applicable to their circumstances which should be prescribed on the drug chart.
1》Constant performance oxygen delivery systems (e.g. Venturi mask) provide a stable concentration of inspired oxygen (FiO2) (24%, 28%, 35%, etc.) for a range of ventilation rates.
These must always be used for hypoventilating patients with elevated PaCO2.
2》Variable performance oxygen delivery systems—the FiO2 varies. The system delivers oxygen at a given rate which mixes with room air at rates dependent on ventilation. Systems include: -Nasal cannulae. Often better tolerated by patients and allow them to eat and talk. With oxygen flows of 1–2L/min, FiO2is usually low (<28%) but can approach 30% if the patient hypoventilates. -Simple face mask, e.g. Hudson. Provides variable FiO2 up to 40%
-Non-rebreathing mask with a reservoir bag. Provides variable FiO2 of up to 60%
Long-term oxygen therapy This improves prognosis in severe COPD and in moderate COPD with features of cor pulmonale.
To reduce pulmonary hypertension, arterial pO2 should be raised to above 8kPa for at least 15h each day. FiO2 of 24–28% usually achieves this. ▪︎ Respiratory depression is very rarely a problem in patients with stable respiratory failure who receive low oxygen concentrations. ▪︎Specific criteria must be met before prescribing LTOT. ~Measure ABGs twice,on air, at least 3 weeks apart and at least 4 weeks after an acute exacerbation. Criteria include: □PaO2 7.3–8.0kPa in COPD with complications such as ~peripheral oedema, ~evidence of pulmonary hypertension, ~or polycythaemia □PaO2 <7.3kPa in COPD without the complications already listed Intermittent oxygen therapy (IOT) This is useful for a variety of cardiorespiratory conditions, e.g. •COPD, •advanced heart failure, •lung cancer. It relieves distress and improves exercise tolerance and mobility. Low concentrations (24–28%) can achieve significant symptomatic benefit. Prognosis is unaltered. Oxygen gas supply Oxygen concentrators are costly to purchase, but running costs are low. They are cost-effective when needing low-flow oxygen for prolonged periods (≥8h perday). Oxygen is piped to convenient position (s) in the home and usually administered via nasal cannulae. Urgent installations can usually be arranged within 24h. Oxygen bottles are useful for: ~Patients on LTOT via an oxygen concentrator who wish to leave their home for short periods. ~Patients needing oxygen as required, who do not meet the LTOT criteria ~Patients who are likely to have a short-term need for continuous oxygen, e.g.for end-of-life palliation, for whom installation of an oxygen concentrator may not be worthwhile. ~A small oxygen cylinder (300L, lasting 2h) is available which is convenient for wheelchair excursions or travel in a car. ► Smokers should stop smoking before beginning oxygen therapy. The risk of burns and household fires is substantially ↑. |
Q |
|
|
|
Asbestos-related disease The period between asbestos exposure and overt (or covert) disease is usually long, often over 20 years. Almost all new cases are in older people because asbestos exposure is so carefully regulated now. The exposure may not be clearly recollected by the patient, but always consider in high-risk occupations, including building, dock work, and heavy engineering. Confirming the diagnosis is important because compensation may be due if disease can confidently be shown to have arisen as a consequence of asbestos exposure. If the diagnosis could not be confirmed during life, then post-mortem confirmation may lead to compensation payments to relatives. Pleural plaques Discrete areas of thickening of the pleura that often calcify. Benign. A marker of asbestos exposure, but of no further clinical significance. Compensation for this is not universally available. Asbestosis Progressive fibrosis, clinically and radiographically similar to idiopathic pulmonary fibrosis. Usually due to prolonged and substantial occupational exposure. Mesothelioma A malignant, incurable tumour of the pleura, presenting as cough, chest pain,effusion, or dyspnoea. Very poor prognosis; few survive over 2 years. Treatment is nearly always palliative; the tumour is not resectable and is relatively insensitive to chemotherapy or radiotherapy. Asbestos exposure may have been only transient. Bronchial carcinoma There appears to be a synergistic effect between asbestos and tobacco. |
Q |
|
|
|
Q |
Q |
|
|
|
Chapter 12 Gastroenterology The ageing gastrointestinal system The elderly mouth Nutrition HOW TO Manage weight loss in older patients Enteral feeding HOW TO Insert a fine-bore NG feeding tube The ethics of clinically assisted feeding Oesophageal disease Dysphagia Peptic ulcer disease HOW TO Investigate and manage persistent unexplained nausea and vomiting The liver and gallbladder HOW TO Approach an older patient with abnormal liver function tests Constipation Diverticular disease HOW TO Image the older colon Inflammatory bowel disease Diarrhoea in older patients HOW TO Investigate and manage chronic diarrhoea Other colonic conditions The ‘acute surgical abdomen’ Obstructed bowel in older patients Obesity in older people |
Q |
|
|
|
The ageing gastrointestinal system Teeth Change colour-yellow and less translucent Become worn (enamel does not regenerate)↓ vascularity and sensitivity of dentine and pulp. Caries periodontitis, and tooth loss are common, but not inevitable in older patients. Being ‘long in the tooth’ refers to gum retraction seen with periodontal disease which ↑ with poor oral hygiene and xerostomia, both common in older people.
Mouth Mucosa-thinner and more friable, rarely a functional problem. Salivary glands do not produce less saliva, but causes of xerostomia are more frequent with ↑age. Bone resorption occurs in the mandible alongside osteoporosis. This is accelerated with periodontitis and progresses fast once teeth are lost, leading to a change in facial appearance. Orofacial muscle tone can diminish with consequent dribbling.
Taste Olfactory function, and hence taste discrimination, ↓ gradually with normal ageing, but an acute change or complete absence of taste should prompt investigations for a cranial tumour.
Oesophagus Slight changes in innervation produce clinically insignificant changes in swallow and peristalsis. The misnamed presbyoesophagus is a disorder of oesophageal motility, not a universal age change.
Hiatus hernias and reflux are very common-probably related to anatomical and postural changes. Stomach↑ incidence of atrophic gastritis (with reduced acid production), but in the absence of disease, most older patients maintain normal pH levels. ▪︎Reduction in gastric emptying is common↑ mucosal susceptibility to damage ↑ Helicobacter pylori carriage, but this is less likely to cause ulceration.
Small intestine Function well preserved, except for calcium absorption which is ↓. ↑ incidence of bacterial overgrowth with malnutrition and diarrhoea. Large intestine ↓ rectal sensation contributes to high incidence of constipation. Pancreas Structural changes including atrophy, but function is well preserved Liver Hepatic weight and volume ↓ by around 25% and there is brown (lipofuscin)pigment build-up, but liver function (and therefore LFTs) is not affected. Some older patients have a slightly low bilirubin and albumin level, but results still remain within the normal range. Gall bladder Incidence of gallstones ↑ (40% ♀ >80), probably related to reduced rate of synthesis and excretion of bile. Most gallstones are asymptomatic |
The elderly mouth Mouth examination Use gloves. Be systematic. Important and often not done-serious pathology may be missed. Check:Parotid glands (enlarged in parotitis, alcoholism, chronic lymphoid leukaemia) Temporomandibular joint (arthritis causes crepitus, subluxation, pain). Dislocation can cause pain and inability to close the mouth Soft tissues: tongue and floor of the mouth most common site for oral cancerin smokers/alcoholics. Angular stomatitisSalivation: (see ‘Xerostomia’, p. 353)Teeth: how many missing, how many restorations, pain/sensitivities. Caries is↑ by poor brushing and low fluoride exposure, diet of soft sweet foods,xerostomia, poor fitting dentures, and infrequent dentist visitsDentures: cleanliness, integrity, and fitGeneral managementNursing help with dental/mouthcare is vital for anyone unable to helpthemselvesReferral to a dentist. Dental check-ups should continue every 6 months,regardless of age/disability. This is very difficult to arrange for inpatients, butmaxillofacial surgeons (who are also trained as dentists) will sometimes helpout in severe/urgent casesConsider chlorhexidine mouthwash for patients with poor oral self-care, e.g.stroke, dementiaSevere periodontal disease may require antibiotics (topical or systemic) andsurgical debridement to arrest progressPoor oral and dental health contributes to poor appetite and malnutrition—consider nutritional support (see ‘Nutrition’, pp. 354–355)Facial painConsider trigeminal neuralgia, TA, parotitis, temporomandibular joint arthritis,dental caries/abscess, aphthous mouth ulcers, or the idiopathic benign ‘burningmouth syndrome’. |
Sore tongue Can be a side effect of drugs, glossitis (B12, iron, or folate deficiency),candida/thrush, especially after antibiotics or in diabetes. A black tongue may bedue to Aspergillus colonization and is treated with nystatin lozenges/mouth rinse.ParotitisAcute bacterial parotitis is not uncommon in frail older patients who are noteating. Low salivary flow (dehydration and not eating) and poor oral hygienepredispose to parotid gland infection with mouth flora (staphylococci andanaerobes). Treat with aggressive rehydration, iv flucloxacillin, andchlorhexidine mouth rinses. Response to treatment is usually dramatic—if not,consider abscess formation or MRSA.XerostomiaPerception of dry mouth is closely related to salivary flow. Saliva is needed for:Taste: dissolves food to present to taste budsSwallow: helps form food bolusProtection of teeth and mucosa: contains antibacterials, buffers, and mucin.Rapid tooth decay is a risk of xerostomiaXerostomia is not a normal ageing change and should always be investigated.Causes include:Drugs with anticholinergic side effects (e.g. tricyclic antidepressants,levodopa)Sjögren’s syndrome (an autoimmune destruction of salivary glands) can be 1°or associated with other autoimmune conditionsIrradiation, salivary stones, tumours, sialadenitis (viral or bacterial infections)Treatment depends on cause—stop or ↓ causative drugs, stimulate saliva withgrapefruit juice/sugar-free sweets or mints, and promote frequent carefulmouthcare. Artificial saliva can provide symptomatic relief for some patients.Oral candidiasisMay manifest as oral thrush (with removable white plaques on an erythematousbase), angular stomatitis (sore cracks in the corner of the mouth), or rarelyatrophic forms (e.g. under dentures, may not have creamy plaque). Consider andreverse risk factors such as antibiotics, steroids, hyperglycaemia, andimmunosuppression, where possible. Use nystatin 1mL qds, rinsed around themouth for several minutes. In cases with painful swallowing/dysphagia (i.e.might have oesophageal involvement) and those that cannot comply with rinses,use oral fluconazole 50–100mg od for 7–14 days. Dentures should be kept out where possible and soaked in chlorhexidine during treatment.Mouth ulcersSimple aphthous ulcers and ulcers due to poorly fitting dentures should betreated with topical anti-inflammatories (salicylate gel or triamcinolone),hydrocortisone lozenges, or steroids. Ulcers can occur as part of a systemicdisease such as inflammatory bowel disease. Any oral lesion persisting >3 weeksmerits referral and/or biopsy to exclude cancer, but most mouth cancers arepainless.Oral manifestation of systemic diseases/drugsA very long list of manifestations, including common and general (e.g. oralcandidiasis in immunosuppression), as well as rare and specific (e.g. oral lichenplanus). Remember that many drugs also affect the mouth, e.g. xerostomia (see‘Xerostomia’, p. 353), tardive dyskinesia with antipsychotics, and gumhypertrophy with phenytoin.Systemic manifestation of dental diseasesPoor oral hygiene with dental or periodontal disease can cause septicaemia orinfective endocarditis. Poor teeth can contribute to poor nutrition. |
|
|
Nutrition With normal ageing, there are: •Reduced calorie requirements due to reduced activity and lower resting metabolic rate (↓ muscle mass) Reductions in appetite (anorexia of ageing) Lower reserves of macro- and micronutrients (vitamins and minerals)
In the presence of disease, older patients quickly become malnourished, which is a powerful predictor of outcome (↑ functional dependency, morbidity, mortality,and use of health-care resources).
Malnutrition is extremely common in the older, frail or institutionalized population, and studies have shown that once in hospital, most patients’ nutritional status actually declines further.
Protein–energy undernutrition affects:15% of community-dwelling older patients 5–12% of housebound patients with multiple chronic problems 35–65% of patients acutely admitted to hospital 25–60% of institutionalized older persons
Nutritional assessment BMI (weight in kg/(height in m)2) is often impractical, as height cannot be accurately measured in immobile patients or those with abnormal posture(although approximations can be made, e.g. using ulnar length)
▪︎Simple weight is still useful, especially if the patient knows their usual weight—rapid weight loss (>4kg in 6 months) is always worrying, even in obese patients.
▪︎Mid-arm circumference can be used to approximate. ▪︎Nutrition screening tools are often employed by nursing staff to target interventions. The MUST score[ ‘Malnutrition universal screening tool (MUST)’] is widely used in UK hospitals and is sensitive for detection of protein–energy undernutrition in hospitalizedpatients ▪︎More complex tools (e.g. Mini Nutritional Assessment) are helpful, but time-consuming, and are rarely used outside research. ▪︎Biochemical measures (e.g. □hypoalbuminaemia, □anaemia, □hypocholesterolaemia develop at a late stage and are confounded by acuteillness.
Nutritional support Identification is key to allow targeted intervention (improves outcome) The cause is usually multifactorial and a holistic approach is needed, e.g. 1》medical (immobile, unwell, reflux, constipation, etc.), 2》social (poverty,isolation), 3》psychological (depression, dementia) 4》age-related (altered hunger recognition)
Involve a dietician early (especially if anorexia is prominent) Record food intake carefully—this highlights deficiencies in intake and helps identify where interventions might help. Make mealtimes a priority (protected mealtimes) and provide assistance with feeding (care assistants or family) Schemes such as using a red tray can highlight those in need of assistance
Establish food preferences and offer tempting, high-calorie foods (e.g.substitute full-fat milk and yogurt if they are on the lower-fat variety)
Prescribe dietary supplements according to patient preference (e.g. milky or fruit drinks, soups, puddings, or high-calorie shots)
Appetite stimulants, e.g. prednisolone, can ↑ weight, but side effects usually outweigh benefits Consider the role of enteral feeding |
HOW TO . . . Manage weight loss in older patients Peak body mass is reached at age 40–50, and weight loss can occur after this due to ↓ lean mass, although the proportion of fat is relatively ↑, so overall weight is often remarkably stable. As a rule of thumb, unintentional weight loss of >2.3kg (5lb)/5% of bodyweight in a month or 4.5kg (10lb)/10% body weight in 6 months is worrying.
Always try to get recorded weight (rather than relying on patient/carer memory)—a search of old out patient clinic and 1° care records can help. Record weight regularly while you investigate to look for ongoing trends. Dramatic weight loss should always prompt a search for remediable pathology. The cause is often multifactorial. It is important to consider: Dementia Depression Malignancy Chronic infection/disease, e.g. COPD, heart failure, TB Inflammatory conditions, e.g. GCA Malabsorption Mesenteric ischaemia (recurrent postprandial abdominal pain) Drug causes, e.g. digoxin, theophyllines, cholinesterase inhibitors Metabolic disorders, e.g. hyperthyroidism, uraemia Swallowing problems Persistent nausea or abdominal pain/reflux Social causes, e.g. inability to cook, poverty, social isolation, alcoholism A careful history (including dietary history and mental state with collateral history where possible),
examination, and routine screening tests will usually give clues of significant underlying pathology.
If preliminary investigations are negative, a ‘watch and re-weigh and wait’ plan is reasonable—be reassured if weight is actually stable or rising; re-examine and re-screen if further loss occurs. Obviously if a remediable cause is found and treated, then weight loss maybe halted or reversed. Where no such cause is found, or where it is not reversible, interventions are still possible. |
Mnemonic causes for weight loss |
|
|
Q |
Q |
|
|
|
Enteral feeding Consider enteral feeding early if there is dysphagia (e.g. stroke, MND,Parkinson’s disease) or failure of oral feeding (e.g. severe anorexia syndromes,intensive care unit) with an intact gastrointestinal tract. There are three common methods: 1》Fine-bore NGTs: simple, quick, and inexpensive. The preferred method for short-term feeding. Some patients (usually confused/drowsy) repeatedly pullout NGTs. Interference with the tube ↑ the risk of aspiration. Persistence,supervision, and careful taping can sometimes help, but often a PEG or RIG is required (also described here). There is ↑ experience using NGTs which are held in place via a nasal loop (Bridle™). Trained practitioners can insert these by the bedside, and removal by the patient is very rare 2》PEG: the risks of insertion include perforation, bleeding, and infection for a patient who is usually already frail. The patient has to be fit to undergo sedation. Problems obtaining consent from a competent patient and‘agreement’ from the next of kin for an incompetent one are not uncommon. Once established, this method is discreet and better tolerated than NGTs and is the method of choice for medium/long-term enteral feeding. 3》RIG: useful if gastroscopy technically difficult (e.g. pharyngeal pouch) and sometimes if small bowel feeding preferred over gastric feeding. Similar complication rate to PEG Complications for all methods include 1》Aspiration pneumonia: there is a common misconception that enteral feeding eliminates aspiration in dysphagic patients. This is not true—reflux of food into the oesophagus is common and this, along with salivary secretions and covert oral intake, may still be aspirated. Always check the position of the tube if the patient becomes unwell, feverish, or breathless. If aspiration is ongoing despite correct tube position, slow the feed, feed the patient sitting upright (i.e. not at night), and add pro-motility drugs, e.g. metoclopramide or erythromycin (pre-meals). A nasojejunal tube or jejunal extension to a PEG tube can also reduce aspiration rates 2》Re-feeding syndrome: occurs when the patient has been malnourished for a long time. When feeding commences, insulin levels cause minerals (especially phosphorus) to move rapidly into the intracellular space and fluid retention occurs causing hypophosphataemia, hypomagnesaemia, and hypokalaemia. ▪︎This, in turn, can cause life-threatening □heart failure, □respiratory failure, □arrhythmias, □seizures, and □coma. ▪︎Avoid by ‘starting low and going slow’ when introducing feed. It is important to check and correct any abnormal biochemistry before feeding starts and then monitor frequently (check urea and electrolytes, calcium, magnesium, phosphate, and glucose daily for a few days, then weekly). ▪︎Supplementation of minerals may be done iv or by adding extra to NG feed. ▪︎Fluid overload and heart failure: ↓ volume and add diuretics 3》Diarrhoea: exclude infection (especially Clostridium difficile). Try slowing the feed rate or changing the feed to one containing more or less fibre. |
Parenteral feeding Should be considered when the gut is not functioning. It requires large venous access and should only be undertaken when supervised by an experienced nutrition team. It is usually a temporary measure, e.g. post-gastrointestinal surgery. Complications such as 1》fluid overload, 2》electrolyte disturbance, and 3》iv catheter sepsis are common in older patients. |
•HOW TO Insert a fine-bore NG feeding tube This task is often performed by nursing staff who may be very experienced. Doctors are often asked to help when insertion is proving difficult. Get the patient’s consent—if they refuse, come back later. They may well have just had several uncomfortable failed attempts. It is rarely appropriate to perform against the wishes of the patient. Have the patient sitting upright with the chin tucked forward (patients often hyperextend their neck which makes it harder). Draw the curtains (this can be an unpleasant procedure to have done or to watch) Leave the guide wire in the tube and lubricate with lots of jelly Feed the tube down one nostril about 20cm (until it hits the back of the throat). In stroke, start with the inattentive side, as it may be better tolerated. If there is a proximal obstruction, try the other nostril. If possible, ask the patient to swallow and advance the wire. Check the back of the throat carefully-you should be able to see a singlewire going vertically down. Start again if there is a loop Secure the tube yourself immediately with tape to both the nose and cheek Once you believe the tube is in place, you need to check it is in the stomach by one or both of the following methods BEFORE you use the tube. Aspiration of gastric contents that are clearly acidic (pH <5) CXR is used if there is no aspirate or the pH is equivocal. If you leave the guide wire in, the tube shows more easily. The tip of the tube should be clearly below the diaphragm. The method of blowing air down the tube and listening for bubbles has now been discredited, as a bubbling sound can be generated due to saliva and pulmonary secretions. |
|
|
The ethics of clinically assisted feeding Feeding is a highly emotive issue. It is seen by many (especially relatives) as a basic need, and hence failing to provide adequate nutrition is seen as a form of neglect or even euthanasia. In contrast, others feel that artificial enteral feeding is a cruel and futile treatment performed on in competent patients that only postpones a ‘natural’ death that involves anorexia or dysphagia. The use of the term ‘clinically assisted nutrition and hydration’ has been suggested by the General Medical Council (UK) to replace the term ‘artificial nutrition and hydration’ underlining the fact that this is a form of treatment. There are numerous high-profile legal cases regarding feeding (usually withdrawal of), and controversial cases that cannot be resolved locally should always be referred to the courts via the local legal team. ► The key to steering a course through this minefield is communication. Initiating treatment Establish if the patient is competent—even dysphasic patients may understand a little with non-verbal cues, etc. If the patient has capacity ensure they understand the chosen method (and its risks) and projected duration of feeding. Patients with dysphagia must realize that they will be expected to dramatically ↓, or stop, oral feeding For patients who lack capacity, ensure you have communicated with all interested carers, family, and the GP. There is sometimes disagreement between interested parties, and these are best detected and ‘thrashed out’ early. A case conference is often helpful. Establish that everyone accepts the indications for feeding and the aims of treatment, and set a date for review, e.g.:2 weeks of NG feeding in a patient with dysphagia following a stroke,which is hoped will resolve. PEG insertion in a patient with MND and malnutrition with recurrent aspiration pneumonia, to be reviewed if the patient requests or if enters the terminal phase of the disease. Do not be afraid of a therapeutic trial (e.g. if you do not know whether the patient’s lethargy/drowsiness/depression is related to malnutrition). Always ensure everyone understands and agrees on review dates and criteria for reassessment. Patients/relatives can be reassured that PEG tubes can be removed if improvement occurs Record discussions and plan carefully in the medical records.If there is still dispute, get a second opinion. As a last resort, legal advice may be needed. |
Withdrawing treatment ► Withholding treatment is not morally different to withdrawing it.There are, however, technical and emotional differences, which is why many more ethical problems arise when withdrawing and why some doctors are resistant to trials of treatment. Artificial feeding can be withdrawn because: It is no longer required (rarely controversial) A therapeutic trial has failed ( this is sometimes controversial) Although feeding is successful, the patient’s quality of life is felt to be unacceptable (nearly always controversial) A patient with a long-term feed is dying from another condition (sometimes controversial) When considering withdrawal of long-term feeding for stable conditions (e.g.persistent vegetative state), decisions should be referred to the courts. Accepting aspiration risk There is also a group of patients who have dysphagia, weight loss, and recurrent aspiration due to progressive neurological conditions such as dementia, who merit special consideration. It is not always appropriate to aggressively manage such patients, who are frequently incompetent and derive pleasure from eating normally. It may be appropriate to allow the patient to eat, accepting that there is a risk of aspiration. ‘Feeding at risk’ protocols are becoming more common to document the process and improve interface communication. Adopting such a palliative policy is impossible, unless everyone, including the whole MDT and relatives, understand and sympathize with the aims of management. |
|
|
Oesophageal disease Gastro-oesophageal reflux disease The symptoms (retrosternal burning, acid regurgitation, belching, atypical chest pain) correlate poorly with the pathology (normal mucosa to severe oesophagitis) Sinister features which might suggest malignancy include sudden or recent onset, dysphagia, vomiting, weight loss, and anaemia.
They should guide management: In the absence of sinister features, a 4-week trial of treatment is given. If there are sinister features, then a gastroscopy should be arranged.
Oesophageal pH monitoring is rarely necessary Treatment ▪︎Check if the patient is taking prescribed or over-the-counter NSAIDs, steroids,or bisphosphonates, and stop or minimize the dose.
▪︎PPIs have revolutionalized treatment, making antacids and H2 blockers, such as ranitidine, almost redundant. They are very effective (for symptoms and healing) and generally safe. ▪︎They are used for prophylaxis with aspirin in high-risk/symptomatic patients-often at lower dose, as well as treatment, and some are licensed for intermittent symptomatic use. Some are available over the counter. ▪︎Rarely elderly patients can have side effects of diarrhoea or confusion
Barrett’s/columnar-lined oesophagus. Gastric mucosa replaces the oesophageal squamous cell mucosa. It is associated with an ↑ risk of malignancy and should have endoscopic surveillance based on Prague Criteria, regardless of symptoms.
Hiatus hernia Very common in older patients, occurring to a degree in almost all. Laxity of structures at the gastro-oesophageal junction allows the oesophago-gastric junction or portions of the stomach to move up (permanently or intermittently) into the thorax. ▪︎May be asymptomatic but often presents with GORD symptoms and occasionally with dysphagia. ▪︎Very large intrathoracic hernias can impair respiratory function and strangulate/perforate.
Diagnosis on CXR (stomach or fluid level behind the heart), at endoscopy, or on contrast radiology.
Treatment To reduce reflux, suggest: lose weight; avoid alcohol and caffeine; eat small meals often; avoid eating before bed; and sleep propped up on pillows or elevate the head of the bed on blocks. PPIs will nearly always relieve symptoms;consider investigations if they do not. Prokinetic agents, e.g.metoclopramide ,sometimes help. ▪︎Younger patients with intractable problems can be assessed for surgery-laparoscopic surgery now available.
Achalasia An idiopathic neurological degeneration causing impaired peristalsis and a lack of lower oesophageal sphincter relaxation, causing a functional obstruction.
Dysphagia for solids and/or liquids is the most frequent presenting complaint. Onset is insidious and slowly progressive. CXR may reveal a dilated oesophagus. Endoscopy is usually performed to exclude malignancy but may be normal. Barium swallow has characteristic abnormalities (dilated oesophagus terminating in a beak-like narrowing) Manometry is the gold standard for diagnosis. Treatment is aimed at facilitating lower oesophageal sphincter relaxation and can include drugs (calcium channel blockers or nitrates), botulinum toxin injection, endoscopic dilation of the sphincter, or surgical myotomy. |
Oesophageal motility disorders Group of disorders where oesophageal motility is significantly different from normal (excluding achalasia, which is a distinct pathological entity)
Incorporates those patients previously described as having presbyoesophagus(motility abnormalities ascribed to age, probably incorrectly)
Presenting features include heartburn, chest pain, and dysphagia
Syndromes include diffuse oesophageal spasm, nutcracker oesophagus, and hypertensive lower oesophageal sphincter
Motility disorders can also arise 2° to other diseases (e.g. diabetes, systemic sclerosis, chronic GORD) Diagnosis may be made with barium swallow, but manometry is the goldstandard. Treatment is difficult and depends on the condition/dominant symptoms. Try prokinetics or PPIs. Calcium channel blockers or tricyclic antidepressants can relieve chest discomfort. Botulinum toxin and endoscopic dilation are also occasionally used.
Oesophageal candidiasis Can present with dysphagia or pain Consider in frail or immunosuppressed patients, especially if oral candidiasisis present Characteristic appearance on endoscopy (biopsy confirms) or barium swallow Treat with fluconazole for 2 weeks |
|
|
|
Q |
Q |
|
|
|
Dysphagia Dysphagia (difficulty in swallowing) is a common symptom in older patients. History Ask what type of food is difficult (solids or liquids) and the level at which food sticks (mouth/throat, retrosternal, or epigastric) Distinguish dysphagia from early satiety and regurgitation (when successfully swallowed food returns after seconds/minutes), which usually occurs with gastric outlet obstruction or pharyngeal pouch. If the swallow ‘tires’ through a meal, consider myasthenia. Cough, wheeze, or recurrent aspiration pneumonia can be a presentation of swallowing problems which cause aspiration. Signs Look for weight loss, oral thrush (may be associated with oesophageal candida), supraclavicular lymphadenopathy, and a gastric splash (implies gastric outlet obstruction).
Watch the patient swallow some water and food-the diagnosis might be clear.
Causes These can be divided into two: ■Structural lesions (worse with solids) ▪︎Oesophageal or gastric cancer ▪︎Benign strictures—scarring following, e.g. oesophagitis,scleroderma, polymyositis, radiotherapy ▪︎Pharyngeal pouch ▪︎Oesophageal candida or ▪︎severe oesophagitis ▪︎Hiatus hernia can produce obstruction symptoms ▪︎External obstruction, e.g. bronchial tumour, aortic aneurysm, or cervical osteophyte Foreign bodies ■Functional problems (often worse with fluids) Pharynx/throat-the most common neurological cause is stroke but can occur in advanced dementia and MND.
Rarer neurological conditions include myasthenia gravis, inclusion body myositis, multiple sclerosis, and parkinsonian syndromes. |
Oesophagus—dysmotility problems are relatively common in older patients and include achalasia and diffuse oesophageal spasm.
Investigations Gastroscopy is now the 1°investigation and is well tolerated, even in frail patients.
Use a barium swallow first if there is felt to be a high risk of perforation with an endoscope (e.g. suspect pouch), but gastroscopy allows biopsy and therapy, e.g. dilation.
Videofluoroscopy provides functional imaging and is useful diagnostically, but the correlation between observed aspiration and clinically significant problems is poor.
Treatment An empirical trial of PPI can be used in patients who are deemed unfit for investigation. If oral thrush is present (suggesting oesophageal infection), try fluconazole fora week. Oesophageal dilation ± stenting can be very successful for benign or malignant strictures. For functional problems, always involve a speech therapist. Changing the consistency of food and fluids, and positioning the patient correctly can minimize problems. Oesophageal dysmotility—try prokinetic/calcium channel blocker Gastroparesis causes early satiety and vomiting. It can be very hard to treat—try a prokinetic. Electrical gastric ‘pacing’ or surgery may provide relief(rarely used) Nutritional support—elderly patients with dysphagia are usually malnourished to start with and are then put nil by mouth for investigations. Refer to the dietician, and consider dietary supplements and early enteral feeding by NGT or PEG . Aspiration pneumonitis is largely a chemical, rather than infective, insult that may be complicated by infection. It is treated by ▪︎Preventing/minimizing aspiration (nil by mouth, NG feeding) ▪︎Oxygen therapy ▪︎Chest PT ▪︎Antibiotics are often given to prevent/treat super infection |
|
|
|
Investigations and treatment more or less the same as for the young and shouldn't delay treatment or investigations because of the old age or comorbidities.
•Peptic ulcer disease This disease is becoming much rarer with the advent of effective medical treatment. It remains predominantly a disease of the elderly population.
NSAID use is the most common cause, followed by H. pylori. H. pylori is a spiral Gram-negative bacterium which colonizes the gastric mucosa causing gastritis. Carriage rates ↑ with age. Infection is usually asymptomatic but is the most common cause of dyspepsia in older patients.
H.pylori is strongly associated with duodenal ulcers and may have a link with NSAID-associated ulceration.
Presentation Acute bleeding, pain (epigastric, retrosternal, or back), indigestion, ‘heartburn’, dysphagia, anorexia, weight loss, perforation (peritonitis), iron deficiency anaemia, or an incidental finding (e.g. on endoscopy). Older patients may present non-specifically (‘off food’ or vague abdominal pains).
Investigations Upper GI endoscopy is very safe and well tolerated in older patients. It cancoften be performed using local anaesthesia in the throat only. H. pylori can be detected with gastric biopsy and histology or with a test for urease activity (Clo test®). Serological tests remain positive, but titres gradually decline after eradication. Breath tests can detect H. pylori colonization but obviously do not demonstrate pathology.
Treatment Dietary restriction is unnecessary (worth specifically mentioning because older patients can remember harsh or bizarre anti-ulcer diets). Stop any NSAIDs. Stop aspirin if possible, and plan for safe reintroduction depending on risk/benefit of the individual patient. Where there is H. pylori & ulceration /gastritis, treat with one of the many ‘triple therapy’ antibiotic PPI regimens. In the absence of H.pylori, just a PPI will suffice. Arrange a repeat scope at 6 weeks to check healing of all gastric ulcers and malignant-looking duodenal ulcers.
For bleeding Effective resuscitation is life saving. Early interventional endoscopy with adrenaline injection (or other modalities,e.g. heater probes or clips) into the bleeding point is suitable for almost all patients-do not delay because of age/comorbidity.
iv PPI reduces the risk of re-bleeding.
Continued bleeding/re-bleeding despite endoscopic treatment is an indication for surgical intervention or radiological embolization.
Scores using clinical, laboratory, and endoscopic features can stratify risk of re-bleeding (e.g. Rockall score)
For perforation Remember ‘silent’ perforation (without signs of peritonitis) is more common in the elderly population (especially if on steroids or diabetic). Mortality is high due to delayed diagnosis, reluctance to perform surgery, andpost-operative complications. |
•HOW TO Investigate and manage persistent unexplained nausea and vomiting This group of patients can be very challenging, but you should actively manage them from an early stage because they are often very uncomfortable and bed-bound. There is frequently reversible disease and they are at high risk of dehydration / malnutrition and complications of immobility.
Nausea and vomiting can be the major presenting feature of illnesses as diverse as pneumonia, MI, intracerebral haemorrhage, Addison’s disease,UTI, and constipation.
▪︎Start with a careful history (especially drug history) ▪︎Thorough examination (including rectal examination and neurological assessment) ▪︎Regular observations of vital signs (looking for intermittent pyrexia, arrhythmia, etc.) ▪︎Screening blood tests (including calcium, thyroid function, CRP, iron studies, liver function, coeliac screen), urinalysis, CXR, and ECG
Drugs Look very carefully at the drug history-almost any drug can cause nausea and vomiting, but digoxin (even with therapeutic serum levels), opiates,tramadol, antiparkinsonian drugs, antidepressants, NSAIDs, and PPIs are some of the common candidates. New drugs are the most likely, but remember poor compliance with drugs at home which are prescribed in hospital, e.g. co-codamol used occasionally at home may be written as 2 qdsin hospital. If there is polypharmacy, try stopping the drugs one at a time,remembering that some drugs can take days to ‘wash out’. Central causes ▪︎Raised intracerebral pressure can occasionally present this way. A CT scan is needed if there is drowsiness, focal neurology, or a past history of intraventricular blood (exclude hydrocephalus). ▪︎If there is vertigo or tinnitus, consider labyrinthitis or posterior circulation stroke
Gut causes Constipation is a very common cause of nausea. Obstruction should be excluded with an abdominal X-ray (AXR). Consider repeating this if symptoms persist and you remain suspicious. Plain radiology will remain normal in high obstruction, and an oesophagogastroduodenoscopy (OGD) or small bowel follow-through may help. Severe gastritis/peptic ulceration can present with nausea and vomiting without pain/bleeding. Gastroparesis is most common in people with diabetes and is very hard to treat-try prokinetics. |
|
|
|
The liver and gall bladder Cirrhosis Chronic liver disease can present for the first time in older people. The presentation is often non-specific. The prognosis is worse than for a youngerperson with the same degree of liver damage. Common causes include alcohol,hepatitis C, autoimmune hepatitis, and non-alcoholic fatty liver. A proportion arecryptogenic (thought to be ‘burnt-out’ autoimmune hepatitis or non-alcoholicfatty liver disease).Hepatitis C may have been transmitted from blood products received before1991 when screening was introduced. Alcohol consumption is known to ↑ thepercentage of those infected with hepatitis C who develop cirrhosisAlcohol excess can present with falls, confusion, and heart failure at any age,but older patients are less likely to volunteer (or be asked) their alcoholhistory. ► Always enquire about alcohol Investigations If you suspect cirrhosis, should include: α-1 antitrypsin, autoimmune profile(ANA, smooth muscle antibody (SMA), liver–kidney microsome antibodies(LKM), antimitochondrial antibody, and immunoglobulins), ferritin and iron studies, caeruloplasmin, hepatitis B and C serology, and ultrasound including Doppler of the portal and hepatic vein.
Non-alcoholic fatty liver disease Is not always a benign condition (half will be progressive and 15% develop cirrhosis; outlook worse with advancing age). Obesity, hyperlipidaemia, and type2 diabetes are risk factors, so this condition is more common in older patients.
If an ultrasound scan reveals fatty liver, advise about weight reduction and alcohol cessation.
Gallstones Very common (1:3 elderly ♀) and mostly asymptomatic, although troublesome symptoms often misdiagnosed as GORD or diverticulitis in older age groups. Management largely as for younger patients, but the risks with endoscopic retrograde cholangiopancreatography (ERCP)/surgical intervention are higher,so conservative/less invasive approaches often adopted. Acute cholecystitis in older patients may present atypically (e.g. without pain) and is not always associated with gallstones. It has a 10% mortality and should be aggressively treated with iv antibiotics and supportive care. Failure to improve should prompt early surgical review. |
•HOW TO Approach an older patient with abnormal liver function tests This is not an uncommon finding, and investigation is as for younger patients.However, the following should be considered: ▪︎Drug injury to the liver is common and may occur up to 6 months after exposure (e.g. statins, co-proxamol, penicillins, some over-the-counter medications) ▪︎Liver metastases may present this way (more common in older patients) ▪︎Deranged LFTs may occur as part of a systemic illness, e.g. ~sepsis(consider gall bladder/biliary infection if the LFTs are very abnormal), ~cardiac failure (with hepatic congestion), ~inflammatory conditions (PMR), ~ischaemic liver damage (after prolonged hypotension), ~Addison’s disease,and ~thyroid disease. The picture is often mixed, with both cholestatic and hepatocellular components. A careful history should be taken, looking for duration of the problem and whether (if old) it has been previously investigated. If new, ask about possible drug or toxin exposure, associated symptoms (e.g. heart failure,sepsis,1°malignancy), and alcohol use (do not make lifestyle assumptions—alcohol excess is under-recognized in older people).
Clarify that abnormality is from the liver:An isolated elevated ALP is often from a bone source (commonly Paget’sdisease), but do not assume this-liver metastases can present this way. Isoenzymes can be measured, but clinical judgement is usually sufficient to guide investigation. Muscle disorders can cause transaminase elevation. Persistent elevation should always prompt investigations: Bloods including electrolytes, clotting, TFTs, autoimmune screen (serum electrophoresis, ANA, antimitochondrial antibodies, SMA, and LMK),viral serology (hepatitis B and C), iron studies (haemochromatosis), caeruloplasmin (Wilson’s disease), coeliac disease screen, and α-1antitrypsin. Liver ultrasound—a good screening test that will usually identify liver metastases, as well as highlighting any biliary duct dilation prompting referral for ERCP. If the liver damage is progressive, or the diagnosis elusive, then refer to a hepatologist for consideration of liver biopsy. |
|
|
|
Constipation The term constipation is used in different ways, indicating one or more of the following:The time between bowel evacuations is longer than normal. The stool is harder than normal. The total faecal mass present within the abdomen is ↑. The most precise definition may be delayed alimentary tract transit time, but this is hard to measure and is delayed in age, in the institutionalized, and in those eating a Western diet. There are said to be three types of constipation: Hard faeces present in the rectum (often in massive amounts) The whole distal large bowel loaded with soft, putty-like faeces that cannot be evacuated. High (proximal) impaction which may be due to obstructing pathology (e.g.diverticular disease, carcinoma) Diagnosis The diagnosis is largely clinical (based on history and examination alone). ► Ask specifically, as some patients are embarrassed to trouble doctors with bowel symptoms. Constipation may rarely be the 1° cause of delirium but commonly contributes to the presentation of frail older patients with other pathology such as sepsis or renal failure. Rectal examination may be diagnostic, and sometimes the rectum will barely admit the examining finger. If the rectum is empty, consider high impaction. In a thin patient, high impaction is unlikely if the loaded colon cannot be felt during abdominal examination. In more obese subjects, a plain AXR will be necessary to confirm high impaction but is insensitive in the very obese. ► Do not exclude constipation as the cause of faecal incontinence until there has been an adequate therapeutic trial for high faecal impaction. Causes Reduced motility of the bowel: ▪︎drugs (e.g. opiates, iron, anticholinergics ,antidepressants, antipsychotics, calcium channel blockers, calcium preparations), ▪︎immobility, constitutional illness, electrolyte disturbances,dehydration, hypothyroidism, lack of dietary fibre, hypercalcaemia. ▪︎Failure to evacuate the bowels fully: any painful condition of the rectum or anus, difficulty in access to the toilet, lack of privacy, altered daily routine. ▪︎Neuromuscular: Parkinson’s disease, diabetic neuropathy pseudo obstruction Mechanical obstruction of the bowel: carcinoma of the colon, diverticular disease. |
Prevention and treatment Precipitating causes, such as dehydration, hypothyroidism, hypercalcaemia, and drugs, should be identified and reversed. Non-pharmacological measures, including regular exercise, improving access to the toilet, adequate dietary fibre, and adequate hydration, are effective. Laxatives should be used in combination with non-pharmacological measures. Unless there are reversible factors, always prescribe regular laxatives. Waiting for constipation to occur, then using ‘PRN’ doses is far less effective. You will need to titrate the laxative dose with time and changing patient circumstances. There is little good evidence to guide the choice of laxative, and prescription varies with geography and personal choice. Here are some guiding principles: •Stimulant laxatives, such as senna or bisacodyl, or stimulant suppositories may be appropriate for those with bulky, soft faecal overloading. •Avoid stimulant laxatives in patients with hard rocks of faeces, as this may produce abdominal pain. •Use a stool softening (osmotic) laxative instead such as lactulose or a macrogol. •Long-term use of stimulant laxatives has been said to cause ‘bowel tolerance’/neuronal damage, leading to a dilated, atonic colon that required even more laxatives. There is very little evidence to support this, and stimulant laxatives are now considered safe, in moderate doses, for long-term use. ▪︎Sometimes stimulant and osmotic laxatives are used in combination, typically in severe constipation (e.g. opiate-induced) that has been unresponsive to asingle drug. •Stool-bulking agents, such as methylcellulose or ispaghula, are useful in prophylaxis but are less effective in treating established constipation; both fibre and other bulking agents will ↑ stool volume and may ↑ problems. •Newer agents with novel mechanisms of action are available as third-line agents (e.g. prucalopride and lubiprostone) Costs of laxatives vary enormously, and there is no correlation between cost and patient acceptability. Try cheaper preparations first (fibre, senna) Faecal retention severe enough to cause incontinence nearly always needs a determined effort to clear the colon. |
|
|
|
Diverticular disease Narrow-necked pockets of colonic mucosa which occur adjacent to blood vessel penetrations of the muscle bands, like ‘blowouts’ on a tyre. Occur anywhere in the large bowel, but most commonly in the sigmoid.
Rare in <40 years, ↑ frequency with age and almost universal in >85 years.
Cause: thought to be raised intraluminal pressure due to low-fibre Western diet. Investigation: colonoscopy/flexible sigmoidoscopy and barium enema are usually diagnostic and rule out other pathology. CT colonography (abdominalCT with oral contrast) is increasingly used as a better tolerated test in older patients ► The majority of cases are asymptomatic the majority of the time. On other occasions, innocent diverticulae are blamed for symptoms that arise from other pathology, e.g. constipation, irritable bowel disease, or gastroenteritis. The previous diagnosis of diverticular disease should not stop the careful evaluation of new bowel symptoms to exclude important diagnoses such as colitis or cancer. Pain may occur and, especially if associated with constipation, can be improved by a high-fibre diet with or without extra stool-bulking drugs (e.g.ispaghula). Complications ▪︎Diverticulitis-Should be thought of as ‘left-sided appendicitis’. Infection occurs within a pocket and may be due to a faecolith blocking the neck, so avoiding constipation is key to prevention. Abdominal pain and tenderness, diarrhoea, and vomiting occur with fever and raised inflammatory markers. Treat with antibiotics(include anaerobic cover)—mild cases oral antibiotics at home, severe cases may need admission for iv rehydration, antibiotics, and liaison with surgical services.
Haemorrhage Selective angiography can be used to demonstrate bleeding point.
Diverticular abscess Ultrasound or CT for diagnosis. Surgical or interventional radiographically guided drainage is required.
Perforation/peritonitis see ‘The “acute surgical abdomen” ’, p. 378. Fistula Most commonly to the bladder, causing urinary infection and bubbles in the urine (pneumaturia). Cystoscopy or CT scan for diagnosis. Surgery is required,but simple defunctioning colostomy is often sufficient. |
HOW TO . . . Image the older colon This requires careful consideration of the risk and discomfort of a test,balanced against the quality of the information obtained. Discussing the pros and cons of each investigation with the patients and/or relatives will often help. It is useful to clarify that a patient is fit for bowel preparation on the request form, if relevant. ▪︎Flexible sigmoidoscopy is safe and generally well tolerated, requiring only an enema in preparation, allowing direct visualization and biopsy of rectal and lower colonic pathology. Sedation is usually not needed. ▪︎Colonoscopy allows direct imaging and biopsy of more of the colon, and is the preferred method for general population investigation but carries an ↑risk of bowel perforation in the over 75s. Full bowel clearance and sedation are required. ▪︎CT colonography involves using a CT scanner to produce two- and three dimensional images of the colon, which are interpreted by a radiologist.This requires full bowel clearance and air insufflation during the procedure,both of which can be difficult to tolerate. It is less invasive than colonoscopy but is equivalent for detection of lesions of a reasonable size. The technique is improving all the time and is the investigation of choice for patients >75 who are able to tolerate full bowel clearance. ▪︎Minimal preparation CT colon involves ingestion of a contrast agent 48–72h before the scan, which then tags the faecal matter and removes the need for bowel clearance. It is therefore a better investigation for frailer patients, although it misses smaller lesions.
▪︎Plain CT abdomen has reasonable sensitivity for large colonic lesions, may reveal other pathologies, and requires no bowel preparation. It is therefore useful in emergency assessment or where the pathology is not clearly colonic. ▪︎Barium enema requires bowel clearance and is less sensitive than CT colonography, so is becoming less frequently used. |
|
|
|
Diarrhoea with blood Systemic manifestations Complications Contrast studies diagnostic. Treat with steroids. Prevent constipation.
Inflammatory bowel disease Ulcerative colitis and Crohn’s disease are chronic, relapsing conditions caused by inflammation of the bowel wall. Inflammatory bowel disease is idiopathic and has an ↑ incidence in the population as a whole. Initial presentation is usually in adolescence, but there is a second peak of incidence in older patients. Diarrhoea and urgency in this age group can be particularly disabling and may result in incontinence and social isolation. Features ▪︎Diarrhoea (often with blood), malaise, weight loss, and abdominal pain. ▪︎Delayed presentation may result from embarrassment or fear of cancer. ▪︎Delayed diagnosis more common in older people because symptoms are ascribed to one of the common differential diagnoses such as diverticular disease, CDAD, colonic carcinoma, and ischaemic colitis. ▪︎Associated conditions include arthritis, iritis, sclerosing cholangitis, ankylosing spondylitis, and skin disorders (pyoderma gangrenosum, erythema nodosum) Complications include ▪︎thromboembolism, ▪︎malabsorption and ▪︎malnutrition, ▪︎perforation, ▪︎stenosis with obstruction, ▪︎fistula formation, and ▪︎colonic and biliary malignancy Investigations ▪︎Exclude infection with stool culture and examination for ova, cysts, and parasites and Clostridium toxin (if in hospital or recent antibiotics) ▪︎ESR and CRP are usually elevated but may be normal in localized disease ▪︎A normochromic normocytic anaemia is common, but if there is excessive bleeding, iron deficiency can develop. ▪︎Plain X-rays are usually normal, but contrast studies are often diagnostic. ▪︎Sigmoidoscopy/colonoscopy and biopsy have high diagnostic yield.
Treatment Confirmed cases are best managed by gastroenterology teams. Treatment in older patients is not greatly different and is aimed at obtaining and then maintaining remission. Some principles for treating older patients include: ▪︎Exacerbations of distal colitis are usually treated with topical mesalazine and steroids given as enemas. this may be impractical in older patients, unless a carer can help, and oral steroids can be a better option ▪︎Budesonide is a steroid with high topical potency (poor absorption and rapid first-pass metabolism), so equivalent doses cause fewer side effects. ▪︎Side effects, drug interactions, and polypharmacy may be more problematic,e.g. always consider bisphosphonates with oral steroids therapy. ▪︎Look for, and treat, proximal constipation which can impair the efficacy of treatment of a distal colitis. ▪︎Oral 5-aminosalicylic acid preparations (e.g. slow-release mesalazine) are often successful (for exacerbations and maintenance) and well tolerated. ▪︎The risk of malignancy is higher the longer the patient has active disease, so theoretically many older patients should be under surveillance by a gastroenterologist. ▪︎Unfortunately the risk of colonic perforation during colonoscopic screening is higher in the elderly population, so many screening programmes stop at age 75. ▪︎For failure of medical management, elective colectomy is well tolerated and may give the best quality of life. ▪︎In contrast, emergency surgery in older patients has high mortality., |
•Diarrhoea in older patients Acute Short-lived bouts of diarrhoea are commonly due to viral gastroenteritis. Supportive management (rehydration, light diet) is usually sufficient for this self-limiting condition. It can spread rapidly in institutions (especially if due to norovirus—responsible for much of the so-called ‘winter vomiting’), and appropriate infection control measures should be put in place. In frail,hospitalized patients, especially with recent antibiotic exposure, consider CDAD earlier rather than later. If diarrhoea persists, always send samples for culture, ova, cysts, and parasites, and C. difficile toxin. Chronic There is a group of elderly people who have chronic or recurring episodes of diarrhoea that merit active investigation untreated, they suffer high morbidity(especially if diarrhoea induces faecal incontinence) and many causes are treatable.
Malabsorption Patients do not always have diarrhoea. Look for low BMI and falling weight despite reasonable oral calorie intake.
Biochemical markers of malnutrition, e.g.hypoalbuminaemia, may be present. Anaemia is caused by malabsorption of iron, B12, or folate and is therefore microcytic, macrocytic, or normocytic.
The common causes of malabsorption in older patients often coexist and include: ▪︎Coeliac disease/gluten-sensitive enteropathy: Peak incidence at age 50 but can manifest for the first time in old age with weight loss, bone pain (osteoporosis), fatigue (anaemia), and mouth ulcers Duodenal biopsy should be performed in all who present with iron deficiency undergoing endoscopy.
Anti-endomysial or tissue transglutaminase antibodies have very high specificity (100%) and reasonable sensitivity (around 85%). False negatives can occur with low immunoglobulin A (IgA), so always check serum immunoglobulins at the same time Pancreatic insufficiency can occur without a history of pancreatitis, alcoholism, or gallstones. Request a faecal elastase a low level supports pancreatic insufficiency.
Bile salt malabsorption. Ileal resection or disease allows bile salts to reach the colon which causes diarrhoea. Bacterial overgrowth is particularly common in any person with an anatomical abnormality of the gut (e.g. post-gastrectomy, small bowel diverticula) but can also occur with normal gut architecture. |
HOW TO Investigate and manage chronic diarrhoea Diagnoses to consider in the elderly population include: □Colonic tumour □Diverticular disease □Chronic infections □Constipation with overflow diarrhoea □Drugs—many drugs can cause diarrhoea; review the list and stop any that may be implicated. Common culprits include laxatives, antibiotics, bisphosphonates, NSAIDs, and PPIs □Inflammatory bowel disease or microscopic colitis □Malabsorption History Ask about foreign travel, antibiotic exposure, full drug history, previous gut surgery/pancreatitis, and family history of inflammatory bowel disease. Ask the patient or carer to make a record of stool frequency/texture. Examination Abdominal and digital rectal examination. If rectum is loaded, be highly suspicious of overflow diarrhoea. Investigations •Stool: culture, C. difficile toxin, ova, cysts, and parasites •Blood tests: FBC (anaemia), haematinics (iron, B12, folate deficiency), tissue transglutaminase antibodies (and IgA levels), CRP, and ESR •Radiology: plain AXR is rarely diagnostic (except unexpected, left-sided faecal loading) •Sigmoidoscopy: biopsy in several places, even if the mucosa looks normal to exclude microscopic colitis More extensive colonic imaging may be needed. Faecal calprotectin is useful to rule out inflammatory bowel disease. Treatment Obviously depends on the cause, but in patients in whom diagnosis is not clear and are not fit for, or refuse, more complex investigations, there is a place for trial of empirical treatment. One such strategy is at least 2-week trials of: Metronidazole (for overgrowth/diverticular disease) Pancreatin, e.g. Creon® (pancreatic disease) Bile acid sequestrants, e.g.colestyramine (bile salt malabsorption) Steroids (for colitis) Pick the most likely, or try each in turn for a few weeks. |
|
|
Q |
Q |
|
|
|
Other colonic conditions ■Irritable bowel syndrome A chronic, non-inflammatory condition characterized by •abdominal pain, •altered bowel habit (diarrhoea or constipation), and •abdominal bloating, but with noidentifiable structural or biochemical disorder. New onset is rare in older age, and this diagnosis should not be made ‘denovo’ in older patients without very careful exclusion of structural disease(particularly colonic tumours and diverticulitis) Lifelong sufferers may continue with symptoms in later life, but if the symptoms change, the patient should also undergo investigations. Pain or diarrhoea that wakes a patient at night, blood in stool, weight loss, or fever are NEVER features of irritable bowel syndrome Some drugs used to treat irritable bowel syndrome (e.g. tricyclic antidepressants) are less well tolerated in older patients. Mebeverine might be better tolerated for spasm. Dietary advice should be given (low fibre for bloating or wind, high fibre for diarrhoea or constipation, exclude exacerbating foods)
Stool-bulking drugs can be useful for constipation Loperamide or codeine can be used for disabling diarrhoea. ■Angiodysplasia Tiny capillary malformations (like spider naevi) that can occur anywhere in the gut are important only because they bleed. Slow blood loss leads to unexplained recurrent iron deficiency anaemia; brisk loss may produce life-threatening haemorrhage. Unless they are inherited in a syndrome [hereditary haemorrhagic telangiectasia), they are acquired and therefore have ↑ prevalence with age.(most cases aged over 70)
Asymptomatic angiodysplasia in older patients is common.Diagnosis is often by exclusion of other causes of iron deficiency anaemia. Many patients are reinvestigated for recurrent anaemia, and the absence of sinister features over a period of time with no demonstrated pathology on standard tests may suggest angiodysplasia is the cause.
Sometimes colonoscopy can visualize lesions (which can then be treated by diathermy), but CT does not reveal this pathology. Selective mesenteric angiography can demonstrate lesions that are actively and rapidly bleeding. Tranexamic acid, oestrogens, and thalidomide are sometimes successful in controlling chronic blood loss.
■Microscopic colitis Also known as collagenous or lymphocytic colitis. An idiopathic condition causing chronic or episodic watery, non-bloody diarrhoea, but with no gross structural changes seen on colonoscopy.Biopsy changes are diagnostic with collagenous thickening of the subepithelial layer and infiltration with lymphocytes. Peak incidence in 50s. There is no ↑ risk of cancer. Keep treatment as simple as possible start with diet and anti-diarrhoeal drugs (e.g. loperamide), then try steroids (e.g. budesonide)
■Intestinal ischaemia There is a variety of presentations. Pain out of proportion to the abdominal examination findings is common in these syndromes and should always make you consider them. An elevated lactate ± acidosis should alert you to the possibility of dead bowel. Intestinal angina results from chronic arterial obstruction of the coeliac axis or superior mesenteric artery. Epigastric pain occurs after eating. Diagnosis is tricky, as the pain is similar to peptic ulcer pain, but angiography is diagnostic. Treat with antiplatelet agents. Angioplasty or stenting may be useful.
Small bowel ischaemia results from mesenteric artery occlusion, often by an embolus (more common in AF).
There is acute colic with rectal bleeding,followed by circulatory collapse. Laparotomy is required, but outcome is poor. Ischaemic colitis is an under-diagnosed cause of acute diarrhoea ± blood in dehydrated, hypotensive elderly patients with vascular disease. Often self-limiting if volume depletion corrected and the bowel is rested. May result in colonic stricture. Colonic gangrene occurs after profound hypotension typically in older ITU patients with heart failure and sepsis. Laparotomy and colectomy are required,but fatality is high. |
The ‘acute surgical abdomen’► Peritonitis/perforation often presents in a non-specific way. Patients often present to medicine, rather than surgery. The diagnosis is easily missed, so always have a high index of suspicion and examine the abdomen carefully and repeatedly in sick elderly patients without a diagnosis.
Common causes in older patients include: Complications of diverticular disease First presentation of a tumour (gut, pancreatic) Ischaemic bowel (emboli in patients with AF) Strangulated hernias (always remember groin examination) Ruptured abdominal aortic aneurysm Duodenal ulcer perforation (becoming less common Biliary stones/sepsis (stones) and pancreatitis Appendicitis Signs Peritonitis/perforation may not have guarding or rigidity, particularly in the very old, those on steroids, or people with diabetes. Lack of bowel sounds can be helpful. Signs may develop with time, so repeated assessments are mandatory. Investigations •Erect CXR can reveal air under the diaphragm (this is sometimes the only indication of a ‘silent’ perforation). •Ultrasound or CT imaging will often reveal the cause. Management Always involve the surgical team, even where the patient is unsuitable for operation, as they can advise on conservative management and occasionally an‘interval’ procedure is appropriate (e.g. gallstone surgery once cholecystitis has settled). ► Ensure that surgical decisions are made on the basis of frailty assessment and comorbidity, not just age alone. Aim to achieve a senior medical, surgical,and anaesthetic consensus about treatment, and then discuss with the patient (ortheir advocate). Medical management involves: Broad-spectrum iv antibiotics Resting the bowel (nil by mouth, NGT if vomiting) |
|
|
|
Obstructed bowel in older patients As with peritonitis, this often presents in a non-specific or non-dramatic way. Common causes in older patients include: Constipation Colonic tumours Sigmoid volvulus Strangulated hernias (remember to examine the groins) Adhesions (look for old abdominal scars Complications of diverticular disease (abscess, localized perforation, stricture) Signs Consider excluding obstruction (with plain X-ray) in any patient with persistent vomiting and/or abdominal bloating (ask the patient if their tummy is a normalsize for them). Pain/colic, absence of defecation, tinkling bowel sounds, and gastric splash are helpful when present (but are often absent). Always examine the groins in both sexes for obstructed herniae.
Investigations Plain AXR shows dilated bowel standing AXRs have fluid levels but are often impractical in older patients and rarely add diagnostic information to a supine film. CT imaging may localize a cause. Contrast radiology and gastroscopy are sometimes useful. Management Always involve the surgical team who can advise on diagnosis and conservative management, e.g. insertion of a flatus tube for sigmoid volvulus. General management usually involves: Resting the bowel (nil by mouth and wide-bore NGT) Careful monitoring of fluid balance—heart failure from fluid overload or renal failure from dehydration are often the mechanisms of death. Therapeutic oral Gastrografin® (hyperosmolar ‘lubricating’ agent and can relieve small bowel obstruction due to adhesions) Consider broad-spectrum antibiotics if there is fever or features of coexistent perforation Prophylactic low-molecular-weight heparin Where conservative management fails and an operation is necessary, less invasive/palliative procedures are often more appropriate (e.g. defunctioning colostomy, rather than anterior resection). Pseudo-obstruction presents with vomiting and dilated bowel on X-ray but is due to an atonic bowel, so bowel sounds are absent or ↓, rather than ↑. Frail,older, immobile, debilitated patients are more at risk. Can occur with electrolyte abnormality (especially low potassium), post-surgery, with drugs (e.g. opiates,anticholinergics), in neurological disease (e.g. Parkinson’s or Alzheimer’s), or any severe illness (e.g. septicaemia). Supportive care with hydration and bowel rest is needed, along with correction of the underlying abnormality. Often resolves with this approach, but decompression or surgery is occasionally needed. |
Q |
|
|
|
Obesity in older people In developed countries, there is a general ↑ in body weight and BMI until about 60 years of age, after which both tend to decline. The body composition also changes, with an ↑ proportion of intra abdominal fat. Weight gain in older people usually relates to a reduction in activity and falling basal metabolic rate, rather than an alteration in calorie intake (which actually tends to get lower with age). The ideal body mass for an older person has not been established, although it is probably higher than that for a younger person. Impact of obesity The relationship between mortality and obesity in older people has not been established. The following should be considered: Weight loss has been reported to ↑ mortality-but all the studies have methodological problems, and weight loss is a marker of underlying disease which may be occult. Those with chronic disabilities and diseases will reduce activity more than fit older people, and so may gain weight more easily. In care home residents, only severe obesity (BMI >40) is clearly associated with an ↑ in mortality. Care homes may need special equipment (e.g. wider frames, wheelchairs) to allow for their care Obesity will ↑ morbidity from conditions such as arthritis and diabetes and ↑cardiovascular risk Obese older people are more likely to have mobility problems, and their quadriceps strength-to-weight ratio is key to standing ability ■Obesity does not exclude frailty. Treatment The goal of treating obesity in older people is to reduce weight without losing lean mass or contributing to frailty— excessive weight loss in older people is associated with an ↑ in mortality. In the very old, there is little to be gained from altering a lifelong dietary and exercise habit where there are few complications from obesity, but younger patients (60s and 70s) with diabetes or vascular disease may benefit greatly from healthier eating habits. ↑ physical activity is the mainstay of treatment.This may ↑ energy expenditure and promote weight loss, improve muscle strength and stamina, reduce intra-abdominal fat, and promote a feeling of well-being. Calorie restriction should be undertaken with caution and include at least 800kcal/day with good fluid intake. Dietary supplements can be used in patients who have a high BMI during acute illness but should not be continued indefinitely. Drugs to enhance weight loss are rarely useful (risk > benefit), although inhibitors of fat absorption (e.g. orlistat) may be useful in people with diabetes Gastric surgery is higher risk in older obese people and not frequently undertaken. |
Q |
|
|
|
Chapter 13Renal medicine The ageing kidney Acute kidney injury Acute kidney injury: management HOW TO Perform a fluid challenge in AKI/anuria Chronic kidney disease HOW TO Estimate the glomerular filtration rate Chronic kidney disease: complications Renal replacement therapy: dialysis Renal replacement therapy: transplantation Nephrotic syndrome Glomerulonephritis Renal artery stenosis |
Q |
|
|
The ageing kidney Kidney function tends to decline with age, but unless there is additional disease,function is usually sufficient to remove waste and to regulate volume and electrolyte balance; it is only when stressed that lack of renal reserve becomes apparent. The relative contribution of cumulative exposure to risk factors(extrinsic ageing), disease acquisition (often occult), and intrinsic ageing is unknown, but not all the changes described are universal in an older population. Falling renal reserve The GFR falls steadily after the age of 40 in most healthy older people, possibly due to the following age-related changes: ▪︎Rise in BP within the normal range ▪︎Numbers of glomeruli fall (~50% fewer at age 70 than at age 30↑ in sclerotic glomeruli Renal blood flow ↓ by around 10% per decade (cortex more than medulla,leading to patchy cortical defects on renal scans).
Lower GFR and renal blood flow are the major causes of reduced renal reserve, with the following clinical implications: Renally excreted substances are likely to be retained longer (especially drugs),making prescription amendments necessary Reduced threshold for damage with ischaemia or nephrotoxins The normal range for plasma urea and creatinine does not change with age. However, as production of urea and creatinine ↓ with falling body muscle mass,renal function is often substantially diminished in an older person, even with apparently normal blood chemistry.
► GFR is a better estimate of renal function than plasma urea and creatinine ▪︎Blunted fluid and electrolyte homeostasis The following changes occur with age: ~A blunted response to sodium loading and depletion, so equilibrium is achieved more slowly ~Reduced ability to dilute and concentrate urine (falls 5% every decade) ~Lower renin and aldosterone levels (30–50% less than in young people) ~Loss of the sensation of thirst, even when plasma tonicity is high (reasons unclear—may relate to altered baroreceptor function, dry mouth, or altered mental capacity) ~Reduced response to vasopressin ~In addition, many commonly prescribed drugs interfere with renal function(diuretics, NSAIDs, ACE inhibitors, lithium, sedatives, etc.)
Hyponatraemia is therefore common (low sodium intake combined with renal sodium wasting), but in times of acute illness (↑ fluid demand and ↓ intake), the slower adaptive mechanisms make hypernatraemic dehydration more common.
Hypokalaemia is common because of poor intake and frequent diuretic use,but lower GFR and hypoaldosteronism lead to vulnerability to hyperkalaemia, especially when exacerbating drugs (NSAIDs, spironolactone, ACE inhibitors)are used.
Structural changes Renal mass falls by 20–30% between 30 and 90 years, making kidneys appear smaller on ultrasound scanning, without necessarily implying disease. Distal nephrons develop diverticulae (three per tubule by age 90) that may become retention cysts (benign finding in older people) Other changes Renal 1-hydroxylase activity ↓ with age, leading to ↓ vitamin D production.Combined with low phosphate intake, this can mildly elevate parathyroidhormone (PTH) levelsThere is loss of the circadian rhythm, owing to altered sodium handling andpatterns of aldosterone secretion, so that over the age of 60, the proportion ofwater, sodium, and potassium excretion occurring at night ↑, causing nocturia |
The aging kidney has become a topic of great interest in geriatric medicine and clinical nephrology. In 1999, glomerular filtration rate (GFR)-estimating equations started to replace serum creatinine for the evaluation of kidney function. Since that time, more and more older adults have been identified as having acute or chronic kidney disease (CKD), and the prevalence of diagnosed kidney disease in this population has increased. About half of adults over the age of 70 years now have a measured or estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, a threshold often used to diagnose CKD.This higher prevalence of diagnosed CKD is not simply due to increased recognition of diseases that tend to cluster in older adults, such as antineutrophil cytoplasmic antibody (ANCA)-positive small-vessel vasculitis, amyloidosis, diabetic nephropathy, and tubulointerstitial disorders. Rather, much of the increased rate of CKD diagnoses in the elderly population results from the normal structural and functional changes that occur in the kidney with aging . Many have argued that this increased recognition of CKD is a positive development and leads to better care in elderly populations. Others have argued that this is a harmful development that has led to unnecessary labeling of far too many older patients as "diseased" without any proven clinical benefit .This review describes the structural and functional changes in the kidney with normal aging and the clinical significance of these changes. NORMAL AGING VERSUS CHRONIC DISEASE — Aging is a natural and inevitable biological process that results in structural and functional changes in many organ systems. The kidney systematically loses function (eg, glomerular filtration rate [GFR]) with age.In addition to specific kidney diseases that are common in older adults, such as diabetic nephropathy, physiological senescence of the kidney occurs, even with healthy aging . A similar process occurs in the lung, which systematically loses function (ie, forced expiratory volume in one second), even among "healthy" adults It can sometimes be difficult to distinguish the structural and functional changes of a kidney affected by a specific preventable or treatable disease from those of a kidney undergoing the inevitable consequences of aging. However, even if the reduction in function is not preventable or treatable, senescent changes in the kidney are relevant and important to managing older patients. Specifically, loss of renal reserves with aging has the following clinical significance : ●More advanced disease if a new specific nephropathy, such as diabetic nephropathy or vasculitis, develops ●Increased susceptibility to acute kidney injury ●Toxic accumulation of renally cleared medications ●The need for age-appropriate criteria in the selection of living kidney donors Living kidney donors as a resource to examine normal aging — Living kidney donors are a unique and important resource for understanding the structural and functional changes of normal aging. Kidney donors are systematically and meticulously evaluated to confirm "health" or at least "healthy for age" as currently understood. This provides the opportunity to characterize the physiological changes in the kidney with optimal aging. Kidney donors undergo kidney function testing, urinalysis, and contrast computed tomography (CT) angiograms as part of their evaluation. In addition, some transplant centers obtain implantation renal biopsies (ie, "time zero" renal biopsies) at the time of surgical donation. Autopsy studies of the general population (identified by sudden deaths) can also characterize structural changes in the kidney with aging, but they lack simultaneous kidney function and other clinical evaluations . By contrast, general population studies of the aging kidney in living humans rely upon nonspecific biomarkers to evaluate the kidney since structural characteristics of the kidney are not typically available.
STRUCTURAL CHANGES OF THE KIDNEYS WITH AGING — Various structural changes occur with aging, including micro-anatomic changes, such as nephrosclerosis and a decline in nephron number, and macro-anatomic changes, such as decreased kidney cortical volume and the development of renal cysts. Micro-anatomical changes of the kidneys with aging — The major micro-anatomical changes that occur with aging are nephrosclerosis and nephron hypertrophy. Nephrosclerosis — The primary structural finding of the aging kidney on light microscopy is nephrosclerosis, characterized by an increased detection of focal and global glomerulosclerosis, tubular atrophy, interstitial fibrosis, and fibrointimal hyperplasia (arteriosclerosis). In a study of kidney transplant donors, nephrosclerosis, defined as the combination of two or more of glomerulosclerosis, tubular atrophy, interstitial fibrosis, or arteriosclerosis, increased from 2.7 percent in kidneys from 18- to 29-year-olds to 73 percent in kidneys from 70- to 77-year-olds. The difference in nephrosclerosis comparing two kidneys separated in age by eight years was roughly equivalent to the difference in nephrosclerosis comparing a kidney from a hypertensive donor with a similarly aged nonhypertensive donor. Nephrosclerosis is due to an ischemic injury of the nephrons that is thought to result from arteriosclerosis and hyalinosis of small arteries. With ischemic injury, the glomerulus develops wrinkled capillary tufts, thickened basement membranes, and pericapsular fibrosis, eventually leading to collapse of the tuft and a diminutive, globally sclerosed glomerulus with collagen deposition filling Bowman's space. Some of these obsolescent glomeruli may subsequently undergo complete absorption.The tubule associated with the sclerotic glomerulus also atrophies, and the interstitium surrounding the atrophic tubule undergoes fibrosis. Glomerulosclerosis, tubular atrophy, and interstitial fibrosis on biopsy of kidney donors correlate with the presence of arteriosclerosis.There is reduction in podocyte density throughout life that may also cause glomerular tuft collapse and contribute to age-related glomerulosclerosis via a nonischemic pathway. The upper reference limit (95th percentile) for the number of globally sclerotic glomeruli expected on biopsy according to age was determined in 1847 normotensive living kidney donors ▪︎Nephron number in aging kidneys —The number of functional nephrons with which a person is born (ie, nephron endowment) varies between 700,000 and 1.8 million per kidney, and the number progressively declines with aging due to nephrosclerosis. As glomeruli sclerose with aging, there appears to be eventual absorption of these sclerosed glomeruli, and the total number of sclerosed and non-sclerosed glomeruli declines with age. Low nephron endowment associated with low birth weight may accelerate the age-related decline in functional nephrons in adulthood .
Nephron hypertrophy — Nephron size (both glomerulus and tubule) increases in a variety of metabolic risk states, including obesity and diabetes, due to cellular hypertrophy and hyperplasia. In parallel with age-related glomerulosclerosis, hypertrophy of the remaining functional nephrons also occurs. However, whether glomerular hypertrophy, in particular, is a consequence of normal aging is less clear.It is possible that, while healthy aging might not induce glomerular hypertrophy, age-related accumulation of comorbid conditions may be associated with glomerular hypertrophy. Other — Microdissection of nephrons from autopsied kidneys shows a strong association between aging and frequency of tubular diverticula Macro-anatomical changes of the kidneys with aging — The major macro-anatomical changes that occur with aging include a reduction in cortical volume and the appearance of renal cysts and tumors. Kidney volume — A decline in total kidney volume with aging was noted in clinical populations using ultrasound or computerized tomography (CT) . In a large study of 1344 potential kidney donors, kidney parenchymal volume was stable before age 50 years but declined after age 50 years . This study also separately measured the kidney cortical and medullary volumes. ~Cortical volume declined throughout the adult age spectrum in both men and women, but with an accelerated rate of decline after age 50 years. By contrast, ~medullary volume increased with age in both men and women until age 50 years, after which it declined in women and remained static in men. ~Renal sinus fat increases with age; this could have masked some of the decline in kidney parenchymal volume with aging in studies that included sinus fat in kidney volume or weight measurements. Micro-anatomical connections to kidney volume — The age-related decline in kidney cortical volume is largely due to underlying nephrosclerosis (senescent global glomerulosclerosis and atrophy of tubules are lesions that decrease the volume occupied by nephrons). Some of the preservation of kidney parenchymal volume with aging can be explained by compensatory hypertrophy of the remaining functional nephrons.Increased medullary volume with aging can be explained by hypertrophy of tubules attached to juxtamedullary glomeruli in compensation for atrophy (glomerulosclerosis and nephrosclerosis) of the more superficial nephrons in the cortex. There appears to be a more accelerated loss of total kidney parenchymal volume after middle age (approximately 50 years). This may be the point at which hypertrophy of functional nephrons no longer compensates for the volume lost from sclerosed and atrophied nephrons . Renal cysts and tumors — Renal parenchymal cysts become more common, more numerous, and larger with aging. Age-related diverticula in renal tubules are the precursor lesion to these renal cysts . Because of this age-related increase in renal cysts, the diagnosis of autosomal dominant polycystic kidney disease (ADPKD) with renal ultrasound imaging requires age-specific criteria (Ravine criteria). CT scans have higher resolution and can detect smaller cysts not seen by ultrasound. Although renal cysts that are not associated with malignancy or a polycystic disorder are often called "benign" or "simple," they frequently reflect chronic underlying parenchymal injury of the aging kidney and are associated with increased urine albumin excretion. In addition to parenchymal simple renal cysts, other renal cysts are also more common with aging, including parapelvic renal cysts (due to lymphatic dilation near the renal sinus), hyperdense cysts, angiomyolipomas, and malignant cysts or tumors [49].Other structural changes — Atherosclerosis of renal arteries (with or without significant stenosis) is more prevalent in older individuals, ranging from 0.4 percent in 18- to 29-year-olds to 25 percent in 60- to 75-year-olds [52]. Fibromuscular dysplasia (FMD) also becomes more prevalent with aging, though it is much more common in women [52]. In addition, parenchymal calcifications and cortical scars (a focal atrophic region of cortex) are more common with aging [52] . |
FUNCTIONAL CHANGES OF THE KIDNEYS WITH AGING — Glomerular filtration rate (GFR) is the primary tool for assessing age-related functional changes in the kidney and is typically the only tool for examining age-related changes since kidney biopsy and renal imaging are not typically justified in routine patient care.GFR declines with normal aging — Rates of GFR decline with aging follow a fairly normal (Gaussian) distribution, suggesting that it is primarily driven by a physiological process [53]. Not only does the lower reference limit for GFR decrease with age, but the upper reference limit also decreases with age, consistent with a universal or near-universal decline in kidney function (table 2) [54,55]. As an example, a longitudinal study of 254 men followed for 23 years found that the average GFR (estimated serially by urinary creatinine clearance) declined by 7.5 mL/minper decade of life [53]. Some of these individuals had diabetes or obesity, which could have impacted the results, and in fact, one-third of the cohort had an increase in their creatinine clearance over time, which likely reflected both hyperfiltration (seen with obesity, early diabetes, and subclinical cardiovascular disease [56]) and imprecision with urinary creatinine clearance measurements. (See "Calculation of the creatinine clearance" and "Mechanisms of glomerular hyperfiltration in diabetes mellitus".)Some investigators have attributed this GFR decline with normal aging to a high-sodium diet or some other pervasive influences of a "modern" or "Western" lifestyle. However, GFR decline with normal aging is also observed in populations unaffected by Western lifestyle factors. In an isolated population of Kuna Indians in Panama, for example, GFR (measured by inulin clearance) declined by 9.5 mL/min per decade of life, despite not having a Western lifestyle and not having an age-related increase in blood pressure that is typical of a Western lifestyle [57-59].Mechanistic basis for GFR decline with aging — Several studies have suggested that the decline in GFR in aging is primarily driven by a vascular (arterial) process. There has long been evidence of altered responses to vasodilators and vasoconstrictors in the aging kidney [60-64]. This is further supported by an increase in the filtration fraction (the fraction of renal blood flow filtered by the glomerulus) and the blunted rise of renal blood flow (but not GFR) in response to an amino acid infusion with aging [60,65-67]. Detecting a link between GFR decline and nephrosclerosis with aging may be challenging because they may be the same process and universal. Nephrosclerosis on renal biopsy associates with decreased GFR, but not independently of older age [7]. Cortical atrophy with aging appears to be along the same biological pathway as GFR decline, but there also appear to be factors that contribute to GFR decline in addition to cortical atrophy [42]. In particular, GFR may decline with aging due to a decreased metabolic demand, as reflected by lower urea generation in elderly patients [68]. Standardizing GFR to metabolic rate instead of to body surface area attenuates much of the GFR decline with aging [69].High proportion of older adults with reduced GFR — The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines define and stage chronic kidney disease (CKD) by levels of GFR and albuminuria (table 3). (See "Definition and staging of chronic kidney disease in adults".)Since nearly all healthy young adults have a GFR ≥90 mL/min/1.73 m2, this GFR range has been defined as "optimal" or "normal." A GFR of 60 to 89 mL/min/1.73 m2has been defined as "mildly decreased relative to young adult levels." Regardless of age, any GFR ≥60 mL/min/1.73 m2 is not considered to be CKD unless some other criteria of "kidney damage" is met (eg, albuminuria) [3]. A study of 610 community-dwelling adults older than 70 years found that half had "CKD" as determined by a measured GFR <60 mL/min/1.73 m2 [1]. Often, a specific disease process for a GFR reduction to <60 mL/min/1.73 m2 in older adults cannot be identified (other than normal aging).Estimated glomerular filtration rate (eGFR) — Direct measurement of GFR is not available in most clinical settings. Instead, GFR is usually estimated from serum creatinine (eGFRcreatinine) using the Modification of Diet in Renal Disease (MDRD) study equation or the 2009 Chronic Kidney Disease Epidemiology (CKD-EPI) equation [70]. As with measured GFR, about half of adults over the age of 70 years have CKD as determined by an eGFRcreatinine <60 mL/min/1.73 m2. Creatinine-based GFR-estimating equations include an age variable to model the decline in creatinine generation (due to decreased muscle mass) with normal aging [71]. This age variable unmasks the age-related decline in GFR that is not detected by serum creatinine alone. Interestingly, the age-related decline in GFR is approximately canceled out by the age-related decline in muscle mass, such that serum creatinine remains stable with healthy aging [72]. The only way a patient can maintain a stable eGFRcreatinine with aging is to have a progressive decrease in his or her serum creatinine level. However, a declining serum creatinine level can represent loss of muscle mass from a disease process. A patient with a stable serum creatinine level who "ages into CKD" based upon eGFRcreatinine <60 mL/min/1.73 m2 could have experienced nothing more than the expected age-related decline in GFR. Ideally, patients would lose neither GFR nor muscle mass with aging, and serum creatinine levels would still remain stable with aging.When and how to confirm CKD in older adults — Uncertainty regarding a reduction in GFR when using eGFRcreatinine may exist when other evidence of CKD such as albuminuria is lacking, or when the muscle mass is more than average for the patient's age. When such uncertainty exists regarding the accuracy of eGFRcreatinine, a urinary creatinine clearance or direct GFR measurement can further assess the kidney function of older adult patients. This may be particularly important when an accurate GFR assessment is needed for a patient who wants to donate a kidney. (See 'Living kidney donor evaluation' below.)Older patients in good health and thus with higher-than-average muscle mass may meet the definition of CKD by eGFRcreatinine <60 mL/min/1.73 m2 but may actually have a GFR ≥60 mL/min/1.73 m2 using a confirmatory test. This can be reassuring to some older patients who are concerned about being diagnosed with CKD. However, if an older patient's urinary creatinine clearance or direct GFR measurement is still modestly reduced (eg, 45 to 59 mL/min/1.73 m2), it is still possible that this could be nothing more than a reduced GFR from normal aging. (See 'What to do with isolated mild reductions in eGFR in older adults' below.)When confirmation of CKD is desired, the KDIGO guidelines suggest using GFR-estimating equations that are based upon cystatin C (eGFRcystatin C) or upon both creatinine and cystatin C (eGFRcreatinine-cystatin C) [73]. Specifically, they suggest these calculations be made in patients with an eGFRcreatinine of 45 to 59 mL/min/1.73 m2 and no other markers of kidney damage [3]. There is improved discrimination of outcomes (end-stage renal disease [ESRD] and mortality) with eGFRcystatin C or eGFRcreatinine-cystatin C compared with eGFRcreatinine [3]. However, the optimal GFR-estimating equation for predicting such outcomes does not necessarily reflect the true association between GFR and outcomes [74]. Unlike an elevated serum creatinine, elevated cystatin C occurs with other chronic diseases besides CKD. Cystatin C is a protease inhibitor that plays a role in atherosclerosis, obesity, and inflammation [75-77], and serum cystatin C associates with both CKD risk factors and the development of ESRD, independent of the patient's underlying GFR (figure 1) [78-81]. Thus, although patients with elevated serum cystatin C have a higher risk for ESRD, cardiovascular disease, and mortality, this risk may be mediated by factors other than kidney function (ie, GFR). As a result, we do not recommend use of eGFRcystatin C or eGFRcreatinine-cystatin C to confirm a diagnosis of CKD.Mortality and kidney failure risk with CKD from aging — It is not clearly evident that the relationships between eGFR and death or ESRD support the use of a common threshold for CKD in all age groups. A meta-analysis by the CKD Prognosis Consortium studied 46 cohorts with 2,051,244 patients and found that a low eGFRcreatinine was associated with mortality and ESRD in all age groups [82]. However, several features of this meta-analysis argue that a separate GFR threshold is needed for older adults:●Among individuals without albuminuria, an isolated mild reduction in eGFRcreatinine of 45 to 59 mL/min/1.73 m2 (compared with 75 to 89 mL/min/1.73 m2) was associated with a 20 percent higher relative mortality in those 75 years and older, but a 179 percent higher relative mortality in those aged 18 to 54 years.●The lowest risk for mortality in adults aged 18 to 54 years was with an eGFRcreatinine ≥105 mL/min/1.73 m2. However, in adults aged 75 years and older, those with an eGFRcreatinine in this range had a 60 percent higher relative mortality. If the separation between "normal" and "disease" is based upon risk, as suggested by KDIGO, then age-specific ranges are justified (figure 2).●In the KDIGO guidelines, an eGFRcreatinine ≥90 mL/min/1.73 m2 is used to define "optimal" or "normal" [3]. However, the meta-analysis performed by the CKD Prognosis Consortium to evaluate risks associated with isolated mild reductions in eGFR did not use this "normal" range as the reference group, thereby limiting the use of these data.Other functional changes — The kidneys of older patients demonstrate impaired renal conservation of sodium in response to an acute reduction of sodium intake, as well as impaired ability to rapidly excrete a large sodium load. Animal studies show a decreased abundance of sodium chloride transporters and epithelial sodium channels with aging [83]. There is also loss of both the maximum urine concentrating ability (from about 1200 to 800 mosm/L)and the maximum urine diluting capacity (from about 50 to 100 mosm/L) [84-87]. The expression of aquaporins is reduced in the medulla with aging in animal models [88]. The reduced diluting capacity of the kidney increases the risk of hyponatremia in older patients, particularly those with a low-protein "tea and toast" diet that is consumed by some older adult patients. Changes in the diluting capacity also contributes to thiazide-associated hyponatremia, which is more common in older adults [89].CLINICAL SIGNIFICANCE OF THE AGING KIDNEY — There are several important issues in the management of patients with age-related changes in kidney structure and function. These include:●There are no proven therapies to halt or reverse age-related declines in glomerular filtration rate (GFR). Any therapy aimed at raising the GFR by causing the remaining functional nephrons to filter more may actually be harmful rather than beneficial to the kidney. Hypertrophy and hyperfiltration of functional glomeruli in animals, for example, can lead to further glomerulosclerosis. (See "Secondary factors and progression of chronic kidney disease" and "Secondary factors and progression of chronic kidney disease", section on 'Intraglomerular hypertension and glomerular hypertrophy'.)This is a key difference distinguishing age-related "chronic kidney disease (CKD)" from other age-related chronic disease such as hypertension or hyperlipidemia. Age-related hypertension and hyperlipidemia can be reversed, and reducing the blood pressure and cholesterol has clinical benefit in older adult patients. By contrast, generic therapies for CKD (such as angiotensin inhibitors and protein-restricted diets) do not reverse age-related GFR decline, and it is not clear that these therapies are of any benefit to an older adult with only age-related "CKD."●Patients with age-related reductions in GFR require medication dose adjustments. (See 'Medication dosing' below.)●The loss of functioning nephrons associated with normal aging puts older adults at higher risk for acute kidney injury. (See 'Acute kidney injury' below.)●A reduction in estimated glomerular filtration rate (eGFR) with aging does not substantially improve the assessment of cardiovascular risk over conventional predictors and does not indicate a high risk of end-stage renal disease (ESRD). (See 'Cardiovascular morbidity and mortality risk' below and 'End-stage renal disease' below.)●Age-related declines in GFR do not typically prevent older adults from becoming living kidney donors. (See 'Living kidney donor evaluation' below.)●Overall, the typical age-related declines in GFR have little, if any, effect on life expectancy, and this point is important in discussions with older adult patients. (See 'Counseling patients with CKD due to normal age-related GFR declines' below.)What to do with isolated mild reductions in eGFR in older adults — There is controversy about whether or not to diagnose older adults with CKD based upon an eGFRcreatinine of 45 to 59 mL/min/1.73 m2 in the absence other evidence of CKD (such as albuminuria). An eGFR of 45 to 59 mL/min/1.73 m2 has a modest though statistically significant association with adverse events such as death and ESRD in older adults, leading many experts and the Kidney Disease Improving Global Outcomes (KDIGO) group to endorse an eGFR <60 mL/min/1.73 m2 for diagnosing CKD regardless of age. (See "Definition and staging of chronic kidney disease in adults".)By contrast, many authorities caution against labeling older patients with a stable and normal serum creatinine as having CKD solely based upon an eGFRcreatininebetween 45 and 59 mL/min/1.73 m2. The major reasons for this caution are as follows:●Estimation of GFR is imprecise and can be inaccurate. A true decrease in GFR in someone with CKD, for example, can be partially cancelled out by a parallel decrease in creatinine generation [78]. In addition, the age variable that is included in the estimating equations can inflate the adverse risks attributed to reduced GFR, particularly in older adults [71]. Many problems associated with estimating GFR using estimation equations apply both to younger and older populations; these are presented in detail separately. (See "Assessment of kidney function" and "Assessment of kidney function", section on 'Estimation of GFR'.)●Because of a decline in GFR with healthy aging, the association of a given eGFR with mortality and ESRD is not identical across the age spectrum (figure 2). The relative risk of mortality is substantially less in older compared with younger patients with an eGFR of 45 to 59 mL/min/1.73 m2. This issue is discussed above in detail. (See 'Mortality and kidney failure risk with CKD from aging' above.)There is concern for both over-diagnosis of CKD in older patients with an eGFR below 60 mL/min/1.73 m2 and under-diagnosis of CKD in younger patients with eGFR above 60 mL/min/1.73 m2. Thus, some have suggested using the upper reference limits for serum creatinine (1.2 mg/dL in men and 1 mg/dL in women [106.3 and 88.6 micromol/L, respectively]) to identify those patients who have CKD resulting from GFR reductions that exceed the expected age-related decline. These reference limits are derived using healthy adults in the general population and in healthy kidney donors [90,91]. The upper reference limits for serum creatinine may be slightly higher in African Americans and slightly lower in Asians, although few studies have assessed reference limits in non-white populations [91].Some have also suggested reporting age-specific lower reference limits for eGFRcreatinine, which can be calculated from the upper reference limits for serum creatinine (table 2) [54,55]. However, few studies have assessed reference limits for serum creatinine in elderly populations (>70 years) [92]. In an elderly patient with an eGFR of 45 to 59 mL/min/1.73 m2 and no albuminuria, we advocate caution in diagnosing CKD (rather than an age-related GFR decline). Approximately half of all older patients will have an eGFR <60 mL/min/1.73 m2, and the relative risks of ESRD and mortality associated with lower eGFR values are substantially less than in younger patients (figure 2) [2].Medication dosing — Dosing with medications that are excreted by glomerular filtration (typically water-soluble medications) should be adjusted to the patient's GFR as indicated by medication dosing guidelines (typically provided by the manufacturer). Historically, most medication dosing guidelines have been developed using the Cockcroft-Gault equation (calculator 1 and calculator 2) [93]. The Cockcroft-Gault equation is not as accurate as the Modification of Diet in Renal Disease (MDRD) study or Chronic Kidney Disease Epidemiology (CKD-EPI) equations for estimating GFR. If GFR is estimated by the MDRD study or CKD-EPI equations, the result must be converted to mL/min by multiplying by the patient's body surface area (calculator 3) and dividing by 1.73. Issues related to estimating GFR as it pertains to drug dosing are discussed elsewhere in detail. (See "Assessment of kidney function", section on 'Drug dosing'.)Acute kidney injury — Loss of renal reserves (functional nephrons) makes older patients more susceptible to acute kidney injury [94,95]. Medications that have potential renal toxicity, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and radiocontrast agents, should be used cautiously in older patients. (See "NSAIDs: Acute kidney injury (acute renal failure)", section on 'Risk factors'.)Cardiovascular morbidity and mortality risk — Age is already included in models that estimate cardiovascular risk. (See "Cardiovascular disease risk assessment for primary prevention: Risk calculators".)Addition of eGFRcreatinine to cardiovascular risk models does not meaningfully improve risk prediction over conventional risk stratification models [96-98]. In addition, the inclusion of cystatin C with conventional factors in cardiovascular risk assessment only minimally improves prediction over conventional risk factors alone [99]. Cystatin C might contribute to the prediction of cardiovascular outcomes along non-GFR biological pathways, such as inflammation and atherosclerosis [75-77]. The issue of CKD as a risk factor for cardiovascular disease is presented in detail elsewhere. (See "Chronic kidney disease and coronary heart disease" and "Chronic kidney disease and coronary heart disease", section on 'CKD as a CHD risk equivalent'.)End-stage renal disease — CKD from aging alone does not, by itself, progress to kidney failure, even in the very elderly [100]. A validated calculator has been developed to estimate the risk of kidney failure [101]. Like most prior kidney failure risk models, both age and eGFRcreatininewere included in the prediction model. While lower eGFRcreatinine is associated with increased risk of kidney failure, older age is actually associated with a lower risk of kidney failure. This occurs because, at the same eGFRcreatinine, older patients as compared with younger patients are more likely to die before developing ESRD [102]. Kidney failure prediction models can help inform decisions about the need to prepare for dialysis in older adult patients (eg, placement of an arteriovenous fistula). These models can also reassure older patients with only age-related reductions in eGFRcreatinine that their risk of progression to ESRD is very low.Living kidney donor evaluation — There are substantial changes in the micro-anatomy (glomerulosclerosis), macro-anatomy (cortical volume loss), and kidney function (GFR decline) with older age, even among healthy adults. Even a kidney donor in his or her thirties will have more nephrosclerosis on renal biopsy than a kidney donor in his or her twenties [7]. Living donors have been reported to have similar long-term mortality and kidney failure risk compared with age-matched controls who do not donate a kidney, despite the fact that a post-donation eGFR <60 mL/min/1.73 m2 occurs in more than 60 percent of donors older than 50 years [103-105]. In particular, a study of 219 living donors aged ≥70 years found a lower risk of mortality compared with age- and comorbidity-matched controls [105]. Conversely, a study of 1901 living donors in Norway found an increased long-term risk for ESRD and mortality with kidney donation, possibly because the age-matched controls in that study were healthier than age-matched controls in prior studies. Whether this risk they detected differs between older and younger donors was not reported [106].Many transplant programs only require the pre-donation GFR to be appropriate for the donor's age (table 2) and permit certain CKD risk factors (such as mild hypertension) to be present in older donors without rejecting them as candidates [107]. From the recipient's perspective, an older allograft (donated kidney) is associated with somewhat higher rates of graft loss [105], and this may be related to the increased parenchymal injury present in the allograft with aging. (See 'Structural changes of the kidneys with aging'above.)Counseling patients with CKD due to normal age-related GFR declines — In older adults who are diagnosed with age-related "CKD," a careful explanation is warranted to reassure the patient that a decline in GFR within the range expected for normal aging will have little, if any, effect on his or her life expectancy. Although they may be at higher risk for ESRD because of lower functional reserve (fewer functioning nephrons), this is still a relatively rare event, particularly in the elderly with an eGFR of 45 to 59 mL/min/1.73 m2 [108]. Context is particularly relevant, as there are no known therapies that can reverse the age-related decline in GFR. It may also be helpful to discuss that senescence occurs in many other organs such as the skin and the lungs. (See "Overview of benign lesions of the skin" and "Selecting reference values for pulmonary function tests".)ALBUMINURIA — Although urine albumin excretion increases with age in the general population [109], 24-hour urine albumin excretion does not increase with age in healthy adults, provided few or no comorbidities are present [7]. Thus, albuminuria may be a useful marker for distinguishing normal aging from another etiology of chronic kidney disease (CKD).However, the random first morning urine albumin-to-creatinine ratio (UACR), which is more widely used in clinical practice than a 24-hour urine collection, is more likely to give a falsely elevated result in an aged population. Unlike the 24-hour urine albumin excretion, UACR usually increases with healthy aging due in part to age-related reductions in muscle mass that result in reduced creatinine generation and excretion [71,110,111]. If a random or morning UACR is used to assess albuminuria in older adults, multiplying the UACR by the expected 24-hour urine creatinine may improve the accuracy of the test for detecting an abnormally high albumin excretion. The expected 24-hour urine creatinine can be calculated from one of several formulas [112,113].SUMMARY AND RECOMMENDATIONS●In 1999, glomerular filtration rate (GFR)-estimating equations started to replace serum creatinine for the evaluation of kidney function. Since that time, more and more older adults have been identified as having acute or chronic kidney disease (CKD), and the prevalence of diagnosed kidney disease in this population has increased. About half of adults over the age of 70 years now have a measured or estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, a threshold often used to diagnose CKD. This higher prevalence of diagnosed CKD is not simply due to increased recognition of diseases that tend to cluster in older adults. Rather, much of the increased rate of CKD diagnoses in the elderly population results from the normal structural and functional changes that occur in the kidney with aging. (See 'Introduction' above.)●It can sometimes be difficult to distinguish the structural and functional changes of a kidney affected by a specific preventable or treatable disease from those of a kidney undergoing the inevitable consequences of aging. However, even if the reduction in function is not preventable or treatable, senescent changes in the kidney are relevant and important to managing older patients. Specifically, loss of renal reserves with aging has the following clinical significance (see 'Normal aging versus chronic disease' above):•More advanced disease if a new specific nephropathy, such as diabetic nephropathy or vasculitis, develops•Increased susceptibility to acute kidney injury•Toxic accumulation of renally cleared medications•The need for age-appropriate criteria in the selection of living kidney donors●Various structural changes occur with aging, including (see 'Structural changes of the kidneys with aging' above):•Nephrosclerosis (see 'Nephrosclerosis' above)•Decline in nephron number (see 'Nephron number in aging kidneys' above)•Decreased kidney cortical volume (see 'Kidney volume' above)•Development of renal cysts (see 'Renal cysts and tumors' above)●Rates of GFR decline with aging follow a fairly normal (Gaussian) distribution, suggesting that it is primarily driven by a physiological process. Not only does the lower reference limit for GFR decrease with age, but the upper reference limit also decreases with age, consistent with a universal or near-universal decline in kidney function (table 2). (See 'GFR declines with normal aging' above.)●The Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines define and stage CKD by levels of GFR and albuminuria (table 3). Approximately half of adults older than 70 years are found to have "CKD" as determined by a measured or estimated GFR <60 mL/min/1.73 m2. Often, a specific disease process for a GFR reduction to <60 mL/min/1.73 m2 in older adults cannot be identified (other than normal aging). (See 'High proportion of older adults with reduced GFR' above.)●Uncertainty regarding the diagnosis of CKD in older adults may exist, particularly when the patient's eGFRcreatinine is barely below 60 mL/min/1.73 m2, when other evidence of CKD such as albuminuria is lacking, or when the muscle mass is higher than average for the patient's age. When such uncertainty exists regarding the diagnosis of CKD based upon eGFRcreatinine, a urinary creatinine clearance or direct GFR measurement can further assess the kidney function of older adult patients. This may be particularly important when an accurate GFR assessment is needed for an older patient who wants to donate a kidney. In contrast to KDIGO suggestions, we do not recommend use of cystatin C to confirm a diagnosis of CKD in older adults. (See 'When and how to confirm CKD in older adults'above.)●There are several important issues in the management of patients with age-related changes in kidney structure and function. These include (see 'Clinical significance of the aging kidney' above):•There are no proven therapies to halt or reverse age-related declines in GFR. Any therapy aimed at raising the GFR by causing the remaining functional nephrons to filter more may actually be harmful rather than beneficial to the kidney. Hypertrophy and hyperfiltration of functional glomeruli in animals, for example, can lead to further glomerulosclerosis. (See "Secondary factors and progression of chronic kidney disease" and "Secondary factors and progression of chronic kidney disease", section on 'Intraglomerular hypertension and glomerular hypertrophy'.)•Patients with age-related reductions in GFR require medication dose adjustments. (See 'Medication dosing' above.)•The loss of functioning nephrons associated with normal aging puts older adults at higher risk for acute kidney injury. (See 'Acute kidney injury' above.)•A mild reduction in eGFR with aging does notsubstantially improve the assessment of cardiovascular risk over conventional predictors and does not indicate a high risk of end-stage renal disease (ESRD) (figure 2). (See 'Cardiovascular morbidity and mortality risk'above and 'End-stage renal disease' above.)•Age-related declines in GFR do not typically prevent older adults from becoming living kidney donors. (See 'Living kidney donor evaluation'above.)•Overall, the typical age-related declines in GFR have little, if any, effect on life expectancy or the future need for dialysis, and this point is important in discussions with older adult patients. (See 'Counseling patients with CKD due to normal age-related GFR declines' above. |
|
Acute kidney injury: management Is this acute kidney injury?Older people are more likely to have underlying CKD, and this confers a worse prognosis. Check old notes; ask the patient, family, and GP about history, and look back at blood test results. The more severe and persistent the episode of AKI, the greater the permanent deterioration in renal function. Generally, management does not differ significantly from younger patients. Treat the cause Older people respond as well to most treatments. Monitor meticulously Pulse and BP, cardiac monitor, input (iv and po), and output (urine, faecal matter, vomit, drains, sweat) May be best done on HDU Aim for euvolaemia (assessed clinically, may need to correct deficit), then maintain by matching input to output on an hourly basis initially.
Management of fluid balance is likely to be harder in older people because of comorbidity (especially heart failure) ► The presence of peripheral oedema does not necessarily indicate fluid overload. Circulating volume is best assessed by BP, pulse, JVP, and skin turgor. May need central venous catheter and urinary catheter initially, but remove as soon as possible because of infection risk. Document daily weight and total fluid balance summary. Be prepared for polyuria in the recovery phase, and ensure that the patient does not become fluid-depleted.
Treat complications Importantly hyperkalaemia, acidosis, and pulmonary oedema. Refer early for further renal support (filtration or dialysis); a patient can remain oliguric for some time while renal recovery is occurring, but it is sensible to make the relevant teams aware of a potential patient.
The indications for renal replacement therapy are as follows: ▪︎Refractory pulmonary oedema (older people are particularly prone to this after over-enthusiastic initial fluid replacement) ▪︎Persistent hyperkalaemia (potassium >7mmol/L) that cannot be controlled by insulin/glucose infusions and iv calcium ▪︎Worsening acidosis (pH <7.2) ▪︎Uraemic pericarditis ▪︎Uraemic encephalopathy |
HOW TO Perform a fluid challenge in AKI/anuria •Many older patients are clearly dehydrated with mildly impaired renal function tests, and these patients can be simply rehydrated orally or parenterally. •If the patient presents with established AKI or is found to be anuric despite simple rehydration, then a fluid challenge should be contemplated. •This is an important clinical skill to develop and requires advanced clinical acumen and an investment of time. •The key is an accurate assessment of fluid status, with the aim of rendering the patient euvolaemic. •A urinary catheter is usually required, and a central venous pressure monitoring device is helpful if facilities exist. •Start by assessing and clearly documenting baseline fluid status, as this will inform your management and will be helpful when the patient is reassessed. •If patient is already fluid overloaded then a single bolus of iv loopdiuretic and early contact with the renal specialist team is needed. •If the patient appears to be hypovolaemic, give 500mL of normal saline(sodium bicarbonate may be considered if they are profoundly acidotic) over 30–60min, and review. •If the patient appears to be euvolaemic, then the fluid challenge should be more cautious.Give a 100mL bolus iv, and review after about 15min. •For the review, repeat and document the fluid status and urine output. •Repeat this cycle of fluid prescription, and do a careful review as the clinical progression becomes clear. |
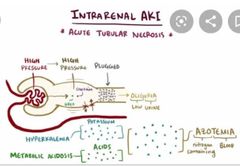
Acute kidney injury—investigations Investigation Rationale Special points in older people •Urea and creatinine Elevated in renal failure Older people with very little muscle mass will have lower baseline levels, so a urea of 10 in a small elderly woman will represent significant renal impairment[normal 8-21mg/dl]
•Urea:creatinine ratio can be useful—elevated in pre-renal and post-renal failure,acting as a marker of dehydration or obstruction. •Electrolytes- Potassium rises dangerously in AKI More prone to cardiac complications of electrolyte disturbance monitor carefully. •Arterial blood gases Monitor pH which falls in AKI pH can also be checked on a venous sample •Inflammatory markers (ESR, CRP,white cell count) Check for infection Common precipitant of AKI in older people(may be occult) •Urine dipstick Check for leucocytes and nitrites(infection), blood, and protein (active renal lesion likely)
High rate of positive urine dipstick in older people does not always imply infection.
Urine microscopy Looking for casts (red cell casts in glomerulonephritis, white cell casts in infection, etc.) and blood cells
Always send for culture, even when the dipstick is negative. Blood and urine cultures Identify microbes Ensure these are sent on all patients (who may have occult infection) prior to starting antibiotics.
Creatinine kinase Elevated in rhabdomyolysis Always check after falls (especially after a long period on the floor before being found). Even if there is not full blown rhabdomyolysis, an elevated CK level indicates the need for hydration and monitoring of renal function. Urinary sodium •Helps distinguish between pre-renal failure (urinary Na <20 mmol/L as kidney still functioning to preserve sodium) and •ATN (urinary Na >40mmol/L as kidney not functioning, so losing Na) Particularly useful in older people where clinical assessment of fluid balance may be harder because of peripheral oedema etc.
Not helpful if the patient has taken diuretics(increase sodium excretion)
CXR Looking for evidence of •cardiac disease, •source of infection, •pulmonary oedema or •pulmonary infiltrates (vasculitis)
More prone to pulmonary oedema-extra caution with fluid replacement if there is cardiomegaly, even where there is no history of cardiac failure
ECG Looking for evidence of cardiac disease and monitoring for hyperkalaemia. Again alerts to occult cardiac disease if ECG is abnormal
Renal ultrasound ▪︎Assess renal size and look for evidence of hydronephrosis. ▪︎Very useful in older people to help establish if renal failure is truly acute (small kidneys with chronic failure). ▪︎Also checks for treatable obstructive causes common inthis age group.
Other tests All should have FBC and LFTs Usually also send autoantibodies(ANA, antineutrophilic cytoplasmic antibodies (ANCA)),immunoglobulins, complement and electrophoresis of blood and urine. |
|
|
Q |
Q |
|
|
|
Q |
Q |
|

