![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
82 Cards in this Set
- Front
- Back
|
Ascending Pain Pathway: Overview
|
Stimulation of nociceptors causes release of substance P and/or glutamate in the dorsal horns
This activates neurons in the ascending spinothalamic tract (AST) The AST projects primarily to the reticular formation and periaqueductal grey (PAG; dull pain) or to the PAG to thalamus to somatosensory cortex (sharp pain) |
|
|
Pain Modulation.
|
The endogenous opioid system is very important in the modulation of pain.
|
|
|
The Modulation of Pain Occurs in Two Ways:
|
Raising the threshold for pain perception
Increasing the tolerance for pain perception |
|
|
Raising the threshold for pain perception
|
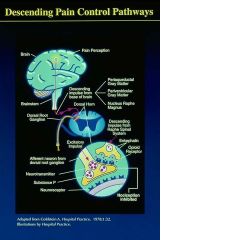
Descending pain pathway
Signals from higher brain centers intercept and modulate incoming nociceptive signals at the level dorsal horns via opioid activity (the Descending Pain Pathway) Pain signals sent to the brain are diminished Peripheral nerve endings Immunocytes release opioids to modulate reactivity of nociceptors |
|
|
There are two components to the perception of pain:
|
1. Sensory (nociception)
•Pain occurs via stimulation of pain receptors •Pain sensations are transmitted via the Ascending Pain Pathway • Pain perception is modulated via the Descending Pain Pathway and directly at peripheral pain receptors 2. Psychological (“affective”) •Related to a person’s state of mind •Modulated by many intrinsic and extrinsic factors |
|
|
Increasing the pain threshold via the Descending Pain Pathway
|
|
|
|
Increasing the pain threshold via the Descending Pain Pathway
|
|
|
|
Summary of the Descending Pain Pathway
|
The perception of pain causes higher brain centers to stimulate the release of enkephalon in the spinal horns to diminish the activity of incoming nociceptive signals
|
|
|
Nociception
|
Nociception is the sensory perception of pain. It occurs through pain receptors known as “nociceptors”.
• Nociceptors are free nerve endings |
|
|
Nociceptors fall into three main classes that correspond to:
|
|
|
|
There are two primary neurotransmitters released from nociceptive fibers in the dorsal horn:
|
1.Substance P
|
|
|
The modulation of pain occurs in two ways:
|
1. Pain modulation by raising the threshold for pain perception (neurochemical modulation)
•Involves the sensation of pain •Two areas of modulation: 1. Raising the threshold for pain via modulation of the Descending pain pathway |
|
|
Pain modulation by increasing the tolerance for pain (“psychological” modulation)
|
•
Pain is still perceived, but the individual is less concerned by it • This is the affective component of pain that occurs in higher brain centers • Pain tolerance is greatly affected by individual temperament (cognitive and emotional disposition), environmental setting, and other factors, for example, anxiety tends to intensify pain • Pain tolerance is modulated by higher brain centers and includes endogenous opioid activity, for example, β-endorphin modulates anxiety in the amygdala |
|
|
Increasing the pain threshold via the modulation of peripheral sensory nerves
|

decreasing overall pain sensation
circulating leukocytes are attracted to injured area and cause release of opioids not Ca dependent |
|
|
Increasing the tolerance for pain perception
|
Pain is still perceived, but the individual is less concerned by it
This is the affective component of pain that occurs in higher brain centers |
|
|
Affective Component of Pain Modulation: Increasing the Tolerance for Pain
|
Pain tolerance is greatly affected by individual temperament (cognitive and emotional disposition), environmental setting, and other factors
For example, anxiety can intensify pain Pain tolerance is modulated by higher brain centers and includes endogenous opioid activity For example, B-endorphin modulates activity in the amygdala, a structure important in fear and anxiety |
|
|
Summary of the Pain System:
|
Sensory component of pain
Transmission through the Ascending Pain Pathway (nociception dorsal horns spinothalamic tract brain) Modulation through opioid receptors in the Descending Pain Pathway Raising threshold for pain Modulation through opioid receptors on Peripheral Nociceptors Raising threshold for pain Affective component of pain Modulation through opioid receptors in higher brain centers Raising tolerance for pain |
|
|
The endogenous opioid system is the main mechanism through which pain is modulated
|
Opioids modulate pain in the brain (increase pain tolerance), in the spinal cord (decrease pain perception), and at sensory nerve endings (decrease pain perception).
|
|
|
OPIOID DRUGS
|
Among pain relief medications, opioids are unique in that they act on both the sensory and affective components of pain. As such, opioid drugs inhibit incoming nociceptive signals at the level of the spinal cord to modulate the sensory component of pain and they also can act at higher brain centers to modulate the affective component of pain
Opioid drugs “take advantage” of the endogenous opioid system to relieve pain. |
|
|
There are three broad classes of opioid drugs:
|
|
|
|
Systemic or local administration of opioid drugs
|
inhibits incoming nociceptive signals (raising the threshold for pain).
|
|
|
Systemically administered opioid drugs act at
|
higher brain centers (e.g., the amygdala) to modulate the affective component of pain (increasing the tolerance for pain).
|
|
|
Opioid drugs fall into two main structural groups:
|
Morphine analogues
*May be agonists, partial agonists, or antagonists Synthetic derivatives with structures unrelated to morphine *May be agonists or partial agonists |
|
|
Opiate drugs
Pure agonists |
Morphine, codeine, oxymorphone, dextropropoxyphene, oxycodone
Methadone Pethidine Etorphine, bremazocine Fentanyl, sufentanil |
|
|
Partial/mixed agonists
|
Pentazocine, ketocyclazocine
Nalbuphine Nalophine Buprenorphine |
|
|
opioid Antagonists
|
Naloxone
Naltrexene, diprenorphine |
|
|
Endogenous peptides
|
β-endorphin
Leu-enkephalin Met-enkephalin Dynorphin |
|
|
Pharmacologic actions of opioid agonists and partial agonists
|
Opioid agonists and partial agonists have a wide variety of effects, both in the central nervous system and in the periphery. The specific effects of any individual drug are due to such factors as opioid receptor binding profile, distribution, and metabolism.
|
|
|
Neuronal activity
central effects of opioids |
•Reduction in neuronal firing
•Inhibition of neurotransmitter release |
|
|
Major Central Effects
analgesia |
Increase in pain tolerance
*Awareness or concern of pain is reduced *Pain is no longer all-consuming *Dependent on many factors (individual temperament, setting, etc.) Increase in pain threshold *Indirect stimulation of serotonin and norepinephrine neurons (PAG, locus coeruleus, Raphe nucleus) *Inhibition of substance P release in axon terminals of nociceptive fibers entering the dorsal horn decreasing pain signals getting to the brain |
|
|
Major Central Effects
respiratory depression |
•Inhibition of brain stem respiratory centers
•Depressed response to PCO2 leading to depressed respiration rate •Slight rise in PCO2 due to decreased respiration rate (this causes cerebral vasodilation which leads to increased CSF pressure; this effect is more pronounced in the presence of head injury) •May not be tolerated in individuals with any kind of respiratory impairment •This effect is not separable from analgesic effects •The effect is additive with other CNS depressants (alcohol, barbiturates, benzodiazepines) •HIGH DOSES OF OPIOID AGONISTS, ESPECIALLY IN COMBINATION WITH OTHER CNS DEPRESSANTS MAY LEAD TO DEATH DUE TO RESPIRATORY FAILURE! |
|
|
what happens to the body's response to CO2 when taking opioids
|
the bady becomes less sensitive so respiration decreases
|
|
|
Major Central Effects
mood changes |
•The majority of users experience euphoria (relaxed, dreamy, floating sensation; freedom from anxiety or stress)
•A minority experience dysphoria (apprehension, anxiety, malaise) |
|
|
Major Central Effects
Sedation |
•Drowsiness, fogginess
•Sleep is frequently induced in the elderly •Degree of sedation varies among the drugs •Normally, the patient is easily aroused •The effect is additive (possibly synergistic) with other CNS depressants |
|
|
central effects of opioids
excitation |
Excitation occurs in some other species (cats, horses, cows, pigs) and in some humans at low doses
|
|
|
central effects of opioids miosis
|
Pupil constriction
Very little tolerance develops to this effect |
|
|
central effects of opioids
N/V |
|
|
|
central effects of opioids
antitussive |
(prevention of cough reflex)
|
|
|
central effects of opioids
endocrine |
|
|
|
central effects of opioids
thermoregulation |
Inhibition of hypothalamic thermoregulatory mechanisms
Ability to maintain constant temperature is impaired Body temperature is dependent on ambient temperature |
|
|
central effects of opioids
sleep |
Disruption of Rapid Eye Movement (REM) sleep
not getting quality sleep |
|
|
peripheral effects of opioids
histamine release |
•Increased release of histamine from mast cells (this effect is unrelated to opioid receptors)
•Causes arterial and venous dilatation •Can potentially cause hypotension, cutaneous flushing, and loss of body heat like an allergic reaction, but not a true one just sensitive to this SE |
|
|
peripheral effects of opioids
GI effects |
•
Contraction of biliary sphincter (may cause increased pain in patients with GI distress) • Contraction of bladder sphincter (may cause painful urine retention) • Increased tone of GI tracts, biliary tracts, ureter • Reduced peristaltic movement causing delayed fecal passage and increased water absorption from large intestine (constipation) |
|
|
peripheral effects of opioids
cardaic affects |
Mild bradycardia (slowing of pulse)
Decrease in blood pressure due to pain relief |
|
|
PO advantages (opioids)
|
Prolonged and smooth effect
Avoidance of discomfort of injection Well absorbed from GI tract May be easily administered by patient |
|
|
PO disadvantages (opioids)
|
Potency of some drugs may be reduced due to first pass metabolism in liver; drugs with free hydroxyl group undergo conjugation with glucoronic acid, for example, morphine
Slow onset of action (up to or over 30 minutes) GI discomfort |
|
|
Subcutaneous or intramuscular
(opioids) advantages and disadvantages |
Advantages
*Rapid onset of action (5-15 minutes) *No reduction in potency due to first pass metabolism Disadvantages *Requires discomfort of injection *May be difficult for patient to self-administer *Increased risk of infection |
|
|
IV (opioids) advantages and disadvantages
|
Advantages
Almost instant onset of action No reduction in potency due to first pass metabolism Disadvantages Requires discomfort of IV catheter May be difficult for patient to self-administer Increased risk of infection Very sudden onset |
|
|
Epidural/intrathecal
(opioids) advantages |
Direct injection into the spinal epidural or subarachnoid space
Mixtures of opioids/local anesthetics may be used Advantages Localized effect (not systemic) Less respiratory depression and nausea |
|
|
Epidural/intrathecal
(opioids) disadvantages |
A small percentage of patients still suffer nausea and/or respiratory depression
“Low pressure” headaches can occur if the dura mater is punctured Certain types of pain are unresponsive Increased risk of spinal cord damage, infection |
|
|
Patient controlled analgesia (PCA) advantages for opioids
|
IV, subcutaneous, epidural routes are available
Provides patient maximal control over analgesia Pre-set “lockout” time to prevent overdose Possibly reduced side effects Simple to use Patient feels more in control |
|
|
Percutaneous admin for opioids
|
Transdermal patches for sustained analgesia (fentanyl; 3 days)
Should not be used immediately following surgery due to respiratory depression associated with its use Very slow onset of action, so oral drugs may be needed initially Works well for our four-legged friends |
|
|
nasal spray for opioids
|
Rapid onset of action
Increased bioavailability (no first pass effect) Very convenient |
|
|
Absorption of opioid drugs
|
Most are well absorbed from GI tract
Serum protein binding varies from drug to drug (30-85% bound) probably contributing to half-life differences Many of the drugs undergo first-pass metabolism decreasing bioavailability Duration of analgesic action ranges from 1-6+ hours |
|
|
Distribution of opiod drugs
|
|
|
|
• Metabolism of opioid drugs
|
|
|
|
Local vs. Systemic Administration of opioids
|
Local (e.g., epidural)
Requires an injection Source of pain must be localized and known Do not get benefit of affective effects Avoids some side effects Systemic (e.g., IV, oral) Increased side effects Does not always have to be injected Do get benefits of affective effects |
|
|
kidney impairment and morphine
|
typically 90% of the active metabolites of morphine (morphine-6-glucuronide and morphine-3-glucoronice) are renally eliminated
therefore when a patient has renal impairment the active metabolites accumulated and enter the CNS |
|
|
Tolerance to Opioid Drugs
|
Tolerance: reduced effect to equivalent dose
Degree of tolerance depends on drug and response being measured Time course: Rapid (2-3 weeks or less): analgesia Slow (>2-3 weeks): respiratory depression, nausea Not at all: miosis, constipation |
|
|
Time course of tolerance in opioids
|
|
|
|
how can tolerance be minimized with opioids
|
minimized with smaller doses and longer dosing interval
|
|
|
is opioid tolerance functional or metabolic
|
functional: so the receptors are being altered
It is not the drug being metabolized more |
|
|
is there cross tolerace with opioids
|
Cross-tolerance develops with all opioid agonists (but not antagonists)
|
|
|
Physical dependence of opioids
|
Prolonged use produces abstinence or withdrawal syndrome:
Sleeplessness, tremor, irritability, hallucinations, seizures Severity is dependent on specific drug and duration of exposure Drugs with a slow elimination produce less severe withdrawal than drugs that act quickly, intensely, and briefly (for example, methadone vs. heroin) Typical time course: 2-3 weeks of consistent use |
|
|
Psychological dependence of opioids
|
|
|
|
Morphine
|
Agonist; high affinity for u receptor
Routes of administration: oral, IM, IV, subcutaneous, epidural, suppository High abuse potential Reduced oral bioavailability (because of glucaronidation) |
|
|
clinical uses of morphine
|
Analgesia (moderate to severe and chronic pain)
Preoperative sedation Myocardial infarction Pulmonary edema Abdominal surgical or cancer patients (epidural) |
|
|
Morphine has some adverse effects and contraindications and so should not be used in individuals with:
|
|
|
|
morpine contraindications
|
Hypersensitivity reactions
Respiratory depression Cranium injuries, raised intracranial pressure Compromised renal function |
|
|
codeine
|
Agonist; morphine analogue
Less potent than morphine Typically administered orally Excellent bioavailability Clinical uses: Mild to moderate pain Antitussive Side effects: Very sedating at analgesic doses one less OH group makes it more bioavaiable |
|
|
Morphine has a reduced oral bioavailabilty due to _____________and an active metabolite ______________ during chronic administration.
|
first pass metabolism (glucuronidation
morphine-6-glucuronide) that accumulates |
|
|
Codeine
|
is a morphine analogue and agonist, but is somewhat less potent than morphine, so it is used primarily for analgesia when the pain is mild to moderate. It is also used as an antitussive. Codeine has few side effects at low antitussive doses, but at analgesic doses it can be quite sedating. It has excellent oral bioavailability and so is typically administered orally. In addition, it produces little euphoria, so the abuse potential is low. It is commonly used in combination with nonopioid analgesics, for example, acetaminophen.
|
|
|
meperidine
|
Agonist; phenylpiperidine
Little antitussive activity or constipative effects Administered orally (reduced bioavailability), IM, subcutaneous Clinical uses: Analgesia for pulmonary patients and cancer patients Side effects: Tachycardia, blurred vision, dry mouth Metabolite (normeperidine) is proconvulsant and hallucinogenic (should not be used in patients with compromised renal or liver function) |
|
|
Fentanyl
|
Agonist; piperidine
Very potent analgesic (80-100 times greater than morphine) Administered transdermally or IV Slow onset of action Clinical uses: Analgesia Adjunct to anesthesia |
|
|
se FENTANYL
|
Possible teratogenic effects
High incidence of SIDS Bradycardia, hypotension |
|
|
SE meperidine
|
Tachycardia, blurred vision, dry mouth
Metabolite (normeperidine) is proconvulsant and hallucinogenic (should not be used in patients with compromised renal or liver function) |
|
|
clinical uses of meperidine
|
Analgesia for pulmonary patients and cancer patients
|
|
|
Levorphanol
|
Agonist; morphine analogue
Potent analgesic (5-7 times greater than morphine) Less nausea than morphine |
|
|
Levorphanol clinical uses
|
Preoperative anxiolytic
Adjunct to anesthesia |
|
|
Methadone
|
Agonist; unique structure
Tolerance and physical dependence slower to develop than morphine Longer duration and better oral bioavailability than morphine |
|
|
methadone clinical uses
|
Replacement therapy in treatment of opioid dependence
|
|
|
drug interacations and methadone
|
Tricyclic antidepressants and benzodiazepines inhibit metabolism leading to increased accumulation, prolonged half-life, intensified effects
|

