![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back
|
What is Kelvin temperature?
|
A measure of temperature that is +273 to Celsius. The pressure of the gas is proportional to its kelvin temperature.
|
|
|
What is absolute zero and how many degrees is it in Kelvin temperature?
|
The point at where particles have no energy (freezing). Absolute zero: -273.
|
|
|
What is a newton (N)?
|
Unit of force (on earth 9.8N per kg)
|
|
|
What is a pascal (Pa)?
|
Unit of pressure (N/m^2, force per area)
|
|
|
What is the formula to calculate density?
|
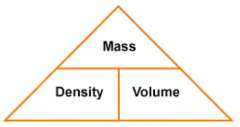
Density = mass/ volume , ρ = m/V
(Units of density- kg/m3, g/cm3) |
|
|
What is the formula to calculate pressure?
|
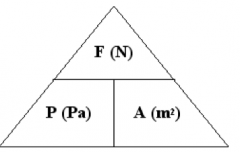
Pressure (p, pascals) = Force (F, newtons) / Area (A, m2)
|
|
|
How does the nature of pressure in liquids or gases at rest help explain the Magdeburg hemispheres?
|
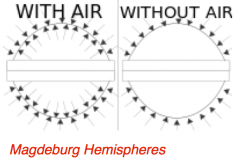
When a liquid or gas is at rest, pressure acts equally in all directions. For example, in a can with holes punched in the bottom at the same depth, water is forced out equally in all directions. This can be seen in a gas using Magdeburg hemispheres.
|
|
|
How does the depth of a liquid or gas effect the pressure?
|
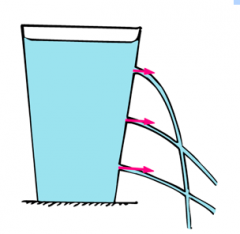
Pressure difference(p,pascals) = Depth(h,metres) x Density(p, kg/m3) x Gravitational Field Strength(g,N/kg)
p = h x p x g Pressure increases with depth |
|
|
Define Brownian Motion.
|
This it the random movement of particles of a liquid or gas that are moving around continually and bump into each other and into tiny particles.
|
|
|
How do particles in a gas act?
|
Molecules in a gas have a random motion and they exert a force caused by collisions between particles and hence a pressure on the walls of the container.
|
|
|
How does the increase of temperature effect the pressure?
|
An increase in temperature results in an increase in the speed of gas molecules as the temperature gives the molecules more kinetic energy, which will in turn increase the pressure against the walls of the container.
|
|
|
What is Boyle's Law?
|
Pressure is indirectly proportional to volume (pressure = 1/volume).
|
|
|
What happens when you change the volume or pressure of a gas?
|
p1V1=p2V2
This means that if you take a fixed mass of gas that has a pressure p1 and a volume V1 and change either the pressure or the volume, the new pressure p2 multiplied by the new volume v2 will be the same as p1V1. |

