![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
58 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Elements of Group 15 |
Nitrogen Phosphorus Arsenic Antimony Bismuth |
N P .. .. .. |
|
|
Chile Saltpetre |
NaNO3 |
|
|
|
Indian Saltpetre |
KNO3 |
|
|
|
P block ranges from? |
Group 13 to 18 |
|
|
|
Group 15 is also called |
Pnictogens |
|
|
|
E.C of group 15 |
s orbital is completely filled ns2np3s orbital is completely filledp orbital is partially filledHence E.C is extra stable ns2np3s orbital is completely filledp orbital is partially filledHence E.C is extra stable p orbital is partially filled Hence E.C is extra stable |
|
|
|
Atomic Radii grp 15 |
Inc down the grp Huge inc from N to P small inc from As->Sb->Bi due to presence of completely filled d and/or f orbitals |
|
|
|
Ionisation Enthalpy (IE) of grp 15 |
Dec downwards with inc of atomic size Greater than grp 14 due to stability by half filled p orbitals 1st IE<2nd<3rd |
|
|
|
Electronegativity of grp 15 |
Dec downwards with inc in atomic size |
|
|
|
Physical Properties of grp 15 |
All eles are polyatomic N2-gas rest are solid Metallic character inc downwards N,P- non metals As,Sb- metalloids Bi- metal BP inc downwards MP inc up to As and dec to Bi Except N all show allotropy |
|
|
|
OS of grp 15 |
-3,+3 and +5- common OS -3- dec downwards, Bi hardly forms compound +5- Dec downwards, Only Bi(V) compound is BiF5 +3- inc downwards due to inert pair effect |
|
|
|
OS of N |
+1,+2,+4 with O2 OS from +1-+4 tend to disproportionate in acid sol: 3HNO2--> HNO3+ H2O+2NO |
|
|
|
OS of P |
+1 and +4 OS in some oxoacids All intermediate OSs disproportionate into +5 and -3 in both alkali and acid |
|
|
|
Anomalous behavior of N? |
Small size High electronegativity High IE No d orbital Unique ability to form pπ-pπ multiple bonds with itself and similar eles Very high bond enthalpy as N(triple bond)N P,As and Sb form single bond Bi forms metallic bonds N-N bond weaker than P-P bond due to the repulsion of non bonding e(-)s and small bond length. Hence catenation tendency is weaker in N. Restricted covalency of 4 due to no d orbital Cannot form dπ-dπ bonds |
|
|
|
Covalency? |
No. of e(-)s shared with other atoms |
|
|
|
Reactivity towards H 15 |
Hydrides of form EH3 Stability dec from NH3 to BiH3 as bond dissociation enthalpy Dec downwards. Reducing character inc downwards Basicity dec downwards. SbH3>=BiH3 |
|
|
|
Reactivity towards O2 15 |
Oxides: E2O3 and E2O5 Acidic character Dec downwards N2O3 and P2O3- purely acidic Sb2O3 and As2O3- amphoteric Bi2O3- basic |
|
|
|
Reactivity towards X 15 |
Halides: EX3 and EX5 Due to no d orbital NX5 not formed EX5 is more covalent than EX3 since in 5 the OS is of +5 due to higher (+)ve OS of central metal atom. The atoms have greater polarity effect. 3 all are stable except NX3. NF3 is stable only. Except BiF3 all 3 are covalent |
|
|
|
Reactivity towards metals 15 |
Form binary compounds with -3 OS Eg. Ca3N2, Ca3P2, Na3As2, Zn3Sb2 and Mg3Bi2 |
|
|
|
Why no NX5 ? |
No d orbital |
|
|
|
BP PH3< BP NH3? |
EH3 stability dec downwards as bond dissociation enthalpy Dec downwards. And no H bonding. |
|
|
|
Pentahalides are more covalent then trihalides? 15 covalent then trihalides? 15 lent then trihalides? 15 |
Due to higher (+)ve OS of central metal atom ie +5. The atoms have greater polarity effect. |
|
|
|
Prep of N2 |
1. By liquefaction and fractional distillation of air. Liquid N2 distils 1st leaving liquid O2. 2. In lab: NH4Cl(aq)+NaNO2(aq)-->N2(g)+2H2O(l)+NaCl(aq) Impurities(HNO3 & NO) formed are removed gas thru aqH2SO4 with K2Cr2O7 3. Thermal decomp of ammonium dichromate: (NH4)2Cr2O7(heat)--> N2(g)+2H2O(l)+NaCl(aq) 4. Very pure N2 by thermal decomp of Na/Ba azide: Ba(N3)2-->Ba+3N2 |
|
|
|
Pure N2 can be obtained by? |
Thermal deconp of Na/Ba azide: Ba(N3)2--> Ba+3N2 |
|
|
|
Prop of dinitrogen |
Colourless, odourless, tasteless and non toxic gas. 2 isotopes 14N & 15N. Low solubility in water Low BP and FP Inert at room temp due to the high bond enthalpy of N triple bond N. Reactivity inc with inc in temp At high temp with metals gives ionic nitrides and with non metals gives covalent nitrides: 6Li/3Mg+ N2--> 2Li3N/Mg3N2 HABER'S PROCESS At 773K with catalyst forms ammonia: N2(g)+3H2(g) --> 2NH3(g) Enthalpy of formation= -46.1 kJ/mol At high temp of 2000K: N2+O2--> NO(g) |
|
|
|
Manufacture of ammonia |
Haber's Process |
|
|
|
Uses of N2 |
Manufacture of ammonia Refrigerant Cryosurgery For inert atmosphere |
|
|
|
N2 is inert at room temperature? |
Due 2 high bond enthalpy of N triple bond N. |
|
|
|
Prep of ammonia |

1. In air and soil on decomp of urea: NH2CONH2+2H2O-->(NH4)2CO3<=>2NH3+H20+CO2 2. Decomp of ammonium salts with caustic soda ( Ca(OH)2): Photo* 3. HABER'S PROCESS N2(g)+3H2-->2NH3(g) Enthalpy of formation -41.6 kJ/mol Temp~700K Pressure of 20*10^5 Pa~200 atm atmTemp~700KCatalyst iron oxide with small amounts of K2O and Al2O3. Earlier Fe was catalyst and molybdenum was promoter.Used for large scale production of ammonia. Catalyst iron oxide with small amounts of K2O and Al2O3. Earlier Fe was catalyst and molybdenum was promoter.Used for large scale production of ammonia. . Earlier Fe was catalyst and molybdenum was promoter. Used for large scale production of ammonia.
|
|
|
|
Haber's Process? |
HABER'S PROCESS N2(g)+3H2-->2NH3(g)Enthalpy of formation -41.6 kJ/molPressure of 20*10^5 Pa~200 atmTemp~700KCatalyst iron oxide with small amounts of K2O and Al2O3. Earlier Fe was catalyst and molybdenum was promoter.Used for large scale production of ammonia.
|
|
|
|
Structure of NH3 |
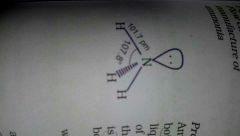
Trigonal pyramidal with N atom at the apex. Has 3 bond pairs and 1 lone pair. |
|
|
|
Phy prop of ammonia |
Colorless gas with pungent smell In solid and liquid states associated by H bonding which results in higher MP & BP than expected. Trigonal pyramidal with N atom at the apex. Has 3 bond pairs and one lp. |
|
|
|
Chem prop of ammonia |
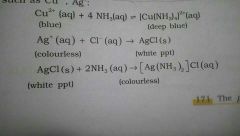
Higly soluble in water: NH3(g)+H2O--> NH4+(aq) + OH-(aq) Forms ammonium salts with acids such as ammonium chloride and Ammonium Sulphate. Ammonium hydroxide is a weak base: Hence it forms Hydroxide of many metals from their salt solution: Zns o4+nh4oh --> zn (oh)2(white solid ppt)+(nh4)2so4 Fecl3+nh4oh-->fe2o3.xh2o(brown solid ppt)+NH4Cl Presence of lp of e(-) on N makes Ammonia a Lewis base. This property is used for detection of metal ions of Copper and silver: photo* |
|
|
|
Uses of ammonia |
Production of nitrogenous fertilizers Manufacturer of inorganic nitrogen compounds like nitric acid Liquid ammonia is used as a refrigerant |
|
|
|
Why does Ammonia act as a Lewis base? |
N and has one pair of electrons available for donation |
|
|
|
Mention the conditions required to maximise the yield of ammonia |
Temp~700KPressure of 20*10^5 Pa~200 atm atmTemp~700KCatalyst iron oxide with small amounts of K2O and Al2O3. Earlier Fe was catalyst and molybdenum was promoter.Used for large scale production of ammonia.Catalyst iron oxide with small amounts of K2O and Al2O3 |
|
|
|
How does Ammonia react with a solution of Cu2+? |

|
|
|
|
Oxides of nitrogen |

|
|
|
|
Resonance structures of Nitrogen oxide |

|
|
|
|
Why does NO2 dimerise? |
It contains odd number of Valence Electrons. It behaves as a typical odd molecule. on dimerisation it is converted to stable N2O4 with even number of electrons. |
|
|
|
Nitrous oxide? |
Dinitrogen oxide N2O |
|
|
|
Nitric oxide? |
Nitrogen monoxide NO |
|
|
|
Prep of Nitrous oxide and phy prop and structure |
Nh4NO3-->N2O+2H2O(heat) Colourless gas neutral Linear |
|
|
|
Prep of Nitric acid and phy prop and structure |
2Nano2+2feso4+3h2so4-->fe2(so4)3+2nahso4+2h2o+2no Colourless gas neutral Linear |
|
|
|
Prep of dinitrogen trioxide and phy prop Nitrogen (III) oxide and structure |
2NO+N2O4-->(250K)2N203 BLUE SOLID ACIDIC Planar |
|
|
|
Preparation of Nitrogen dioxide and physical properties and structure Nitrogen (IV) oxide |
2pb(no3)2-->(673K)4no2+2pbo+O2 Brown gas acidic Angular |
|
|
|
Dinitrogen tetroxide preparation and physical properties and structure Nitrogen (IV) oxide |
2no2-->(cool)<--(heat)n2o4 Colourless solid or liquid acidic Planar |
|
|
|
Dinitrogen pentoxide preparation and physical properties and structure Nitrogen (V) oxide |
4 hn O3+P4 o10-->4 HP O3+2 n2o5 Colourless solid acidic Planar |
|
|
|
Which N oxide is a blue solid? |
N2O3 |
|
|
|
Which N oxide is a brown gas? |
NO2 |
|
|
|
Hyponitrous acid |
H2 N2 O2 |
|
|
|
Nitrous acid |
H No 2 |
|
|
|
Preparation of nitric acid |
1. By heating Chile saltpetre and Indian salt battery with concentrated H2 S o4 in a glass retort: Nano3+h2so4-->nahso4+HNO3 2. Large scale by OSTWALD'S PROCESS which is catalytic oxidation of NH3 by atmospheric oxygen: 4nh3+5o2-->(pt/Rh gauge catalyst and 500K, 9 bar) 4 NO+6h2o 2NO+O2<=>2NO2 3NO2+H2O--> 2HNO3+NO NO formed is recycled and the aq hno3 can be concentrated by distillation of 2 68% by mass. 98% concentration can be achieved by dehydration with concentrated H2 s o4. |
|
|
|
Explain OSTWALD'S PROCESS |
Large scale preparation of Nitric acid by OSTWALD'S PROCESS which is catalytic oxidation of NH3 by atmospheric oxygen:4nh3+5o2-->(pt/Rh gauge catalyst and 500K, 9 bar) 4 NO+6h2o2NO+O2<=>2NO23NO2+H2O--> 2HNO3+NONO formed is recycled and the aq hno3 can be concentrated by distillation of 2 68% by mass. 98% concentration can be achieved by dehydration with concentrated H2 s o4. |
|
|
|
Properties of nitric acid(physical) |
Colourless liquid Planar molecule Strong acid Strong oxidising agent and attacks most Noble metals |
|
|
|
Chemical properties of nitric acid |
Hn O3+H2O-->h3o+ + no3- With metals: 3 CU+hn O3 dilute-->3 CU (no3)2+2 no+4 H2O Cu+4HNO3(CONCENTRATED)-->Cu(NO3)2+2NO2+2H2O 4Zn+10HNO3(dilute)-->4Zn(NO3)2+5H2O+N2O Zn+4HNO3(CONCENTRATED)-->Zn(NO3)2+2H2O+2NO2 Metals like Cr and Al don't dissolve in concentrated nitric acid because of the formation of a passive film of oxide on the surface. With non metals: I2+10HNO3-->2HIO3+10NO2+4H2O C+4HNO3-->CO2+2H2O+4NO2 S8+48HNO3-->8H2SO4+48NO2+16H20 P4+20HNO3-->4H3PO4+20NO2+4H20 |
|
|
|
Explain the brown ring test |
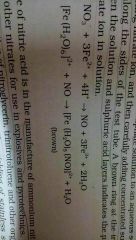
This is a test for nitrates depending on the availability of Fe2+to reduce nitrates to nitric oxide which reacts with Fe2+to form a brown coloured complex. Aq solution containing nitrate ion is mixed with dilute ferrous sulphate solution along with a few drops of concentrated sulphuric acid. A brown ring at the interface between the solution and Sulphuric Acid layers indicates the presence of nitrate ion in a solution. Reaction:*photo |
|
|
|
Uses of nitric acid |
Manufacture of Ammonium Nitrate for fertilizers explosives and pyrotechnic. In a preparation of nitro organic compounds Pickling of stainless steel etching of metals and as an oxidizer in rocket fuel |
|

