![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
6 Cards in this Set
- Front
- Back
|
What are the main gases that make up the air? Give the percentages. |
Nitrogen- 78.1% Oxygen- 21% Argon- 0.9% Carbon Dioxide- 0.04% |
|
|
A metal oxide is formed when a metal and oxygen are reacted together. When metal oxides are dissolved in water, are they acidic, neutral or alkaline? |
Alkaline |
|
|
What happens when you combust a non metal with oxygen? |
You get a dioxide (acidic). |
|
|
Describe an experiment that proves that the air is 20% oxygen. |
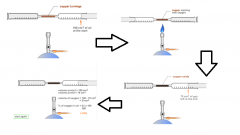
-Pushing air with syringes over copper -Copper reacts with oxygen -After all copper has reacted, 20% of the air will gone as it has solidified to form copper oxide
|
|
|
How can oxygen be created in the lab? |

This experiment uses the downward displacement of water to collect a gas. This is a very common way to collect a gas in chemistry. The oxygen that is evolved in this reaction moves along the delivery tube and then collects in the top of the test tube. It does this because it is lighter than water and so displaces the water downwards. If you were to blow out a lighted splint, and then put it in the oxygenated test tube (quick enough so that the oxygen does not escape) it will relight; this again proves that oxygen has been made. |
|
|
How can carbon dioxide be created in the lab. Give the equation. |
By reacting a carbonate and an acid in a test tube with a side arm. The side arm should be attached (by a coil) to another test tube containing limewater. Carbon dioxide will be formed and will turn the limewater from clear to cloudy. E.g. Calcium carbonate + Hydrochloric acid --> Calcium chloride + Water + Carbon dioxide |

