![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
What does the IR spectra measure?
|
The infrared spectrum of a sample is recorded by passing a beam of infrared light through the sample. When the frequency of the IR is the same as the vibrational frequency of a bond, absorption occurs. Examination of the transmitted light reveals how much energy was absorbed at each frequency (or wavelength). Analysis of the position, shape and intensity of peaks in this spectrum reveals details about the molecular structure of the sample.
|
|
|
What is the IR spectra of a carboxylic acid?
|
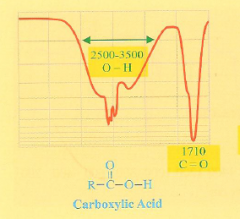
2 major dips. A sharp dip at 1700 cm-1 (C=O) and an O-H dip at 2500-3500 cm-1)
|
|
|
What is the IR spectra of an aldehyde?
|
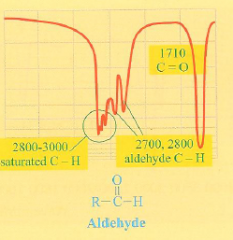
A sharp dip at 1700 cm-1 (C=O) and wide dip with pointy fingers at 2800-3000 cm-1 representing a saturated C-H bond.
|
|
|
What is the IR spectra of an alcohol?
|
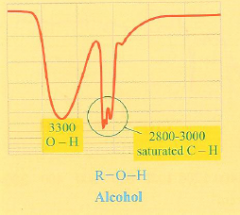
wide dip with pointy fingers at 2800-3000 cm-1 representing a saturated C-H bond and an OH dip around 3300 cm-1
|
|
|
What is the IR spectra of an amine?
|
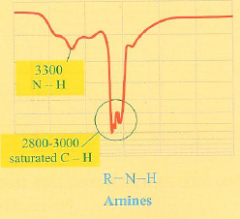
Exactly same as for an alcohol but the dip for N-H is much smaller. wide dip with pointy fingers at 2800-3000 cm-1 representing a saturated C-H bond and an N-H dip around 3300 cm-1
|
|
|
What is the IR spectra for a nitrile?
|
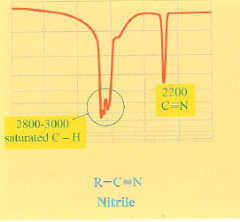
New dip to remember around 2200 cm-1. Don't forget the C-H bond at 2800-3000 cm-1
|
|
|
What is the IR spectra for an amide?
|
-This is going to get messy. There are C-H (2800-3000cm-1). A C=O bond (1700cm-1) and an bond and a N-H (3300cm-1) bond. Here the N-H bond dip is much larger and really looks like the O-H dip except it has an invagination. Not a perfect, uniform dip so watch out for that.
|
|
|
What is an amide
|
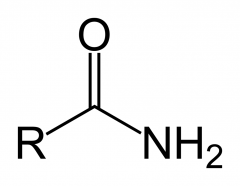
amine to ketone
|
|
|
electron withdrawing groups
|
..
|
|
|
electron donating groups
|
..
|

