![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
6 Cards in this Set
- Front
- Back
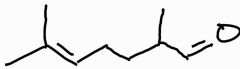
What is the systematic name for melonal |
2, 6 - dimethylhept-5-enal |
|
|
A pratical measure to maximise the yield of melonal |
Distill off to prevent further oxidation. So carboxylic acid doesn't form |
|
|
D |
D |
|
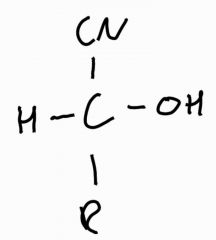
Would you expect this molecule to rotate in plane polarized light |
No the molecule is planar, so can be attacked from both sides. The racemic gives equal amounts of each isomer |
|
|
State and explain how you would quench the reaction of iodine with propanone |
Add excess sodium hydrogen carbonate, to remove acid |
|
|
Propanoic acid and methanol react to form an ester, name the ester |
Methyl proponate |

