![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
- 3rd side (hint)

|
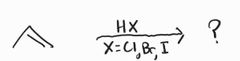
|
Hydrohalogenation -Markovnikov Addition -Racemic Mixture -Susceptible to carbocation rearrangement |
|

|
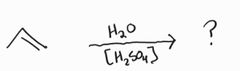
|
Acid Catalyzed Hydration -Markovnikov -Racemic -Carbocation Rearrangement |
|

|
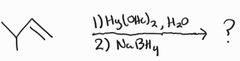
|
Oxymercuration-Demercuration -Markovnikov -No Carbocation Rearrangement |
|
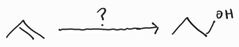
|
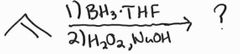
|
Hydroboration Oxidation -Anti-Markovnikov -Syn Addition |
|
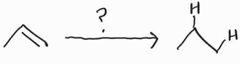
|
|
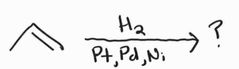
Catalytic Hydrogenation -Syn Addition |
|
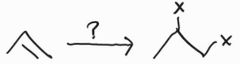
|
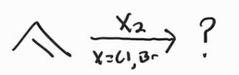
|
Halogenation -Anti Addition |
|
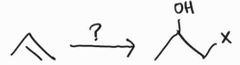
|
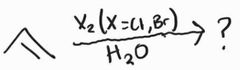
|
Halohydrin Formation -OH group at more substituted position -Anti Addition |
|

|
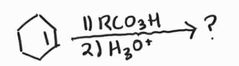
|
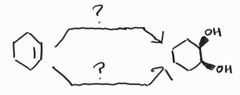
Anti Dihydroxylation -Epoxide intermediate -MCPBA is a common peroxy acid |
|
|
|
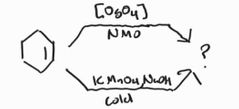
Syn Dihydroxylation -OsO4 acts as a catalyst when paired with a co-oxidant |
|

|
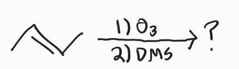
|
Ozonolysis |
|

|
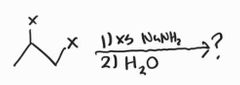
|
Preparation of Alkynes -E2 reaction with a strong base (NaNH2) |
|
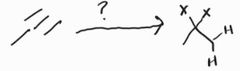
|
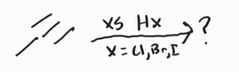
|
Hydrohalogenation (Excess) -Markovnikov |
|
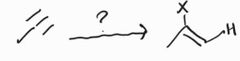
|
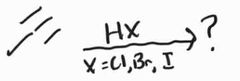
|
Hydrohalogenation -Markovnikov |
|

|
|
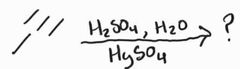
Acid-Catalyzed Hydration -Markovnikov -Acid-catalyzed keto-enol tautomerization favors ketone product |
|
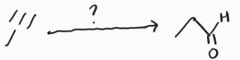
|
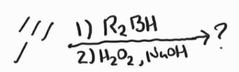
|
Hydroboration Oxidation -Anti-Markovnikov -Disiamylborane and 9-BBN are examples of R2BH -Undergoes base-catalyzed tautomerization |
|
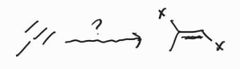
|
|
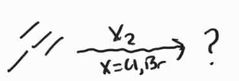
Halogenation -Anti Addition |
|

|
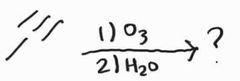
|
Ozonolysis -Forms carboxylic acids/carbon dioxide |
|

|
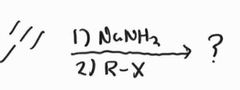
|
Alkylation |
|
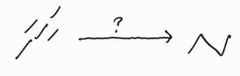
|
|
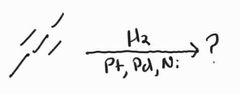
Catalytic Hydrogenation |
|

|

|
Catalytic Hydrogenation (Poisoned Catalyst) -Lindlars Catalyst: Pd prepared with CaCO3 -Syn Addition |
|

|
|
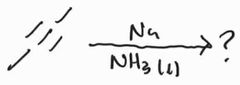
Dissolving Metal Reduction -Anti Addition |
|

|
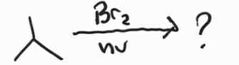
Radical Bromimation -Occurs at more substituted position -Racemic |
|
|

|

|
Anti-Markovnikov Addition of HBr -Requires trace amounts of alkyl peroxide |
|

|

|
Allylic Radical Bromimation -Avoids the addition of Br2 across the pi bond -NBS is an alternative source of radical bromine |

