![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
Functional group
|
Reactive part of the molecule
|
|
|
Family of compounds
|
Contains the same functional group
|
|
|
Homologous series
|
The compounds belong to the same family and only differ by the length of the carbon chain i.e, a -CH2
|
|
|
General formula
|
Formula representing a homologous series of a family of compounds.
|
|
|
General formula - Alkanes, alkenes and alcohols
|
Alkanes - CnH2n+2
Alkenes - CnH2n Alcohols - CnH2n+1OH |
|
|
Structural isomerism
|
Same number and type of atoms can be arranged in different ways to form structural isomers.
|
|
|
Functional group and systematic name - alkane and alkene
|
Alkane: C-C -ane
Alkene: C=C -ene |
|
|
Alcohol - functional group + systematic name
|
F.group: -OH Name: -ol
|
|
|
Halogenalkane - Functional group + systematic name
|
F.name: -Cl Name: chloro-
-Br bromo- -I iodo- -Fl fluro- |
|
|
Ketone - Functional group + systematic name
|
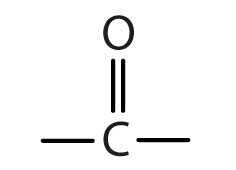
Name: -one
|
|
|
Aldehyde - Functional group + systematic name
|
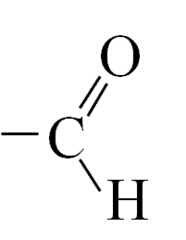
Name: -al
|
|
|
Carboxylic acid - functional group + systematic name
|
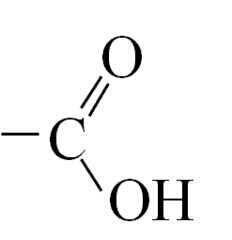
Name: -oic acid
|

